Nanometer iron-lithium oxide composite negative electrode material and preparation method thereof
A negative electrode material and lithium oxide technology, which is applied in the field of nanometer iron-lithium oxide composite negative electrode material and its preparation, can solve the problems of low electrochemical activity, reduced electrochemical performance of iron-lithium oxide, low capacity retention rate, etc. The preparation process is simple, the electrochemical performance is good, and the effect of a wide concentration range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
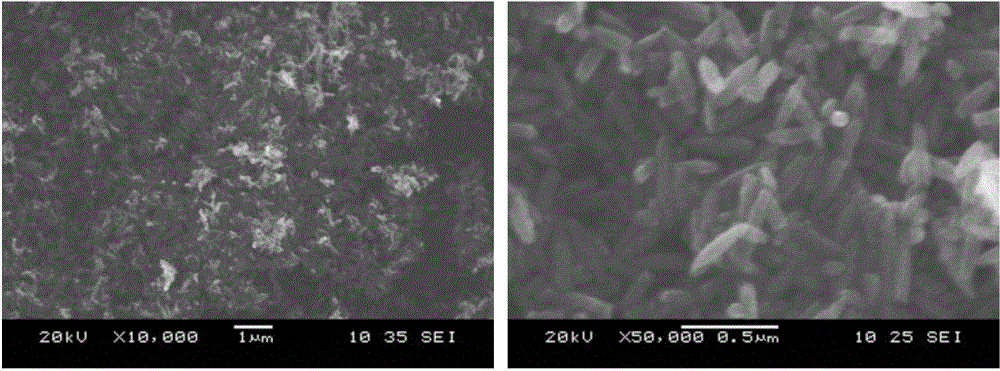
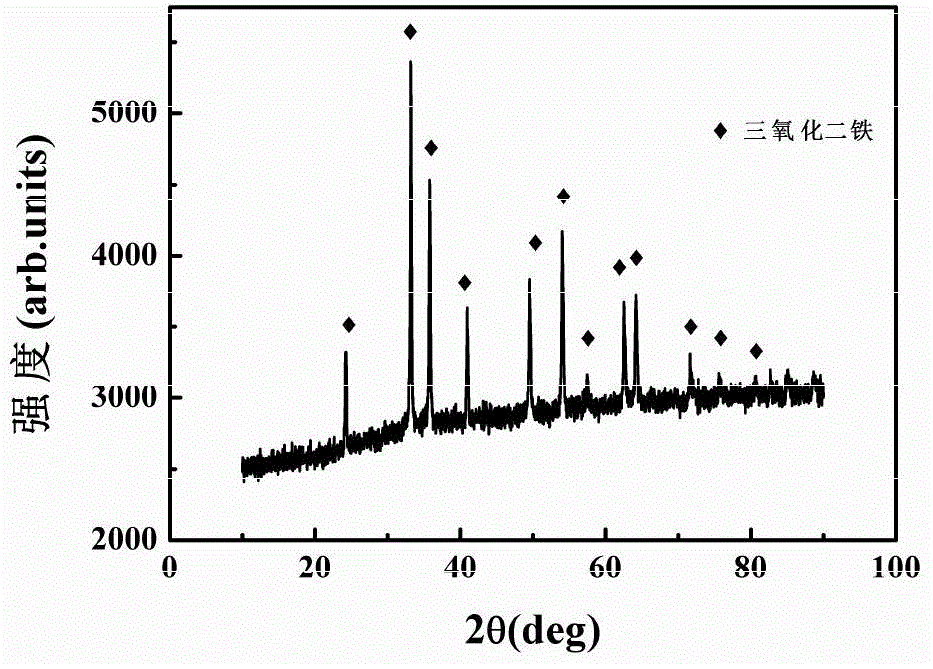
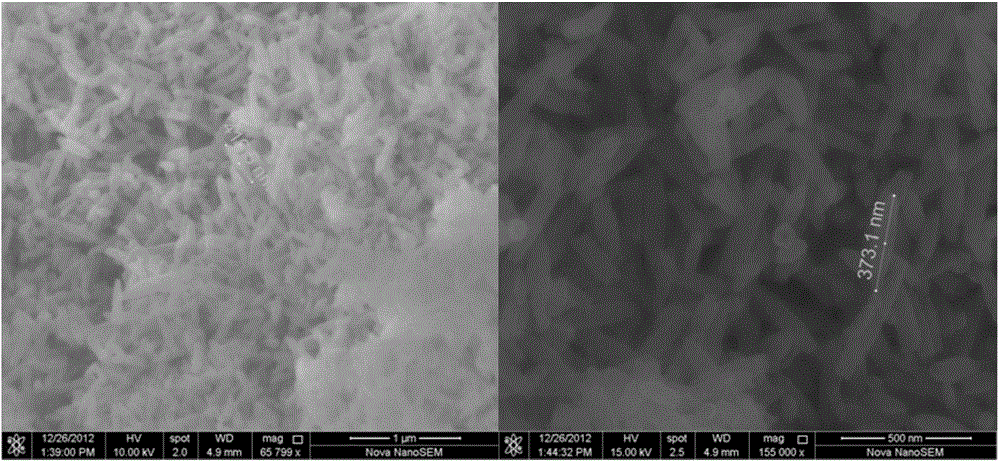
[0044] Ferric chloride hexahydrate (FeCl3 6H2O), ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) (the molar ratio of ferric chloride hexahydrate to ammonium dihydrogen phosphate is 26:1) and deionized water were used as the experimental raw materials, put them into the reaction kettle, at 220°C, the hydrothermal reaction time was 4h, and naturally cooled to room temperature. After the precipitate was separated by washing with deionized water and alcohol, iron oxide (Fe 2 o 3 )Nano stave. Then the iron oxide nanorods prepared by hydrothermal synthesis method and lithium hydroxide monohydrate (LiOH·H 2O) (the molar ratio of iron oxide nanorods and lithium hydroxide monohydrate is 1:2) was used as the raw material, and uniformly mixed. Finally, the mixed samples were calcined in air at 700°C for 10 hours. After cooling to room temperature, the obtained reddish-brown product is taken out, and the obtained reddish-brown product is the nano-iron lithium oxide composite negative...
