Method for Screening Active Components, Synergistic and Antagonistic Components in Compound Panax notoginseng Using Rat Blood Stasis Model
A technology for promoting blood circulation and removing blood stasis and active ingredients, which is applied in the field of in vivo evaluation of the pharmacological activity of traditional Chinese medicines, can solve the problems of unclear composition and composition of drug-containing serum, inability to evaluate the efficacy of drugs, etc., and achieves objective and effective evaluation results of traditional Chinese medicines. The effect of technical bottlenecks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
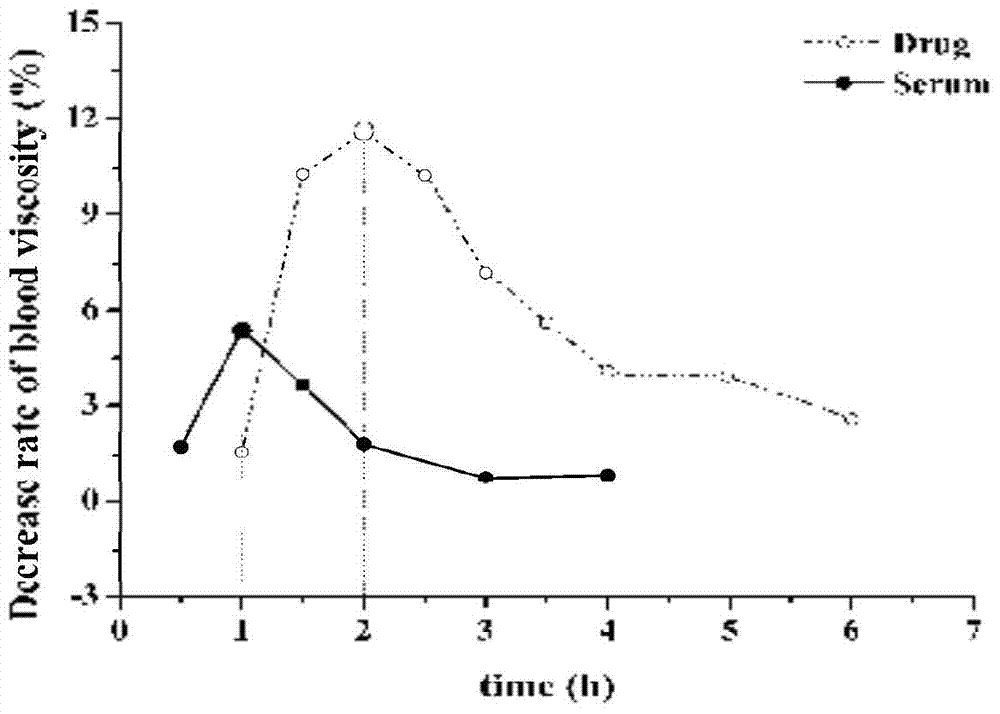
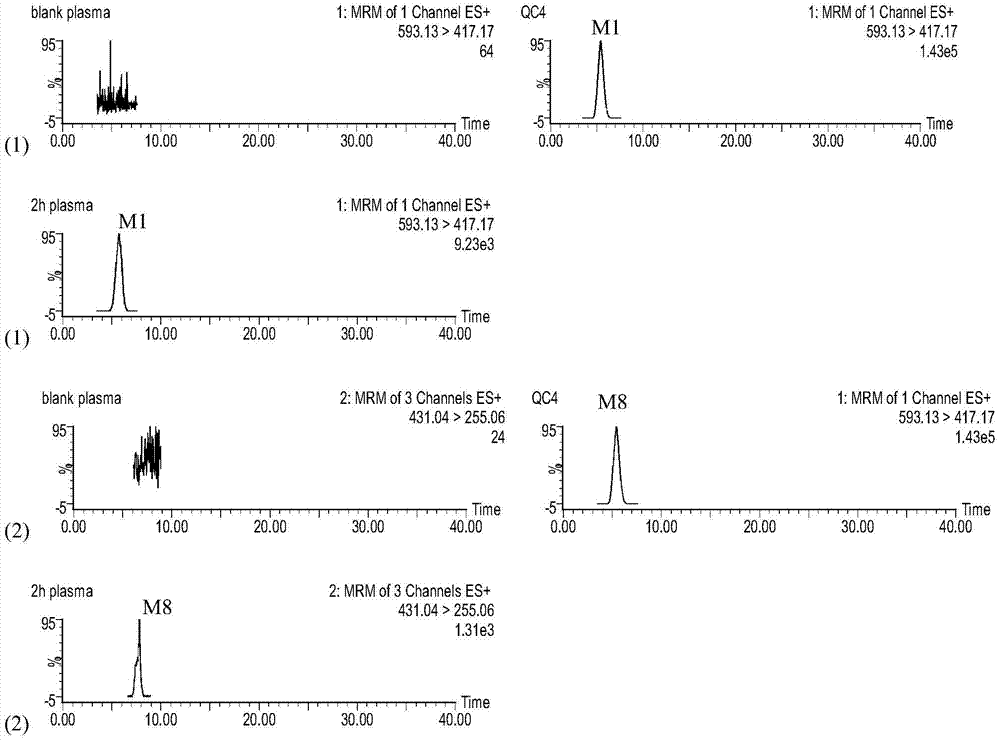
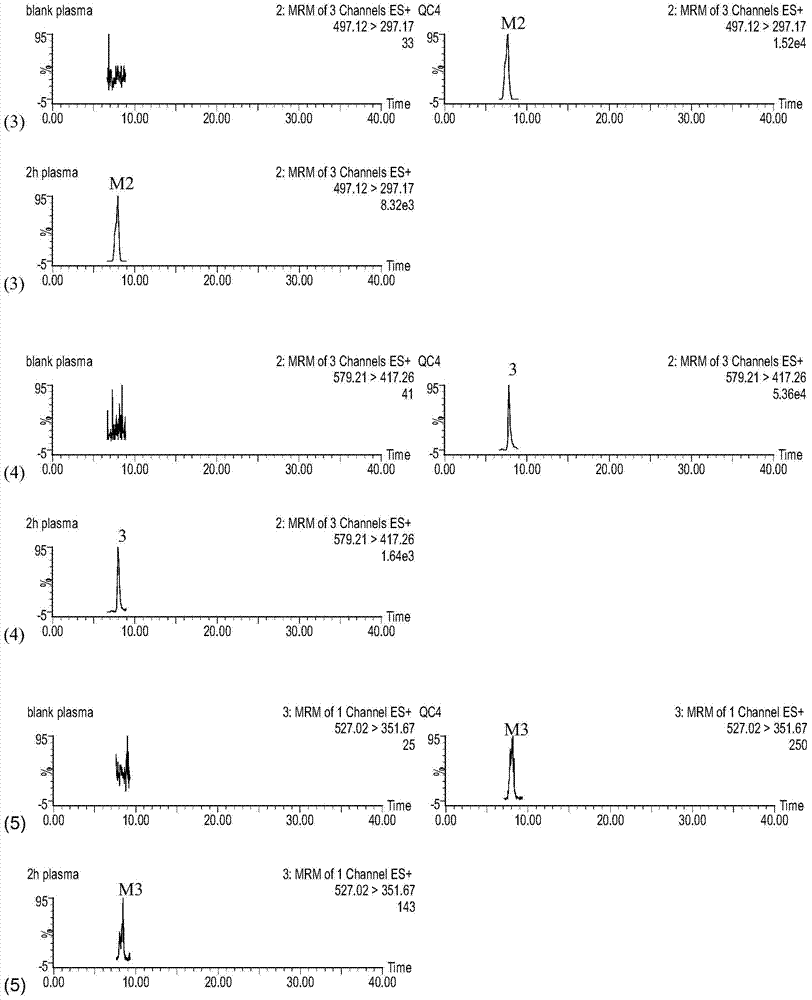
[0038] Example 1. After the blood stasis model rats were given compound Sanqi, the drug-containing serum extract was reinjected to evaluate its effect of promoting blood circulation and removing blood stasis
[0039] 1 Pharmacodynamic evaluation after administration of original traditional Chinese medicine
[0040] 1.1 Blood stasis model preparation
[0041] Male Wistar rats, weighing 240±20g, were randomly divided into 9 time groups ranging from 1.0 to 6.0 hours, with 12 rats in each group. Rats in each group were subcutaneously injected with epinephrine hydrochloride 0.8mg / kg (2ml / kg) twice with an interval of 4h. During the interval, the rats were placed in an ice bath at 0°C and swam for 5min to prepare a rat model of acute blood stasis.
[0042] 1.2 Pharmacodynamic evaluation of promoting blood circulation and removing blood stasis
[0043] Eighteen hours after modeling, the rats in each group were divided into two groups, and were injected with normal saline solution (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com