Method for synthesizing liraglutide
A synthetic method, liraglutide technology, applied in the field of peptide synthesis, can solve problems such as low yield, incomplete reaction, adverse effects on drug quality, etc., achieve high yield, reduce production cost, and shorten synthesis time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
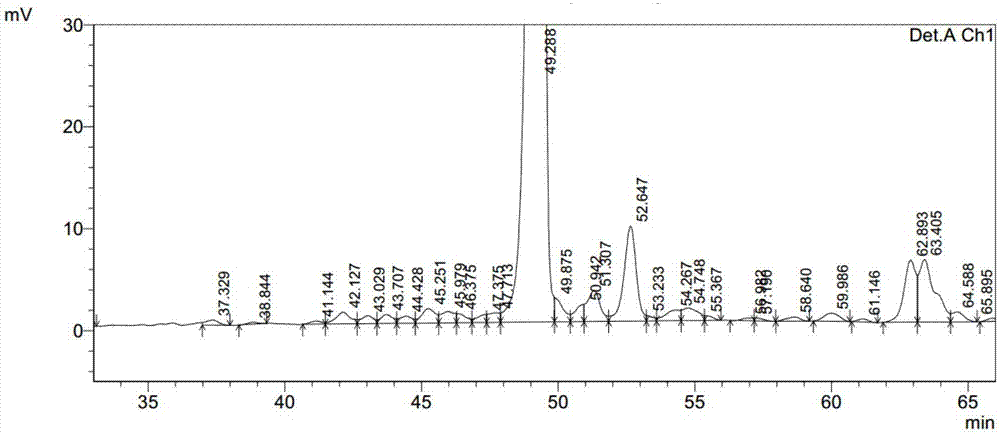
Embodiment 1
[0040] 1) Take 4.25g of Fmoc-Gly-Wang resin (0.279mmol / g) and load it into a solid-phase reaction column, wash twice with DMF, swell with DMF for 30 minutes, deprotect DBLK (5+7min), wash with DMF for 6 times, indene Tested positive for triketones;
[0041] 2) Fmoc-Arg(pbf)-OH (3.245g, 5mmol) and HOBt (0.426g, 3.15mmol) were dissolved in 15mL of DMF, and DIC (0.49mL, 3.15mmol) was added for activation under ice-cooling conditions for 5 minutes, and the activated The good solution was added to the above-mentioned solid-phase reaction column, stirred and reacted for 2 h under nitrogen gas, and the ninhydrin test was negative, and Fmoc-Arg(pbf)-Gly-Wang resin was obtained. The reaction solution was drained, washed 3 times with DMF, DBLK was deprotected (5+7min), washed 6 times with DMF, and the ninhydrin test was negative to obtain H-Arg(pbf)-Gly-Wang resin;
[0042] 3) Fmoc-Gly-OH, Fmoc-Ala-Trp(Boc)-Leu-Val-Arg(Pbf)-OH, Fmoc-Ala-Lys( Alloc)-Glu(OtBu)-Phe-Ile-OH, Fmoc-Ala-OH, F...
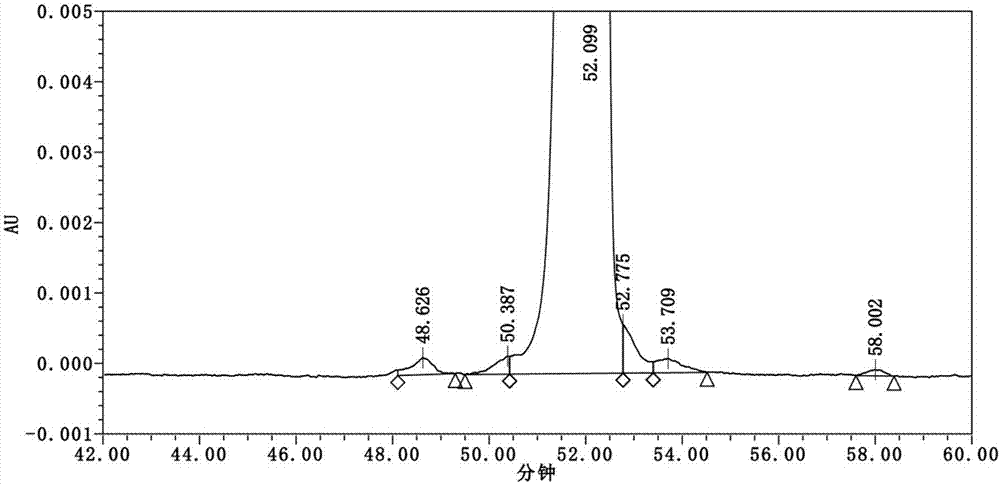
Embodiment 2
[0052] 1) Synthesize H-Arg(pbf)-Gly-Wang resin with reference to the method of Example 1, and couple Fmoc-Gly-OH and Fmoc-Arg(pbf) sequentially on the H-Arg(pbf)-Gly-Wang resin -OH, Fmoc-Val-OH, Fmoc-Leu-OH,, Fmoc-Glu(OtBu)-Phe-Ile-Ala-Trp(Boc)-OH, Fmoc-Lys(Alloc)-OH, Fmoc-Ala-Ala -OH, Fmoc-Gln(Trt)-OH, Fmoc-Gly-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Leu-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Ser(tBu)-OH , Fmoc-Ser(tBu)-OH, Fmoc-Ser(tBu)-Asp(OtBu)-Val-OH, Fmoc-Thr(tBu)-OH, Fmoc-Thr(tBu)-Phe-OH, Fmoc-Gly- OH, Fmoc-Ala-Glu(OtBu)-OH;
