Method for separating and measuring acetylcysteine enantiomers
An acetylcysteine and enantiomer technology, applied in the field of analytical chemistry, can solve problems such as unavailability of normal phase conditions, limited types of mobile phases, and difficulty in detection sensitivity, achieving stable product and fast reaction speed. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
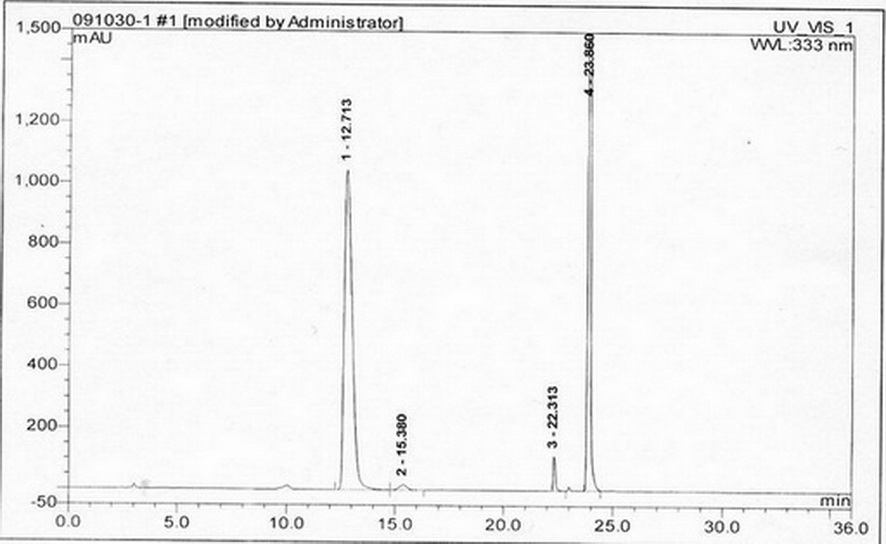
[0028] (1) Instruments and conditions
[0029] Diane UltiMate 3000 high performance liquid chromatography system and workstation in the United States, automatic sample injection, the chromatographic column uses octadecylsilane bonded silica gel column; UV detection wavelength: 333nm. Mobile phase: Use 0.02mol / L ammonium acetate solution (adjust the pH value to 6.8 with phosphoric acid or ammonia water) as mobile phase A, acetonitrile as mobile phase B, and perform linear gradient elution in the following table
[0030] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
80
20
18
80
20
19
50
50
25
50
50
26
80
20
36
80
20
[0031] (2) Experimental steps
[0032] Take 11mg of D-acetylcysteine, add water and dissolve it into 25ml solution, add L-acetylcysteine 82mg and dissolve it to make a mixed solution, take 1ml of the mixed solution and put it in a 10ml measuring bottle, add 2...
Embodiment 2
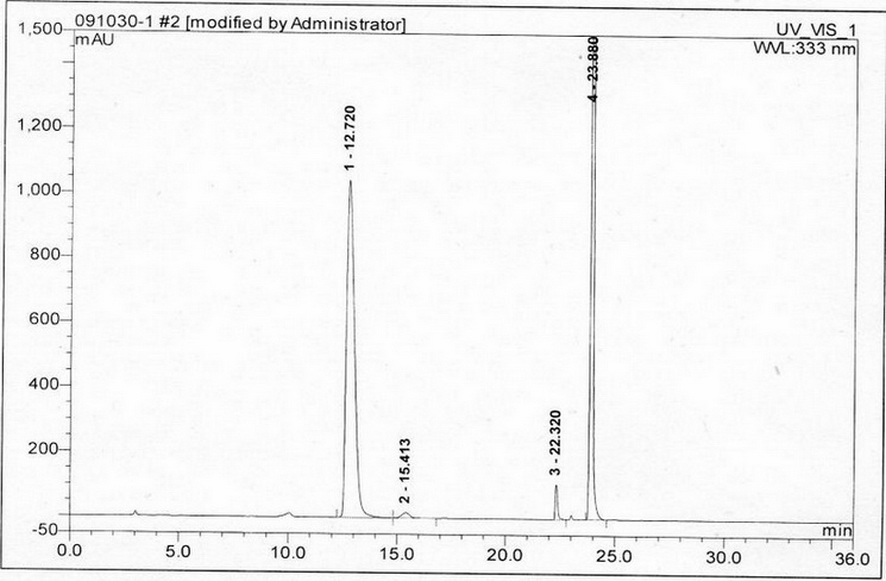
[0034] (1) Instruments and conditions
[0035] Diane UltiMate 3000 high performance liquid chromatography system and workstation in the United States, automatic sample injection, the chromatographic column uses octadecylsilane bonded silica gel column; UV detection wavelength: 333nm. Mobile phase: use 0.03mol / L ammonium acetate solution (adjust the pH value to 5.0 with phosphoric acid or ammonia water) as mobile phase A, acetonitrile as mobile phase B, and perform linear gradient elution in the following table
[0036] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
80
20
18
80
20
19
50
50
25
50
50
26
80
20
36
80
20
[0037] (2) Experimental steps
[0038] Take 82mg of acetylcysteine and put it in a 10ml measuring bottle, add 2ml of 1mol / L sulfuric acid solution, shake to dissolve, and neutralize with 1mol / L potassium hydroxide solution to pH 6.0, add water to dilute to th...
Embodiment 3
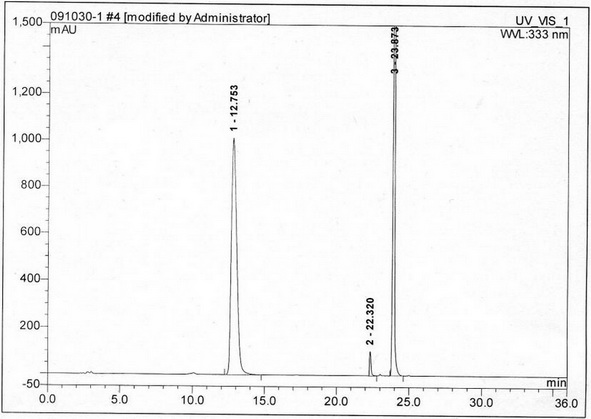
[0040] (1) Instruments and conditions
[0041] Diane UltiMate 3000 high performance liquid chromatography system and workstation in the United States, automatic sample injection, the chromatographic column uses octadecylsilane bonded silica gel column; UV detection wavelength: 333nm. Mobile phase: use 0.04mol / L ammonium acetate solution (adjust the pH value to 7.0 with phosphoric acid or ammonia water) as mobile phase A, acetonitrile as mobile phase B, and perform linear gradient elution in the following table
[0042] time (minutes)
Mobile phase A (%)
Mobile phase B (%)
0
80
20
18
80
20
19
50
50
25
50
50
26
80
20
36
80
20
[0043] (2) Experimental steps
[0044] Take 82mg of acetylcysteine granules, put them in a 10ml measuring bottle, add 2ml of 1mol / L formic acid solution, shake to dissolve, neutralize with 1mol / L ammonia solution to pH 7.0, add water to dilute to the mark, sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com