Magnetic resonance contrast agent based on hyaluronic acid as well as preparation method and application of magnetic resonance contrast agent
A technology of hyaluronic acid and sodium hyaluronate, applied in the field of medical imaging, can solve the problems of further improvement of safety and use effect, achieve excellent fibrotic tissue targeting ability, and achieve the effect of accurate staging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1, targeting molecule mal-ONH 2 Synthesis
[0112] Targeting molecule mal-ONH 2 The synthetic route is as follows:
[0113]
[0114] 1. Preparation of mal-ONHBoc
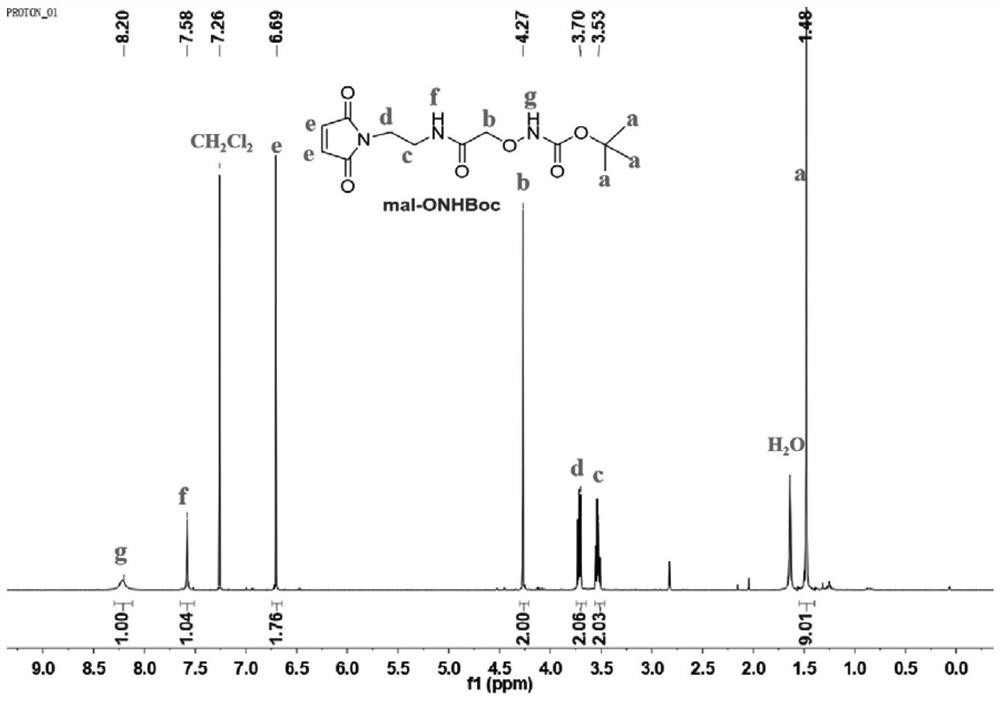
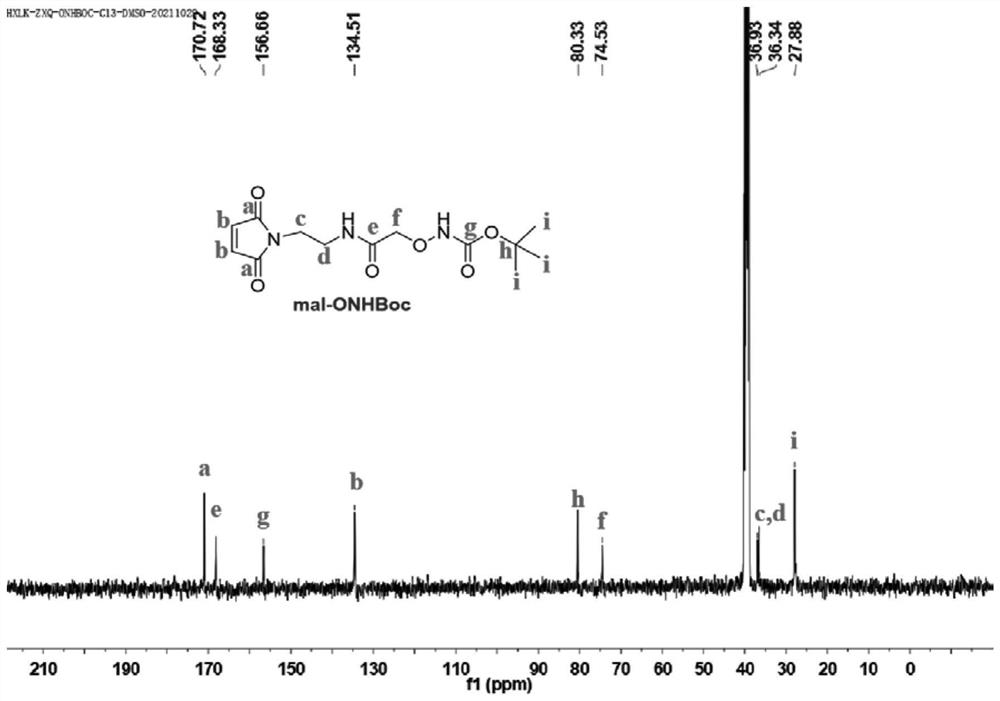
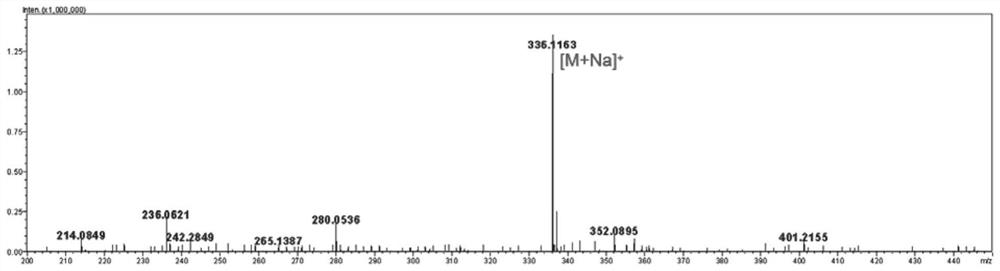
[0115] N-(2-Aminoethyl)maleimide trifluoroacetic acid (284 mg, 1.2 mmol), [(tert-butoxycarbonyl)aminooxy]acetic acid (191 mg, 1 mmol), HBTU (569 mg, 1.5 mmol) and HOBT (203 mg, 1.5 mmol) were placed in a reaction flask, and N 2 Protect. 2 mL of N,N-dimethylformamide (DMF) was then added under an ice bath, followed by 0.69 mL of DIEA. The reaction solution was stirred at room temperature for 2 hours, then 50 mL of CH 2 Cl 2 Diluted with water, then saturated NaHCO 3 solution, 1M HCl solution was washed. CH 2 Cl 2 solution with anhydrous