Method for preparing vinylacetylene through acetylene dimerization by utilizing modified nieuwland catalyst
A vinyl acetylene and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve problems such as low single-pass conversion rate, reduced catalyst service life, low selectivity of vinyl acetylene, etc., to achieve The catalyst is easy to prepare, the amount of polymer produced is small, and the catalyst cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
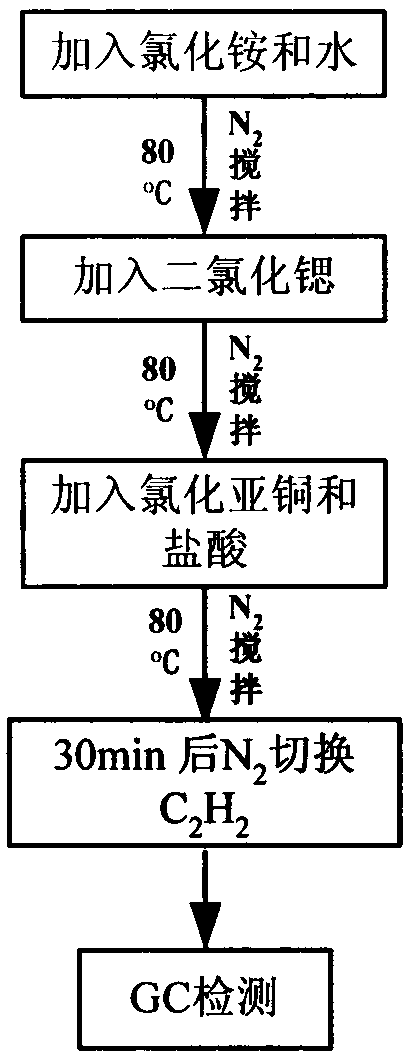
[0020] (1) Add 15mL of water into the reactor, then add 0.1049mol of ammonium chloride, at 80°C, under nitrogen for 110h -1 ~155h -1 Stir with air to dissolve it all.
[0021] (2) Keep the reaction temperature at 80°C, add 0.1049mol of cuprous chloride and 0.1mL of 37%-38% concentrated hydrochloric acid to the catalyst, and then continue to feed into the reactor for 110h -1 ~155h -1 Nitrogen for 30 minutes to fully dissolve the cuprous chloride and activate the catalytic system.
[0022] (3) use 206h -1 Acetylene is used instead of nitrogen, and the reaction temperature is maintained at 80°C. Acetylene undergoes dimerization to form vinyl acetylene.
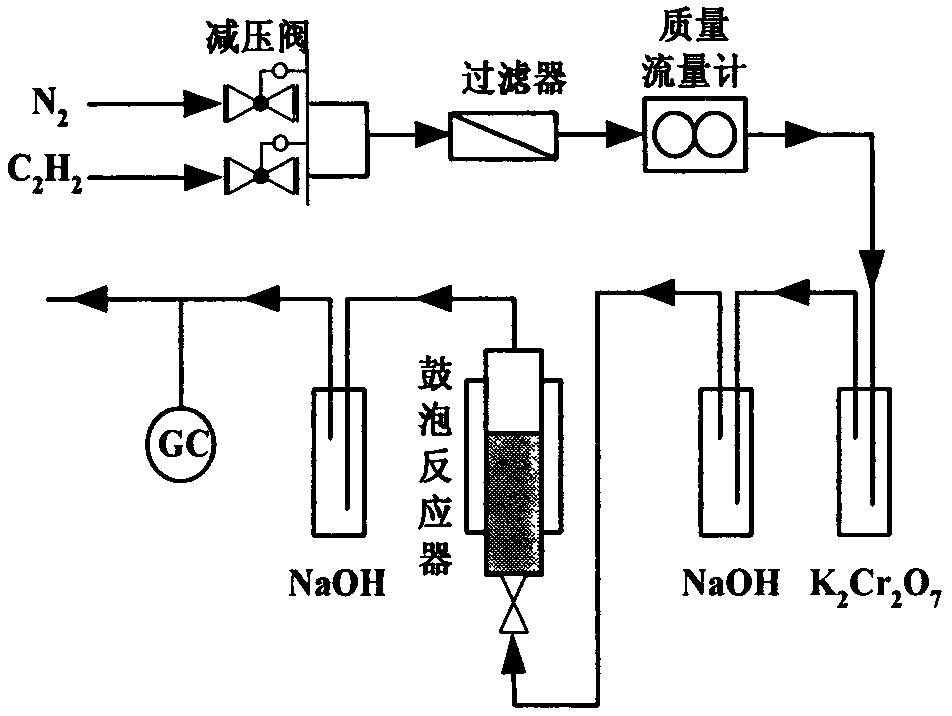
[0023] (4) The gas at the reactor outlet was detected online by Shimadzu GC2014-C gas chromatography, FID detector, and GDX-301 packed column.
specific Embodiment 2
[0024] (1) Add 15mL of water into the reactor, then add 0.1496mol of ammonium chloride, at 80°C, under nitrogen for 110h -1 ~155h -1 Stir with air to dissolve it all.
[0025] (2) Keep the temperature at 80°C, add 0.01496mol strontium dichloride to the ammonium chloride aqueous solution, and keep the nitrogen gas for 110h -1 ~155h -1 Stir with air to dissolve.
[0026] (3) Keeping the reaction temperature at 80°C, add 0.1496mol of cuprous chloride and 0.1mL of 37%-38% concentrated hydrochloric acid to the catalyst, and then continue to feed into the reactor for 110h -1 ~155h -1 Nitrogen for 30 minutes to fully dissolve the cuprous chloride and activate the catalytic system.
[0027] (4) use 206h -1 Acetylene is used instead of nitrogen, and the reaction temperature is maintained at 80°C. Acetylene undergoes dimerization to form vinyl acetylene.
[0028] (5) The gas at the reactor outlet was detected online by Shimadzu GC2014-C gas chromatography, FID detector, and GDX-3...
specific Embodiment 3
[0029] (1) Add 15mL of water into the reactor, then add 0.1144mol of ammonium chloride, at 80°C, under nitrogen for 110h -1 ~155h -1 Stir with air to dissolve it all.
[0030] (2) Keep the temperature at 80°C, add 0.01144mol strontium dichloride to the ammonium chloride aqueous solution, and keep the nitrogen gas for 110h -1 ~155h -1 Stir with air to dissolve.
[0031] (3) Keep the reaction temperature at 80°C, add 0.1144mol of cuprous chloride and 0.1mL of 37%-38% concentrated hydrochloric acid to the catalyst, and then continue to feed into the reactor for 110h -1 ~155h -1 Nitrogen for 30 minutes to fully dissolve the cuprous chloride and activate the catalytic system.
[0032] (4) use 206h -1 Acetylene is used instead of nitrogen, and the reaction temperature is maintained at 80°C. Acetylene undergoes dimerization to form vinyl acetylene.
[0033] (5) The gas at the reactor outlet was detected online by Shimadzu GC2014-C gas chromatography, FID detector, and GDX-301 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com