Embodiment 2
[0051] Ferric chloride hexahydrate (FeCl 3 ·6H 2 O), ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) (the molar ratio of ferric chloride hexahydrate to ammonium dihydrogen phosphate is 26:1) and deionized water are used as experimental raw materials, put into the reaction kettle, at 200 ℃, hydrothermal reaction time 3h, and naturally cool to room temperature. After the precipitate was separated by washing with deionized water and alcohol, iron oxide (Fe 2 o 3 )Nano stave. The prepared nano-iron oxide (Fe 2 o 3 ) nanorod morphology such as Figure 6 shown. Then the iron oxide nanorods prepared by hydrothermal synthesis method and lithium hydroxide monohydrate (LiOH·H 2 O) (the molar ratio of iron oxide nanorods and lithium hydroxide monohydrate is 1:2) was used as the raw material, and uniformly mixed. Finally, the mixed samples were calcined in air at 700°C for 10 hours. After cooling to room temperature, the obtained reddish-brown product is taken out, and the obta...
Embodiment 3
[0053] Ferric chloride hexahydrate (FeCl 3 ·6H 2 O), ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) (the molar ratio of ferric chloride hexahydrate to ammonium dihydrogen phosphate is 30:1) and deionized water are used as experimental raw materials, put them into the reaction kettle, at 240 ℃, hydrothermal reaction time 10h, and naturally cool to room temperature. After the precipitate was separated by washing with deionized water and alcohol, iron oxide (Fe 2 o 3 )Nano stave.
[0054] The prepared nano-iron oxide (Fe 2 o 3 ) nanorod morphology such as Figure 7 shown. The subsequent calcination process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com