Na 2 SO 4 dried and concentrated. The final product was purified by column chromatography (eluent was CH 2 Cl 2 / CH 3 OH, the volume ratio is 10 / 1) to obtain 170 mg of white solid with a yield of 54%. mal-ONHBoc in CDCl 3 middle 1 H NMR ( figu...
Embodiment 2
[0118] Example 2. Synthesis of magnetic resonance contrast agent HA-Target-Cy5.5-DOTA-Gd (HTCDGd) of the present invention
[0119] The synthetic route of HA-Target-Cy5.5-DOTA-Gd(HTCDGd) is as follows:
[0120]
[0121] in,
[0122] n is an integer from 0 to 50;
[0123] m is an integer from 0 to 18;
[0124] o is an integer from 0 to 3;
[0125] p is an integer from 0 to 10;
[0126] n≥m;
[0127] m≥o+p.
[0128] 1. Preparation of HA-SSPy
[0129] HA.Na 20K (2.4 g) was dissolved in 240 mL MES buffer (100 mM, pH 5.5) to give a 1% w / v solution, DMTMM (2.5 g, 1.5 equiv.) was added and stirred for 30 min to activate the carboxyl. 2-(Pyridin-2-yldisulfanyl)ethylamine hydrochloride (2.1 g, 1.5 equiv.) was then dissolved in MES buffer and added dropwise to the reaction solution. The reaction mixture was stirred at 25°C for 24 hours and then washed with reverse osmosis (RO)H 2 O was dialyzed (3.5 kDa MWCO) for 48 hours and lyophilized to obtain HA-SSPy as a white spongy ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com