Compound tablet of pioglitazone hydrochloride, glimepiride and preparation method thereof
A technology of pioglitazone hydrochloride and glimepiride, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pill delivery, etc., can solve the problem of unsatisfactory dissolution of glimepiride and glimepiride Stability impact, poor dissolution of glimepiride and other issues, to achieve the effect of improving product stability, improving solubility, and being suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Based on 1000 pieces
[0030] Glimepiride and Sodium Lauryl Sulfate Mixed Powder 4.0g
[0031] Pioglitazone Hydrochloride 33.0g
[0032] Mannitol 280g
[0033] Croscarmellose Sodium 5.5g
[0034] Magnesium Stearate 5.5g
[0035] Preparation:
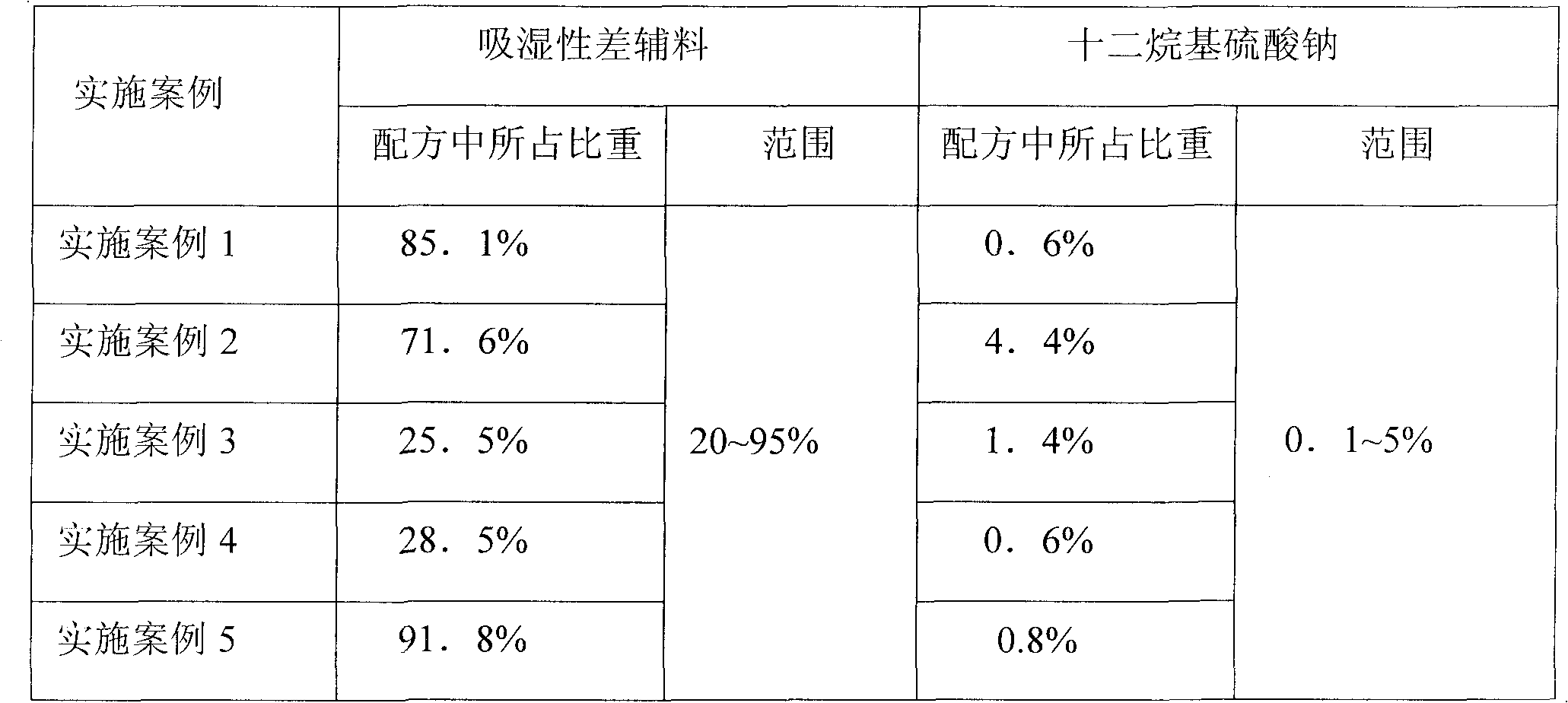
[0036] Step 1: Mix glimepiride and sodium lauryl sulfate at a ratio of 1:1 and pulverize through 200 mesh, and set aside.
