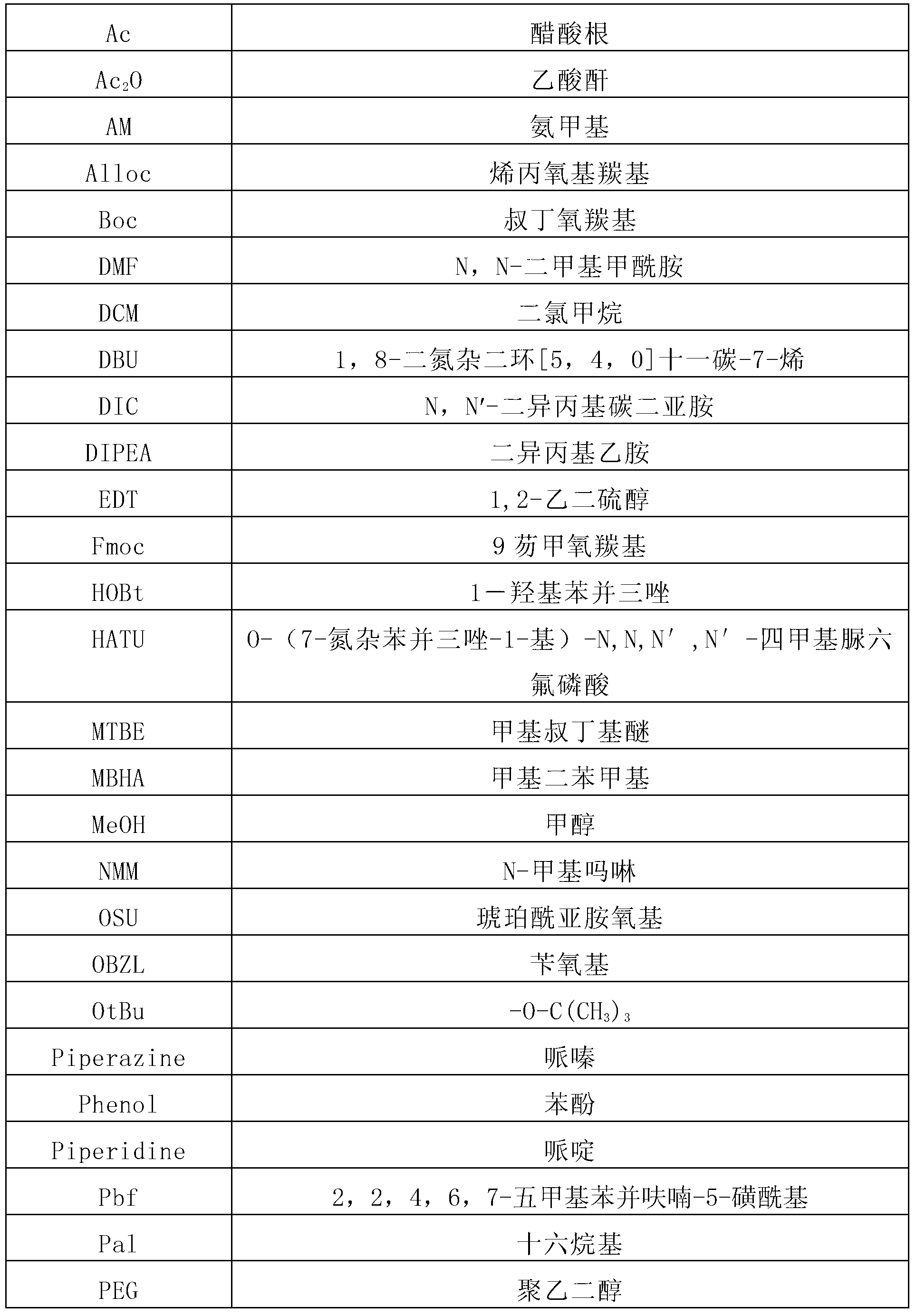
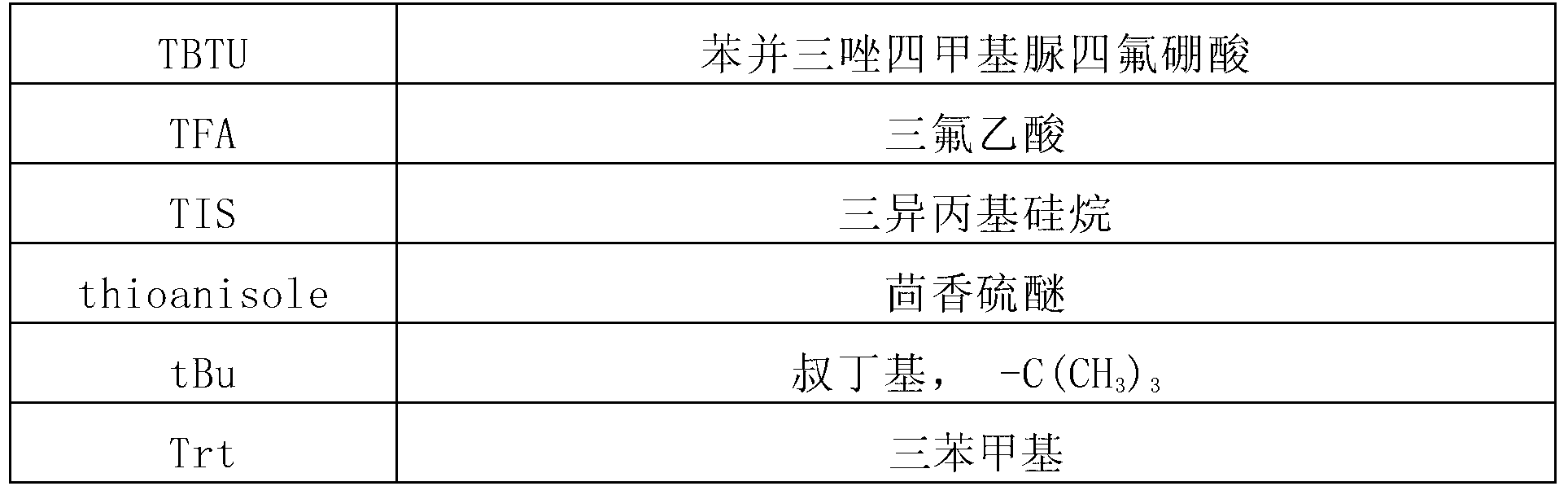
Synthetic method of liraglutide
A technology of liraglutide and synthetic methods, which is applied in the field of chemical synthesis of pharmaceutical polypeptide raw materials, and can solve problems such as difficult removal of impurities, introduction of viruses, unfavorable medicinal purposes of products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The liraglutide solid-phase synthesis method provided by the present invention uses Fmoc-Gly-solid-phase synthetic resin as a starting material, and undergoes the following steps:
[0079] Deprotection step: mixing and swelling the protected amino acid or polypeptide-solid-phase synthetic resin and DCM in the reactor, adding piperidine / DMF solution for deprotection;
[0080] Feeding and condensation step: the protected amino acid, HOBt and DIC with protecting group are dissolved in the mixed solution of DMF / DCM and poured into the above-mentioned reactor, and the ninhydrin detection is used to judge whether the condensation is complete; DMF / DCM (30 %-70%)
[0081] Repeat the deprotection and condensation steps to extend the peptide chain from the C-terminus to the N-terminus until the 1# amino acid is synthesized to obtain a directly connected peptide with a protecting group.
[0082] In the above deprotection step, sufficient swelling is required;
[0083] In the abo...
Embodiment 1
[0119] Weigh 19.23g (5mmol) Fmoc-Gly-Wang Resin into the reaction column, add 20% piperidine / DMF (V / V) solution twice for deprotection. After washing, take out a small amount of resin indene test, it is positive. Weigh 9.73g of Fmoc-Arg(Pbf)-OH and 2.43g of HOBt, add about 50mL of DMF to dissolve, add 