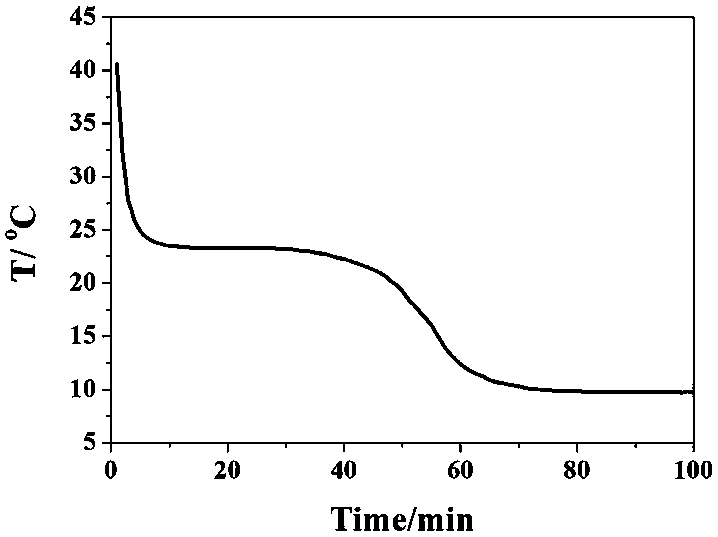
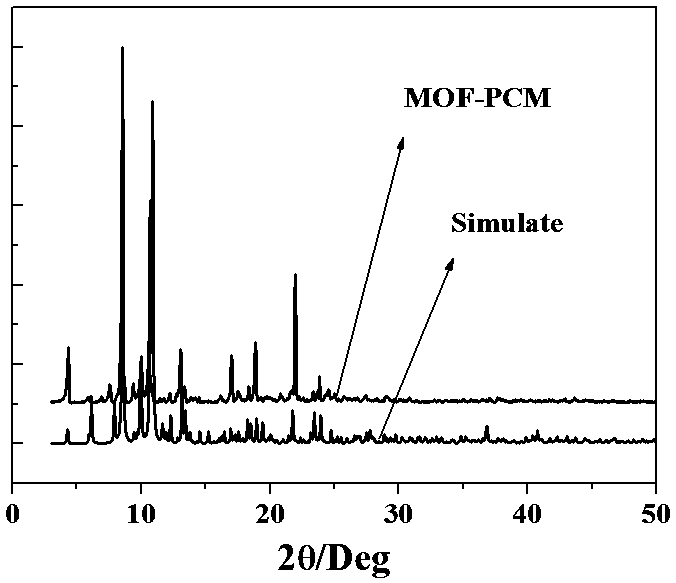
Composite phase-change material of encapsulating calcium chloride hexahydrate by calcium-based metal organic framework
A composite phase change material, calcium chloride hexahydrate technology, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of reducing the service life of inorganic hydrated salt phase change materials, limiting the wide use, etc., to achieve the goal of expanding The effect of scope of use, low price, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) After fully mixing 20mmol ethanol and 6mmol water, slowly add 0.4mmol glacial acetic acid under stirring state, add 2mmol adipic acid under continuous stirring, and finally add 1mmol calcium chloride quickly under vigorous stirring, the calcium chloride is completely dissolved and After the solution was clarified, stirring was continued for 15 min.
[0037] (2) Introduce the above solution into a sealed Teflon reactor, and raise the temperature from room temperature to 120 at a rate of 5K / min o C, keep the temperature for 6 hours, and raise the temperature to 160 at the speed of 10K / min o C, kept at constant temperature for 4h, and then quenched to room temperature to obtain a calcium-based metal-organic framework.
[0038] (3) Use a hydrophobic polypropylene hollow fiber membrane as a fixed membrane and carbon tetrachloride as an extraction phase to extract the calcium-based metal-organic framework and the solution as a whole for 1 hour, and extract the unreacted ...
Embodiment 2
[0042] (1) After fully mixing 25mmol ethanol and 8mmol water, slowly add 0.3mmol glacial acetic acid under stirring, add 2mmol suberic acid under continuous stirring, and finally quickly add 1.5mmol calcium chloride under vigorous stirring, and the calcium chloride is completely dissolved And the solution was clarified and continued to stir for 15 min.
[0043] (2) Introduce the above solution into a sealed Teflon reactor, and raise the temperature from room temperature to 120 at a rate of 5K / min o C, keep the temperature for 6 hours, and raise the temperature to 160 at the speed of 10K / min o C, kept at constant temperature for 6h, and then quenched to room temperature to obtain a calcium-based metal-organic framework.
[0044] (3) Use a hydrophobic polypropylene hollow fiber membrane as a fixed membrane, carbon tetrachloride as an extraction phase, extract the calcium-based metal-organic framework and the solution as a whole for 2 hours, and extract the unreacted adipic acid...
Embodiment 3
[0048] (1) After fully mixing 20mmol ethanol and 10mmol water, slowly add 0.2mmol benzoic acid under stirring state, add 2mmol sebacic acid under continuous stirring, and finally add 2mmol calcium chloride quickly under vigorous stirring, the calcium chloride is completely dissolved and After the solution was clarified, stirring was continued for 15 min.
[0049] (2) Introduce the above solution into a sealed Teflon reactor, and raise the temperature from room temperature to 120 at a rate of 10K / min o C, keep the temperature for 6 hours, and raise the temperature to 160 at the speed of 10K / min o C, kept at constant temperature for 8h, and then quenched to room temperature to obtain a calcium-based metal-organic framework.
[0050] (3) Using polysulfone hollow fiber membrane as the fixed membrane, and chloroform as the extraction phase, the calcium-based metal-organic framework and the solution as a whole were extracted for 2 hours, and the unreacted adipic acid in the solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com