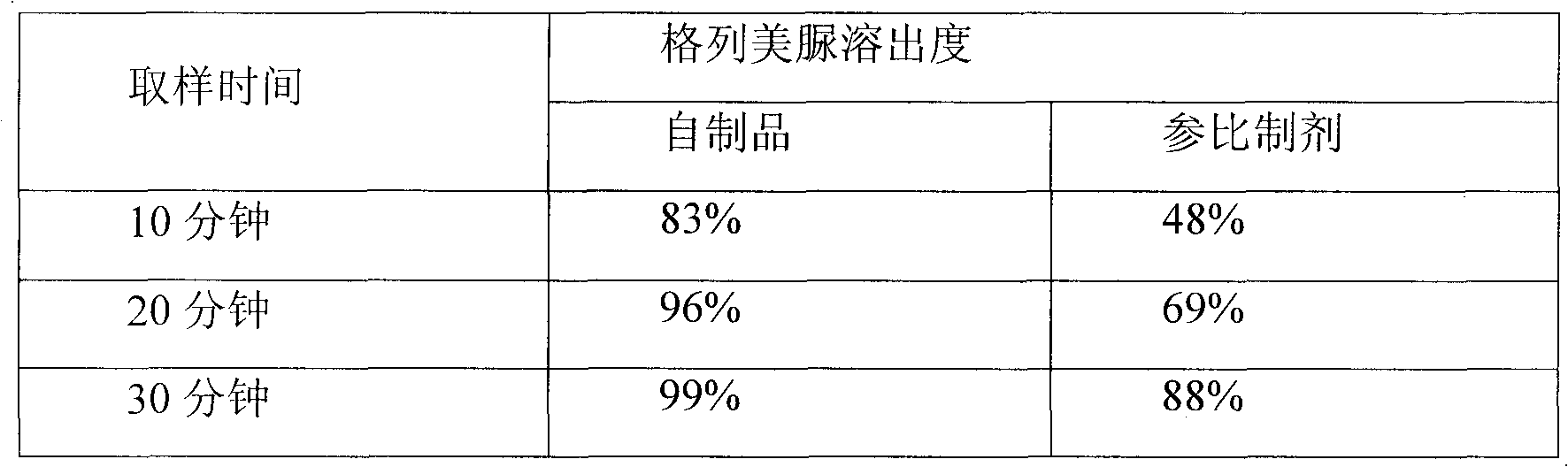
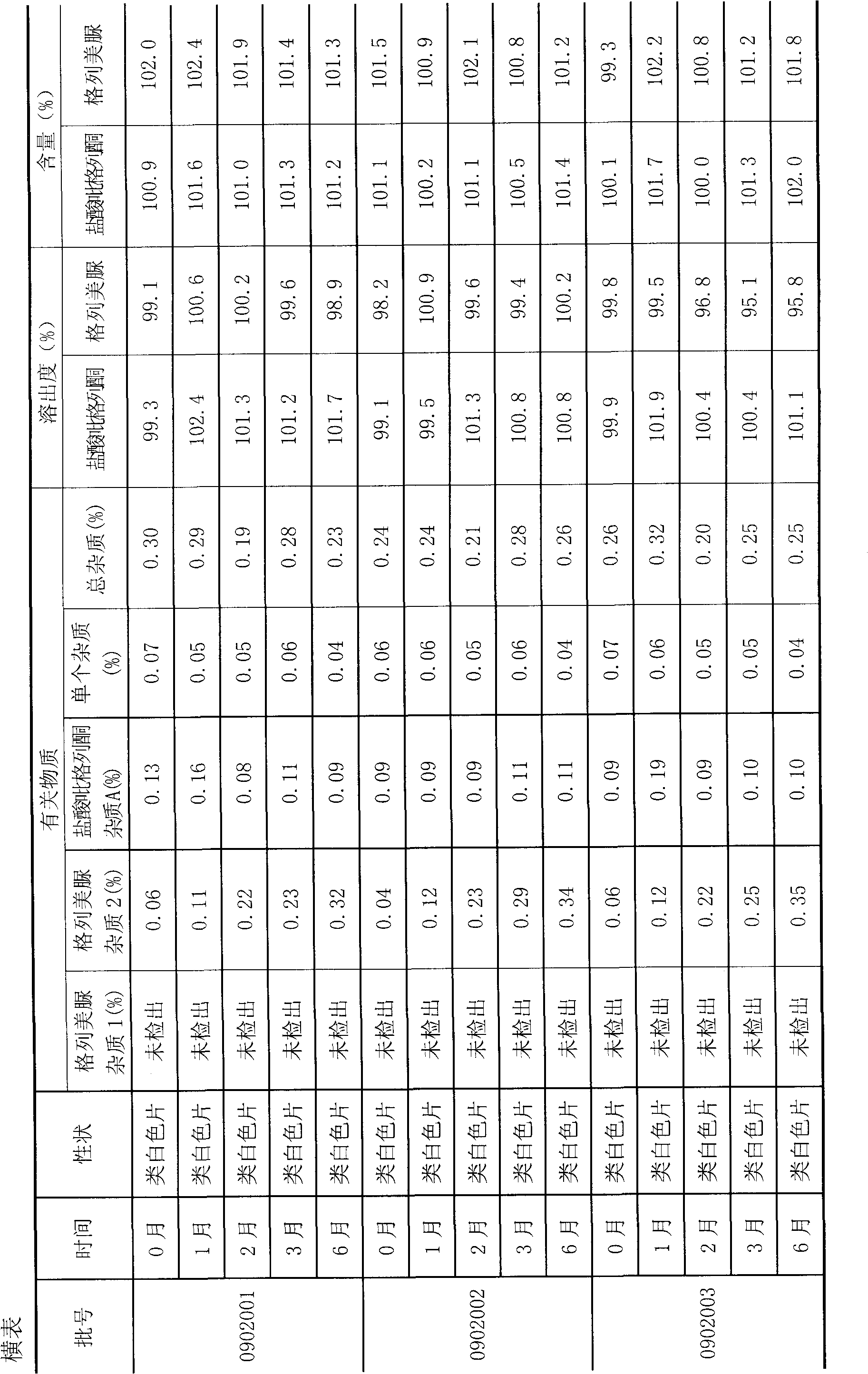
[0037] The second step: Weigh the mixed powder of glimepiride and sodium lauryl sulfate prepared in the first step in the mixer, and add the formula amount of pioglitazone hydrochloride, mannitol, croscarmepiride sodium, lauryl sulfate Sodium sulfate and magnesium stearate are mixed evenly, and the granule content is measured, and the direct tableting method is adopted to obtain the product.
Embodiment 2
[0039] Based on 1000 pieces
[0040] Glimepiride and Mannitol Mixed Powder 8.0g
[0041] Pioglitazone Hydrochloride 33.0g
[0042] Mannitol 125g
[0043] Sodium carboxymethyl starch 10.0g
[0044] Sodium Lauryl Sulfate 8.0g
[0045] Magnesium stearate 0.5g
[0046] Preparation:
[0047] Step 1: Glimepiride and mannitol are mixed and pulverized at a ratio of 1:1 to pass through 200 mesh, and set aside.
[0048]The second step: Weigh the mixed powder of glimepiride and mannitol prepared in the first step in the mixer, and add the formula amount of pioglitazone hydrochloride, mannitol, sodium carboxymethyl starch, sodium lauryl sulfate and stearic acid Magnesium, after mixing evenly, measure the particle content, and adopt the direct compression method to obtain the product.
Embodiment 3
[0050] Based on 1000 pieces
[0051] Glimepiride and Sodium Lauryl Sulfate Mixed Powder 8.0g
[0052] Pioglitazone Hydrochloride 33.0g
[0053] Mannitol 70g
[0054] Crystal Cellulose PH102 75g
[0055] Lactose 80g
[0056] Sodium carboxymethyl starch 10.0g
[0057] Magnesium Stearate 5.5g
[0058] Preparation:
[0059] Step 1: Mix glimepiride and sodium lauryl sulfate at a ratio of 1:1 and pulverize through 200 mesh, and set aside.
[0060] The second step: Weigh the mixed powder of glimepiride and mannitol prepared in the first step in the mixer, and add the formula amount of pioglitazone hydrochloride, mannitol, sodium carboxymethyl starch, crystal cellulose, lactose, dodecane Sodium hydroxysulfate and magnesium stearate, mix evenly, measure the granule content, and adopt the direct compression method to get final product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com