2.8mL of DIC to activate, pour it into the reaction column at room temperature for 2h, and detect ninhydrin. washing. Repeat the above steps of deprotection, washing and condensation until the condensation of N-terminal Fmoc-His(Trt)-OH is completed, the protected amino acid at position 20 is Fmoc-Lys(Boc)-OH, the N-terminal Fmoc protecting group is removed and DMF is added for washing. Weigh 4.25g of Z(2-Cl)-OSu and dissolve it with an appropriate amount of DMF, add it to the above resin for reaction, detect ninhydrin, and dry to obtain 41.2g of liraglutide intermediate peptide resin. The yield of peptide resin is about 91%. The above resin was added to the cleavage formula re...
Embodiment 2
[0122] Weigh 27.0g (10mmol) Fmoc-Gly-WangResin and put it into the reaction column, add appropriate amount of DCM to dissolve it, and filter it with suction. Add 20% piperidine / DMF (V / V) solution to deprotect twice. Then add DMF, MeOH, DCM to wash in sequence, take a small amount of resin indene after extraction, it is positive. Weigh 12.98g of Fmoc-Arg(Pbf)-OH, 7.21g of HBTU and 2.70g of HOBt, add about 80mL of DMF to dissolve, bath in ice water for 2min, add 3.5mL of DIPEA for activation for about 10min, pour it into the reaction column for 2h at room temperature, ninhydrin detection. After the reaction was completed, the reaction solution was withdrawn, added DMF to wash twice, and the above steps of deprotection, washing and condensation were repeated until the condensation of the N-terminal His was completed. 20# and 1# protected amino acids are Fmoc-Lys(Dde)-OH and Z-His(Trt)-OH respectively.
[0123] Prepare 100 mL of 2% hydrazine hydrate / DMF solution and add it to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com