Solid-phase synthesis method of liraglutide
A technology of liraglutide and solid-phase synthesis, which is applied to the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of high cost of reagents, many side reactions, production restrictions, etc. The effect of small quantity, beneficial to industrial production, and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
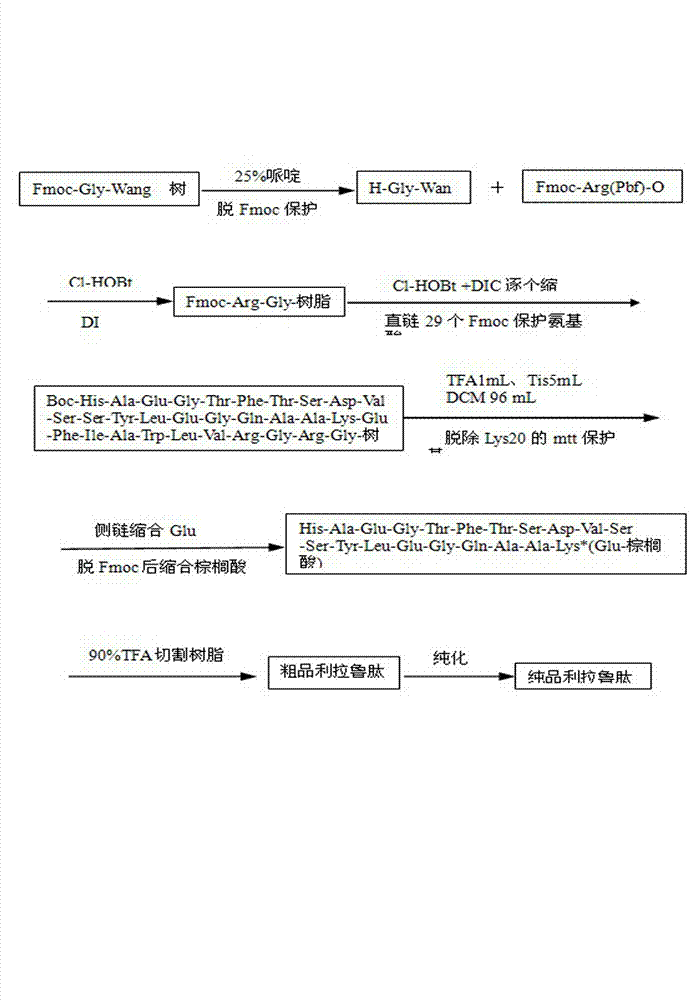
[0025] Example 1 Condensation of linear liraglutide
[0026] Weigh 370mg (0.1mmol) Fmoc-Gly-Wang resin (0.27mmol / g) into the reactor, and swell the DMF solution for 30min. Weigh the amino acids to be reacted in each step in advance, and the dosage is 5 times that of the resin (0.5mmol). The first amino acid Gly at the C-terminal is already on the resin, and the reaction starts from Arg. Reaction steps: 25% piperidine / DMF solution to deresin Fmoc protecting group twice, each time for 15min, wash the resin twice with DMF, methanol, DCM successively, drain, add the first step reactant Fmoc-Arg(Pbf) -OH and condensing agent: 1.1mL of Cl-HOBt and DIC solution (DIC solution: 8mL DIC plus DMF to 100mL; Cl-HOBt solution: 8.5g Cl-HOBt plus DMF to dissolve to 100mL;), shake the reaction at room temperature for 2h, DMF , methanol and DCM to wash the resin twice, and drain. According to the amino acid sequence of liraglutide straight chain His-Ala-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Se...
Embodiment 2
[0027] Example 2 Removal of Mtt protecting group and condensation side chain g-glutamic acid and palmitic acid on Lys20
[0028] Add about 10 mL of newly prepared cleavage reagent A: (TFA 1 mL; Tis 5 mL; DCM 96 mL), shake at room temperature for 5 times, each time for 5 min, wash with DCM, methanol, DMF, DCM twice each, and drain. Add Fmoc-Glu-OtBu (0.213 g) and condensing agent (1.1 mL of Cl-HOBt and DIC solution) to react for 2 hours, de-Fmoc twice with 25% piperidine / DMF solution, drain, wash the resin with DMF, methanol and DCM for 2 hours Once, drained, add 10 times the amount of palmitic acid (0.256 g), 2.2 mL of Cl-HOBt and DIC solution to react for 3 h, wash and drain the polypeptide resin.
Embodiment 3
[0029] Example 3 Cutting the polypeptide from the resin
[0030]Liraglutide peptide resin, add cleavage reagent B: (TFA: 9mL; anisole: 0.25mL; phenol: 0.25g; Tis: 0.25mL; water: 0.25mL) at room temperature for 2 hours, take the filtrate to a 50mL centrifuge tube , wash the resin twice with a small amount of TFA (1 mL), and combine the filtrates. Add anhydrous diethyl ether (cooled in ice in advance) directly to the centrifuge tube to more than 45mL, and a slightly pinkish white precipitate can be seen immediately. After mixing thoroughly, place the centrifuge tube in ice to cool for 30min. After centrifugation, discard the supernatant, wash the crude peptide precipitate with ether for 2-3 times, and then dry it in the air. Finally, 332.7 mg of crude product was obtained, and the yield of crude peptide was 88.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com