Phase change material and preparation method thereof
A technology of phase change materials and raw materials, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve problems affecting the timely release and utilization of heat, complex preparation process of phase change materials, and many types of raw materials used, so as to achieve fewer types , the phase change process is reversible, and the effect of large heat storage capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
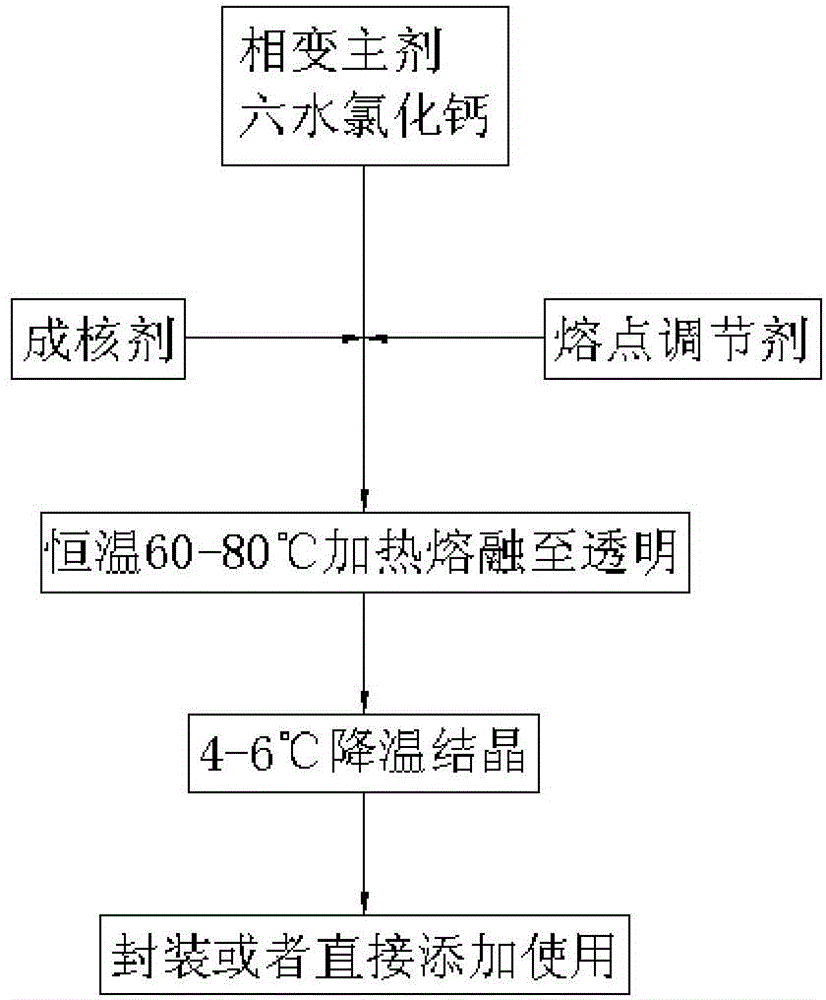
[0027] see figure 1 , the invention also discloses a preparation method of a phase change material, comprising the following steps:
[0028] First, weigh the calcium chloride hexahydrate GaCl 2 ·6H 2 O, the strontium chloride hexahydrate SrCl 2 ·6H 2 O. The melting point regulator is prepared by mixing the above raw materials according to the parts by mass to prepare a solution. The melting point regulator can be the magnesium chloride hexahydrate MgCl 2 ·6H 2 O, the magnesium nitrate Mg (NO 3 ) 2 , the potassium nitrate KNO 3 , one of the sodium chloride NaCl.
[0029] Wherein, the calcium chloride hexahydrate GaCl 2 ·6H 2 O is prepared by cooling crystallization method. anhydrous calcium chloride GaCl 2Grind it into powder to prepare a solution, heat the solution, prepare a supersaturated solution according to its solubility, and then put it in a cold water bath to cool down until it is completely crystallized, pour off the residual solution, and dry it at low t...
Embodiment 1
[0036] First, weigh the phase change main agent calcium chloride hexahydrate GaCl 2 ·6H 2 O15g, nucleating agent strontium chloride hexahydrate SrCl 2 ·6H 2 O 0.75g, melting point regulator sodium chloride 0.5g, thickener sodium hydroxymethylcellulose (carboxymethylcellulose sodium, NaCMC) 0.25g and water 2.5g, the above raw materials were mixed and prepared into a solution.
[0037] Then, heat the prepared solution in a constant temperature water bath at 60-80° C. and keep stirring until all the raw materials are melted and the solution becomes a transparent liquid.
[0038] Next, crystallize the transparent liquid at a low temperature of 4-6°C to complete the storage and release of initial energy and obtain a phase change material.
[0039] The phase change temperature of the prepared phase change material is 31° C., and the latent heat of phase change is 168 J / g.
Embodiment 2
[0041] First, weigh the phase change main agent calcium chloride hexahydrate GaCl 2 ·6H 2 O15g, nucleating agent strontium chloride hexahydrate SrCl 2 ·6H 2 O0.45g, melting point regulator magnesium nitrate Mg (NO 3 ) 2 0.6g and 2.5g of water, the above raw materials were mixed and prepared into a solution.
[0042] Then, heat the prepared solution in a constant temperature water bath at 60-80° C. and keep stirring until all the raw materials are melted and the solution becomes a transparent liquid.
[0043] Next, crystallize the transparent liquid at a low temperature of 4-6°C to complete the storage and release of initial energy and obtain a phase change material.
[0044] The phase change temperature of the prepared phase change material is 30° C., and the latent heat of phase change is 150 J / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com