[0053] 2) Boc-His(Trt)-OH (2.49g, 5mmol) and HOBt (0.426g, 3.15mmol) were dissolved in 15mL DMF, and DIC (0.49mL, 3.15mmol) was added for activation under ice-cooling conditions for 5 minutes. The activated solution was added to the solid-phase reaction column, stirred for 2 hours under nitrogen gas, the ninhydrin test was negative, washed 4 times with DMF, and washed 2 times with DCM; the remaining method steps refer to steps 5-8 in Example 1, and dried under reduced ...
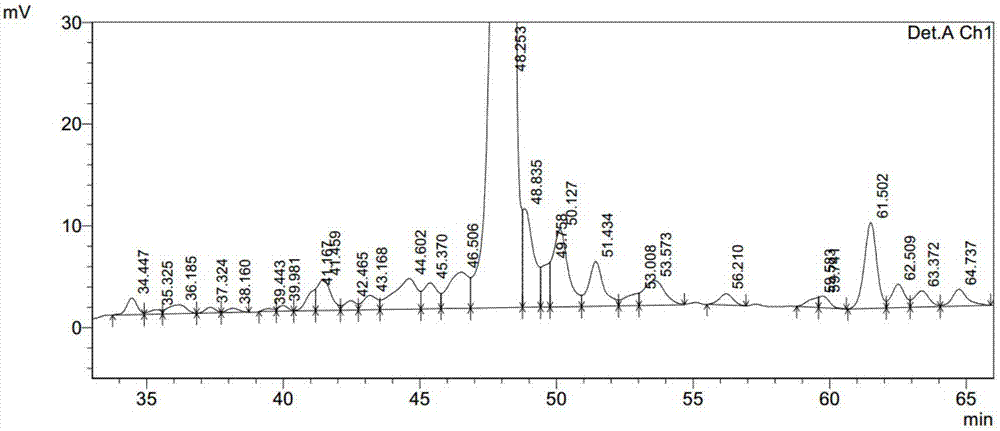
Embodiment 3
[0056] 1) Take 3.58g of Fmoc-Gly-2-CTC resin (0.279mmol / g) and load it into a solid-phase reaction column, wash twice with DMF, swell with DMF for 30 minutes, deprotect DBLK (5+7min), and wash with DMF for 6 times , positive for ninhydrin;
[0057] 2) Fmoc-Arg(pbf)-OH (3.245g, 5mmol) and HOBt (0.426g, 3.15mmol) were dissolved in 15mL of DMF, and DIC (0.49mL, 3.15mmol) was added for activation under ice-cooling conditions for 5 minutes, and the activated The good solution was added to the above-mentioned solid-phase reaction column, stirred and reacted for 2 h under nitrogen gas, and the ninhydrin test was negative, and Fmoc-Arg(pbf)-Gly-2-CTC resin was obtained. Drain the reaction solution, wash 3 times with DMF, deprotect DBLK (5+7min), wash 6 times with DMF, and the ninhydrin test is negative to obtain H-Arg(pbf)-Gly-2-CTC resin;
[0058] 3) Fmoc-Gly-OH, Fmoc-Ala-Trp(Boc)-Leu-Val-Arg(Pbf)-OH, Fmoc-Glu( OtBu)-Phe-Ile-OH, Fmoc-Lys(N-ε-(N α -Palmitoyl-L-γ-glutamyl-OtBu)), Fm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com