Fusion protein and method for preparing liraglutide intermediate polypeptide
A fusion protein and intermediate technology, which is applied in the field of genetic engineering and polypeptide preparation, can solve the problems of long dissolution and renaturation time of inclusion bodies, unsuitable for large-scale production, large renaturation volume, etc., and is conducive to industrial scale-up , Reduce the renaturation process and reduce the effect of the enzyme digestion system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
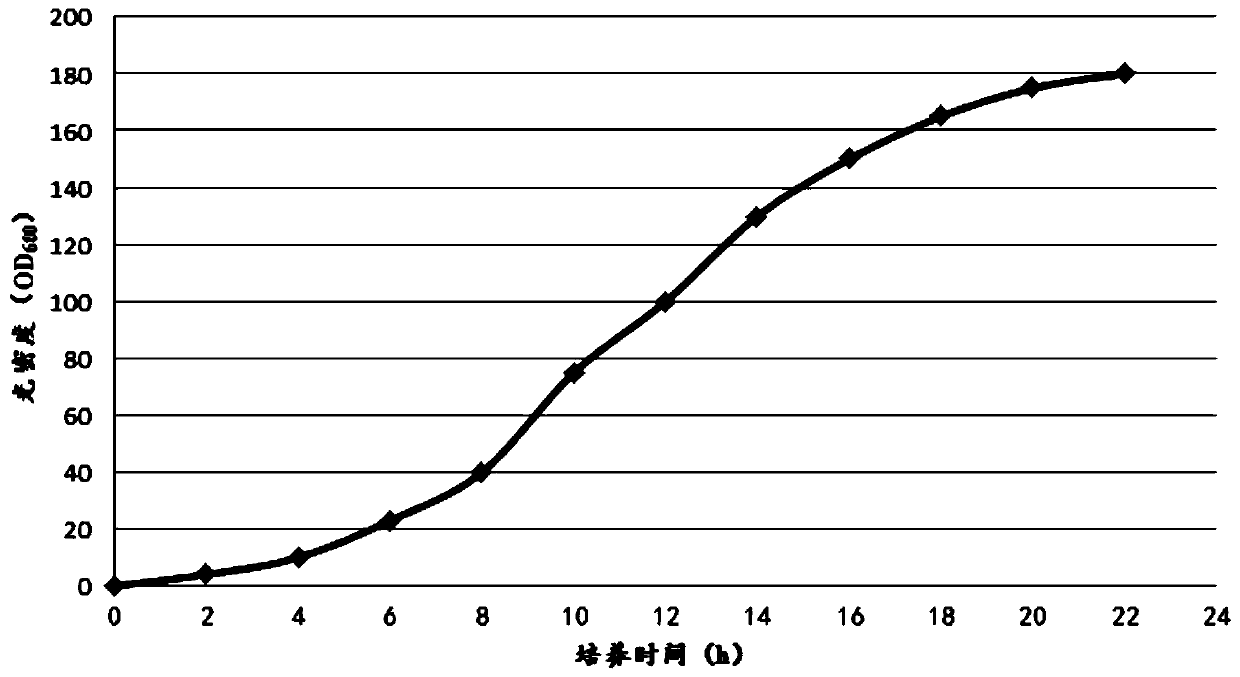
Examples
Embodiment 1
[0061] The construction of embodiment 1 recombinant engineering bacterium
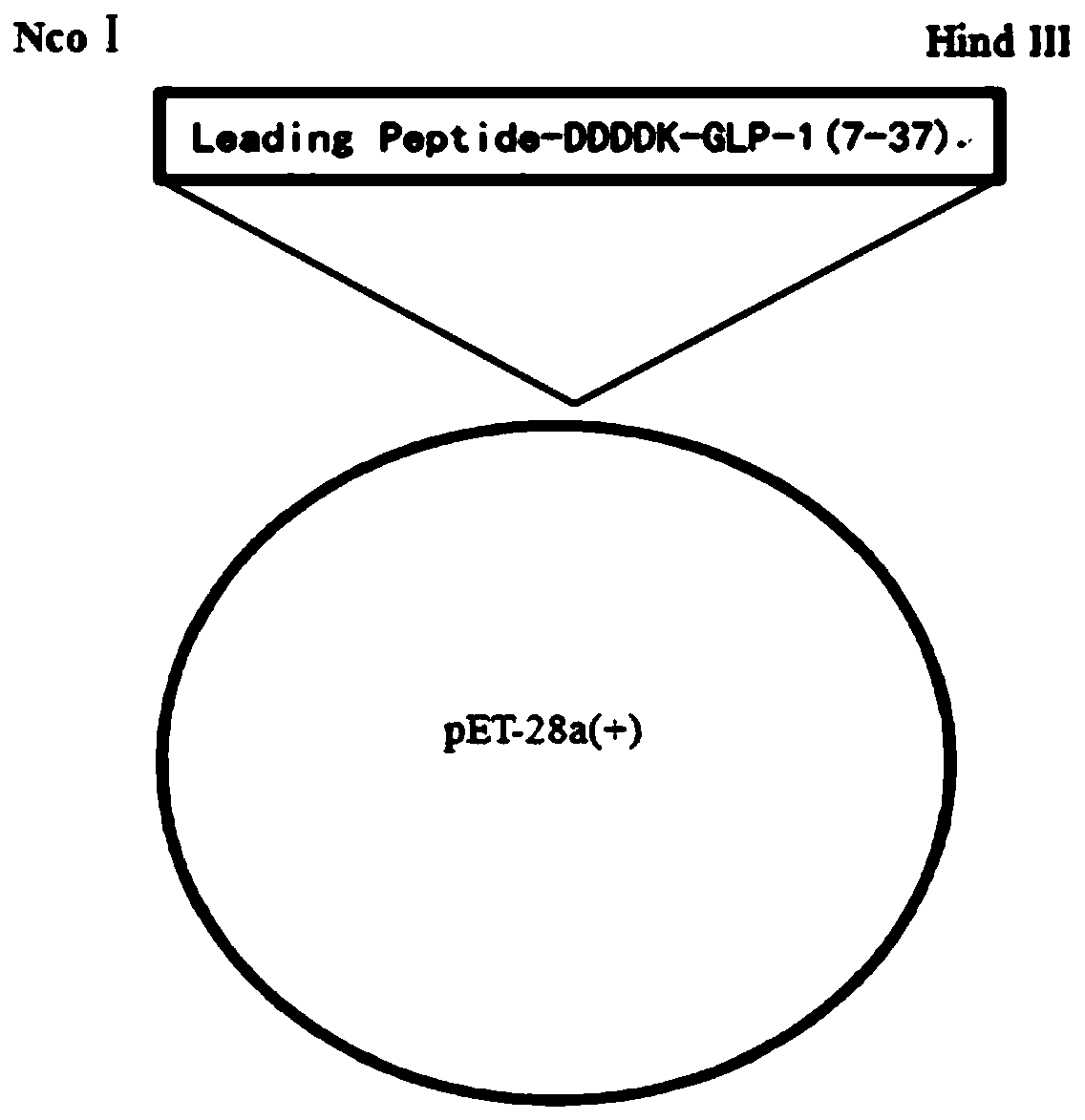
[0062] Design of a fusion protein sequence to the propeptide of liraglutide for expression in E. coli. The N-terminal leader peptide sequence can enhance expression and protect the liraglutide propeptide fusion protein from being degraded by Escherichia coli. A preferred leader peptide sequence is MATHAVSVLKGDGPVQGIINFEQHESNGPVKVWGSIHGLTEGLHGFHVHKFVNQHLCGSHLVALYLV (SEQ ID NO. 3). The C-terminus of the leader peptide sequence is connected to the liraglutide propeptide through DDDDK residues, and the leader peptide is removed by enzymatic digestion with enterokinase. Therefore, the full sequence of the fusion protein Leading Peptide-DDDDK-GLP-1 (7-37) with the leading peptide SEQ ID NO.3 is SEQ ID NO.9, and the specific sequence is as follows:
[0063] MATHAVSVLKGDGPVQGIINFEQHESNGPVKVWGSIHGLTEGLHGFHVHKFVNQHLCGSHLVALYLVDDDDKHAEGTFTSDVSSYLEGQAAKEFIAWLVRGRG;
[0064] The gene fragment of the above-mentio...
