Preparation method of liraglutide intermediate polypeptide
A technology of expressing vectors and recombinant vectors, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Construction of Recombinant Engineering Bacteria
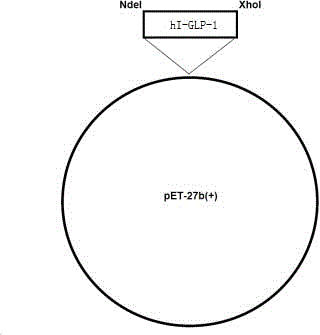
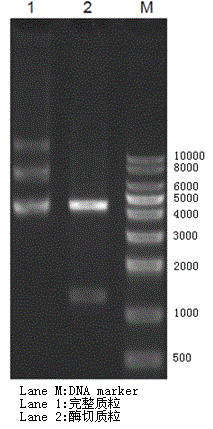
[0038] Synthesize the gene of hI-GLP-1 fusion protein by conventional chemical synthesis method, insert the obtained sequence cDNA into the corresponding restriction site of plasmid pET-27b(+) through NdeI / XhoI restriction site, and construct the Recombinant plasmids such as figure 1 As shown, the electrophoresis after digestion of the recombinant plasmid is as follows figure 2 As shown, the recombinant plasmid inserted into the gene encoding hI-GLP-1 fusion protein was transformed into the host Escherichia coli by conventional chemical transformation method to construct recombinant engineering bacteria. like Figure 3-4 As shown, after sequencing, the sequence in the engineered bacteria was consistent with the design.
Embodiment 2
[0039] Example 2 High-density fermentation
[0040] Inoculate the positive clone of the recombinant engineered bacteria obtained in Example 1 in 10 mL of LB medium, 30 ° C, 250 rpm for overnight culture, then inoculate in 250 mL of LB medium at a ratio of about 0.4%, and shake to OD 600When the value reached 2, it was used as a seed liquid, and was inserted into 12L of fermentation medium for high-density cultivation. The initial fermentation temperature is 30°C, the stirring speed is 300rpm, the ventilation rate is 30L / min, and the pH is 6.7. Afterwards, the stirring speed and ventilation rate are continuously increased to 1000rpm and 80L / min respectively to maintain the dissolved oxygen above 30%. Because of high-density fermentation A large amount of oxygen is needed. If the oxygen supply is insufficient, it will not only inhibit the respiration of the bacteria, limit the growth and reproduction of the bacteria, but also accumulate harmful substances that will poison the ba...
Embodiment 3
[0046] Embodiment 3 Purification of polypeptide intermediate
[0047] After leaving the tank, the cells obtained in Example 2 were collected by centrifugation, and the crushing buffer was added at a weight-to-volume ratio of 1:10. The high-pressure homogenizer was crushed twice under a pressure of 700 bar, and the inclusion body precipitate was collected by centrifugation. The wet weight of the inclusion body was 30 g / L fermentation broth. The precipitate was added to the washing buffer at a weight-to-volume ratio of 1:10, stirred magnetically at room temperature for 1 hour, and the precipitate collected by centrifugation was washed twice with the washing buffer. The inclusion body dissolving buffer was then dissolved overnight at a weight-to-volume ratio of 1:10. After the dissolved inclusion bodies are centrifuged and ultrafiltered to remove impurities, such as Figure 9 As shown, dilute to a protein concentration of 0.2 mg / ml, refold overnight at 4°C in refolding buffer, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com