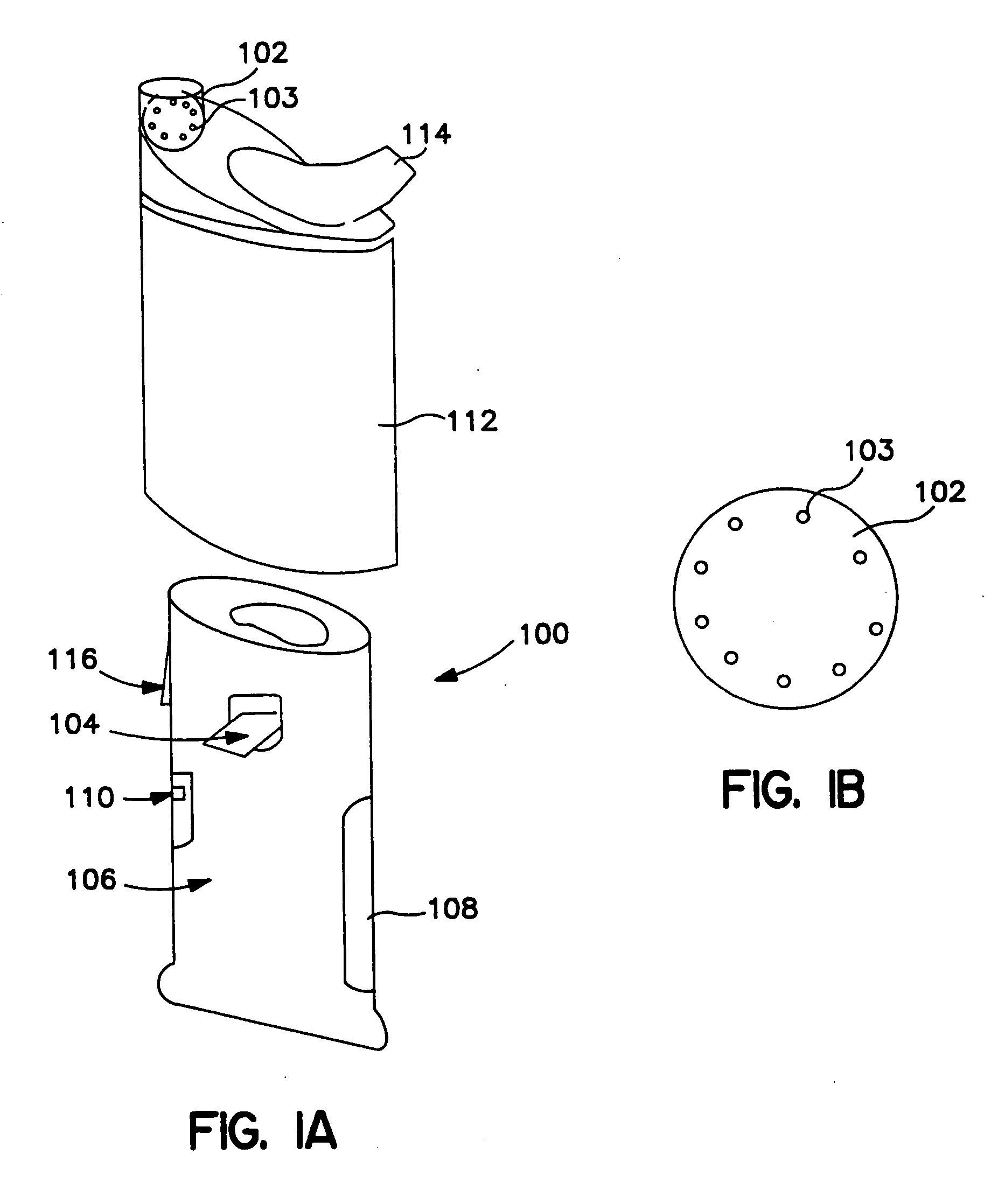
Aerosolized active agent delivery
a technology formulations, which is applied in the direction of aerosol delivery, parathyroid hormones, extracellular fluid disorder, etc., can solve the problems of undesirable insulin injection, low patient acceptance, and inability to adequately process, so as to increase the bioavailability of aerosolized active agents, increase the flow rate, and increase the resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Insulin Powders
[0070] Insulin powders were prepared as described above and administered to patients also as described above. The bioavailibilities, peak insulin concentrations and time to peak insulin concentrations were as shown in Table I below. The figures show, surprisingly, that inspiratory flow rates of 10 liters per minute or less achieved higher bioavailabilities of insulin (AUC), and higher peak concentrations of insulin (Cmax) than did inspiratory flow rates of 17.0 liters per minute or greater. Further, blood glucose level control (AUC) was greater for an inspiratory flow rate of 9.1 liters per minute than for the higher flow rates, and the maximum concentration (Cmax) was lower for the lower flow rate. Accordingly, a flow rate below 17 liters per minute, preferably 10 liters per minute or less is desired for optimal insulin delivery and glucose blood level control.
TABLE 1Summary of Pharmacokinetics and Pharmacodynamic Factors for Insulin aerosolInhaled at Different In...
example 2
Human Calcitonin Powders
[0071] Human calcitonin powders are prepared as described above. Upon administration to patients, flow rates below 17 liters per minute will result in higher bioavailabilities and lower times to peak concentration than those above 17 liters per minutes.
example 3
Heparin Powders
[0072] Low molecular weight heparin powders are prepared as described above. Upon administration to patients, flow rates below 17 liters per minute will result in higher bioavailabilities and lower times to peak concentration than those above 17 liters per minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com