Embodiment 2
[0065] The construction of embodiment 2 recombinant engineering bacteria
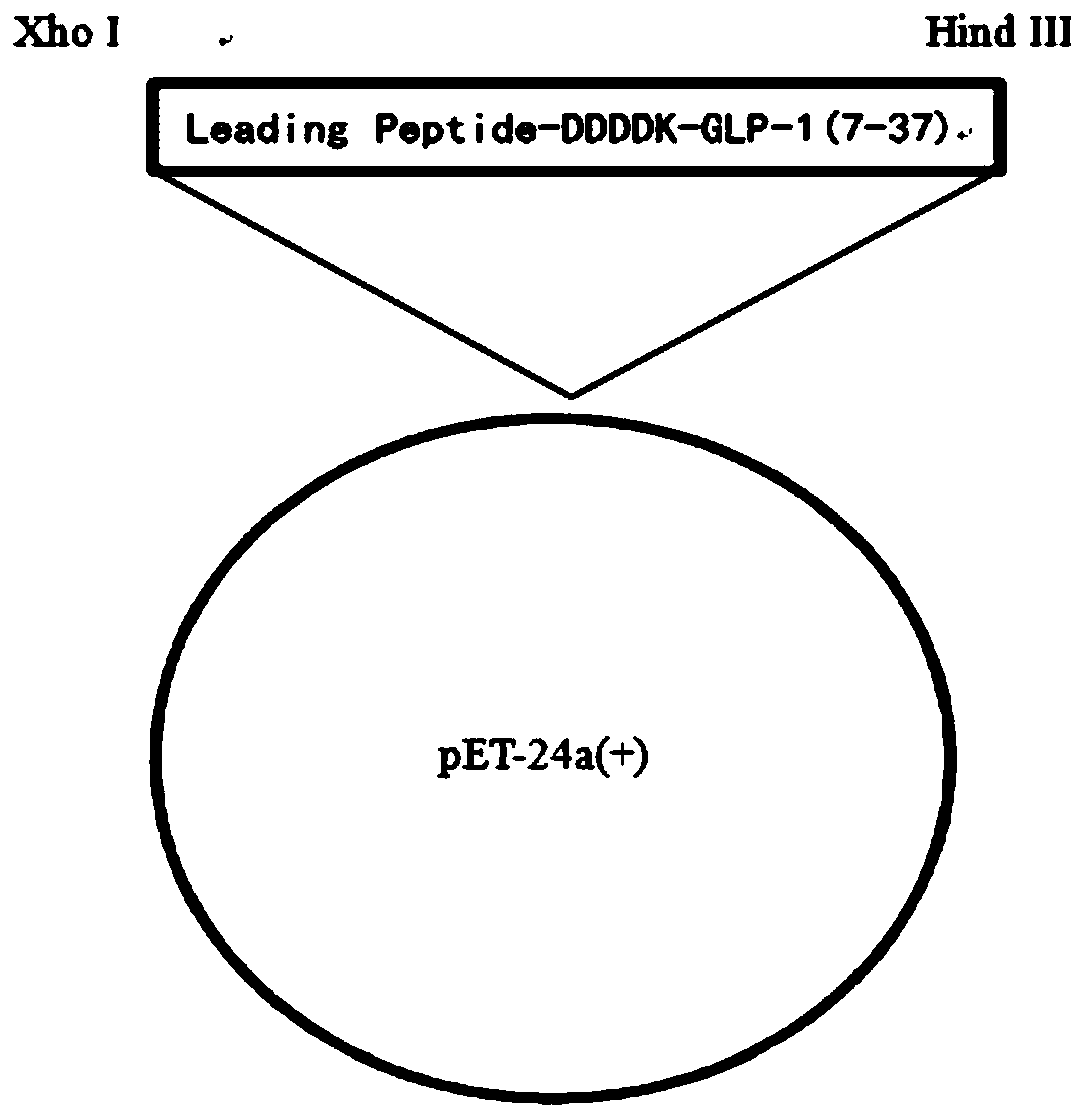
[0066] Refer to the same method as in Example 1 to design the full sequence of the fusion protein LeadingPeptide-DDDDK-GLP-1 (7-37) comprising the leading peptide SEQ ID NO.4.
[0067] The gene of the above-mentioned Leading Peptide-DDDDK-GLP-1(7-37) fusion protein was synthesized by conventional fusion PCR technology, and the obtained sequence cDNA was inserted into the plasmid pET-24a(+ ) in the corresponding restriction site, the constructed recombinant plasmid is as follows figure 2 As shown, the recombinant plasmid inserted into the gene encoding the fusion protein of Leading Peptide-DDDDK-GLP-1 (7-37) was transformed into the host Escherichia coli HMS174 (DE3) by conventional genetic engineering techniques.
Embodiment 3
[0068] The construction of embodiment 3 recombinant engineering bacteria
[0069] Referring to the same method as in Example 1, the full sequence of the fusion protein LeadingPeptide-DDDDK-GLP-1 (7-37) comprising the leading peptide SEQ ID NO.5 was sequentially designed.
[0070] The gene of the above-mentioned Leading Peptide-DDDDK-GLP-1 (7-37) fusion protein was synthesized by conventional fusion PCR technology, and the obtained sequence cDNA was inserted into the plasmid pET-29a (+ ) into the corresponding restriction site, and insert the recombinant plasmid encoding the Leading Peptide-DDDDK-GLP-1 (7-37) fusion protein gene into the host Escherichia coli JM109 (DE3) by conventional genetic engineering techniques.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com