Method for assaying impurities in apremilast and preparations thereof through liquid chromatography
A liquid chromatography and impurity technology, applied in the field of analytical chemistry, can solve the problems of difficulty in effectively separating apremilast from impurities, affecting the quality control of apremilast and its preparations, increasing the difficulty of detection, etc., and achieving high column efficiency, Enhanced retention and improved resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
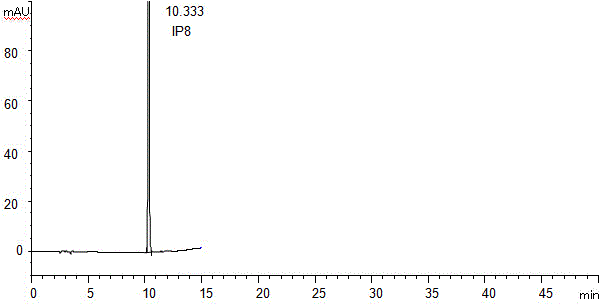
[0052] Apparatus and conditions
[0053] Agilent1200 liquid chromatograph and ChemStation; automatic sampling; AgilentEclipsePlusC18 column (5mm, 250×4.6mm) as the separation chromatographic column; UV detector wavelength: 230nm; mobile phase: 0.01mol / L potassium dihydrogen phosphate solution (Adjust the pH value to 2.3 with phosphoric acid solution) as mobile phase A, methanol-acetonitrile (45:55; V / V) as mobile phase B, gradient elution; 0 minutes, mobile phase A is 95% (V / V) ), mobile phase B is 5% (V / V); 0 minutes to 8 minutes, mobile phase A linearly decreases to 80% (V / V), mobile phase B linearly increases to 20% (V / V); 8 minutes To 40 minutes, mobile phase A linearly decreases to 20% (V / V), mobile phase B linearly increases to 80% (V / V); 40 minutes to 50 minutes, mobile phase A is 20% (V / V), Mobile phase B is 80% (V / V); 50 minutes to 53 minutes, mobile phase A linearly increases to 95% (V / V), mobile phase B linearly decreases to 5% (V / V); 53 minutes to 60 Minutes, mobile...
Embodiment 2
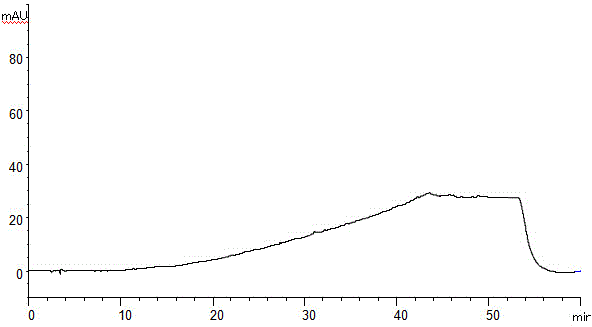
[0060] Determination of impurities in the API of API.
[0061] Take about 10mg of Apster, accurately weigh it, put it in a 50ml measuring flask, add diluent (acetonitrile: water = 20: 80V / V) ultrasonic treatment to dissolve and dilute to the mark, shake it, as the test solution; Accurately weigh 10 mg of the aprester reference substance, put it in a 50 ml measuring flask, add diluent (acetonitrile: water = 20: 80V / V) ultrasonic treatment to dissolve and dilute to the mark, shake it, as a control solution; follow the example Perform liquid chromatography analysis under the chromatographic conditions of 1. If there are impurity peaks (except solvent peaks) in the chromatogram of the test solution, the peak area after the known impurity is multiplied by the correction factor shall not be greater than 1 / 2 of the main peak area of the control solution ( 0.5%), the peak area of a single impurity shall not be greater than 1 / 2 (0.5%) of the main peak area of the control solution, a...
Embodiment 3
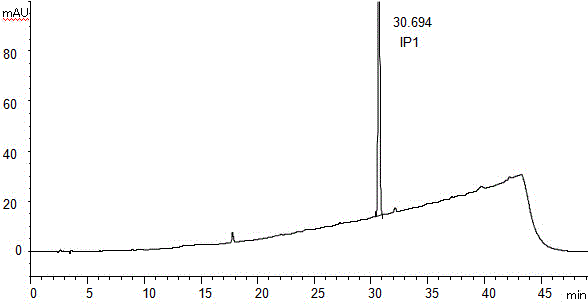
[0063] Liquid chromatography was used to determine the impurities in Apexit tablets.
[0064] Take an appropriate amount of this product (approximately equivalent to 10mg of Aplast), put it in a 50ml measuring bottle, add diluent (acetonitrile: water = 20: 80V / V) ultrasonic treatment to dissolve and dilute to the mark, shake it, as a test Product solution; another accurately weighed 10mg of apreste reference substance, put it in a 50ml measuring flask, add diluent (acetonitrile: water = 20: 80V / V) ultrasonic treatment to dissolve and dilute to the mark, shake it, as a control solution ; In addition, take an appropriate amount of blank excipients according to the prescription ratio, prepare the blank excipients for the test solution in the same way as the test solution; perform liquid chromatography analysis according to the chromatographic conditions of Example 1, if there are impurity peaks in the chromatogram of the test solution (Except solvent peak and blank auxiliary materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com