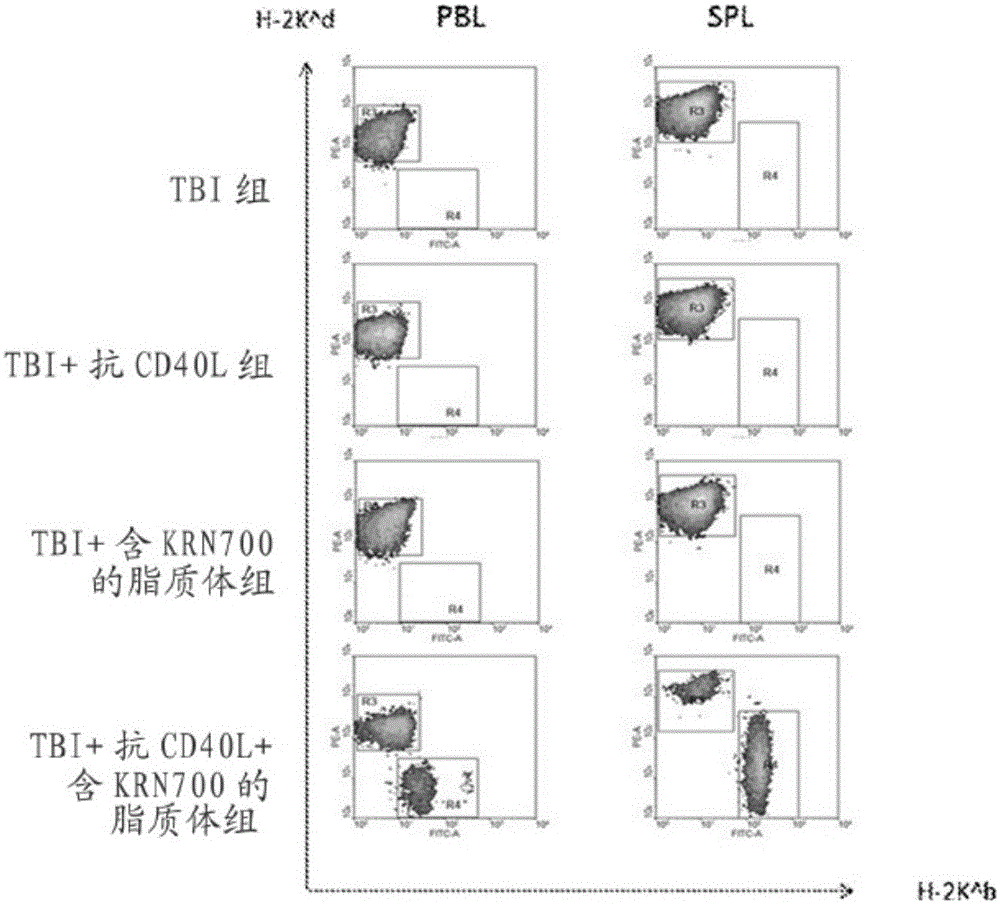
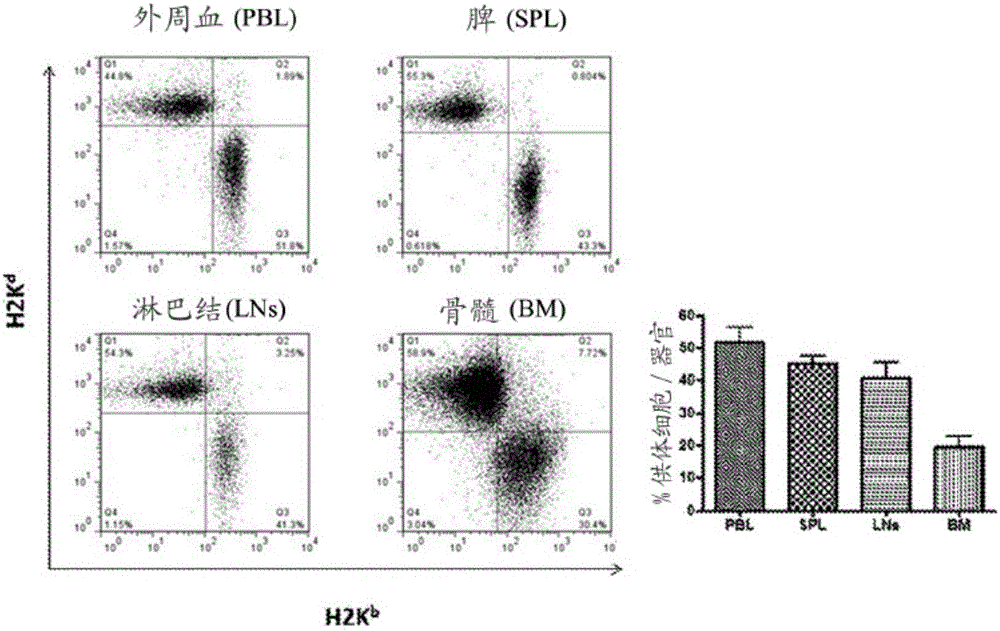
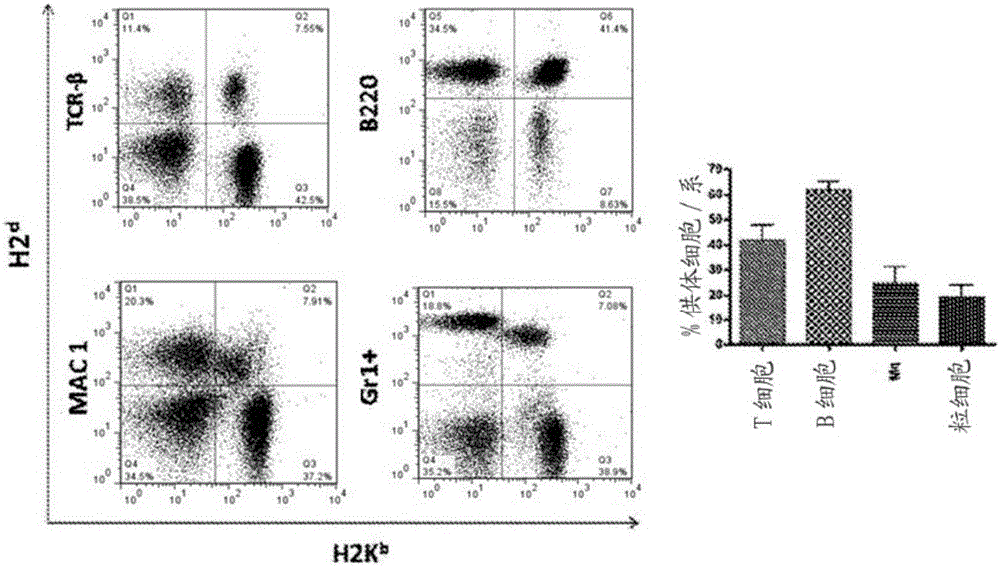
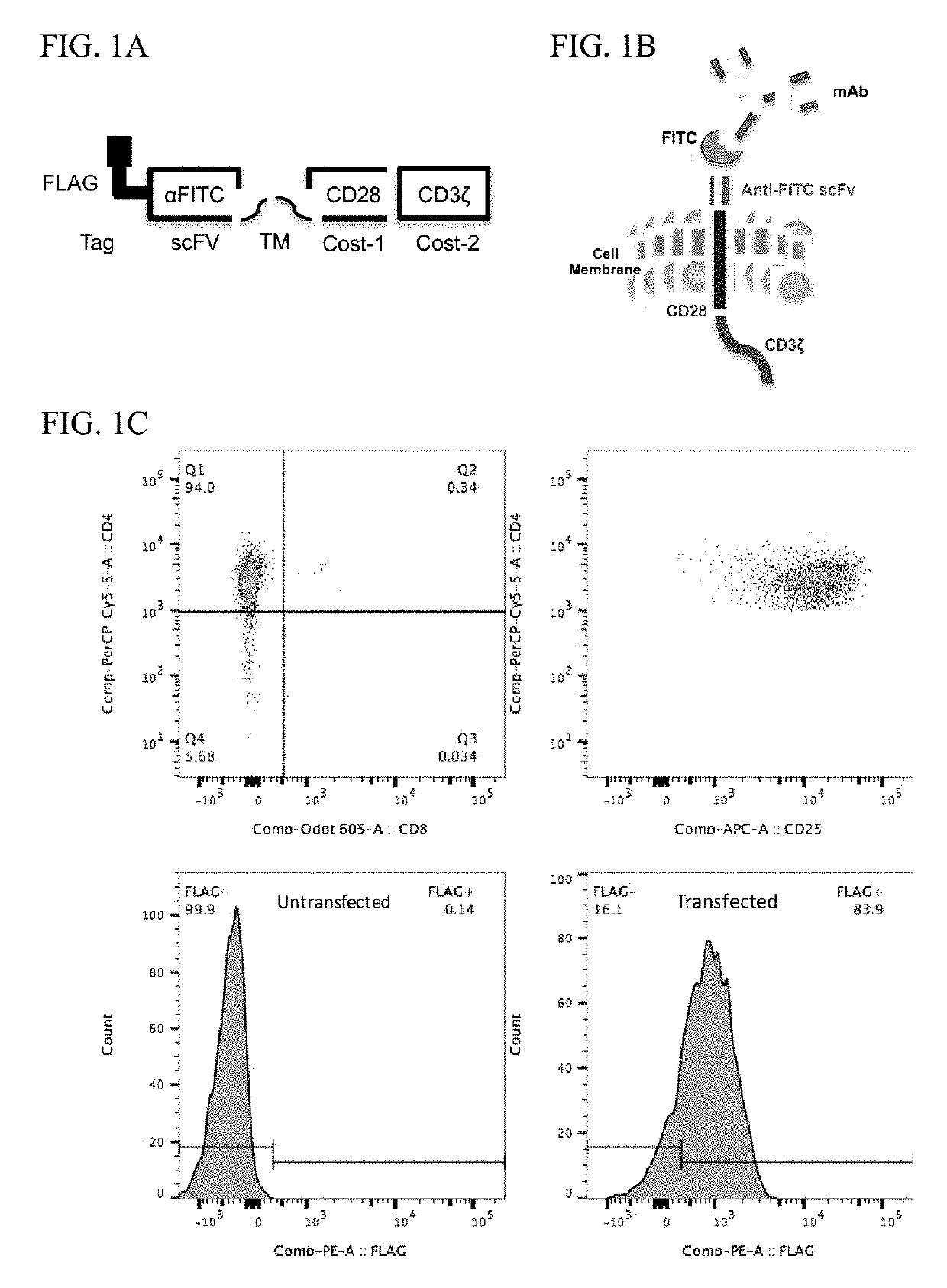
Patents
Literature
32 results about "Haematopoietic cell transplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combined organ and hematopoietic cells for transplantation tolerance of HLA mismatched grafts
ActiveUS9504717B2Organic active ingredientsEnergy modified materialsImmunosuppressive drugHematopoietic cell
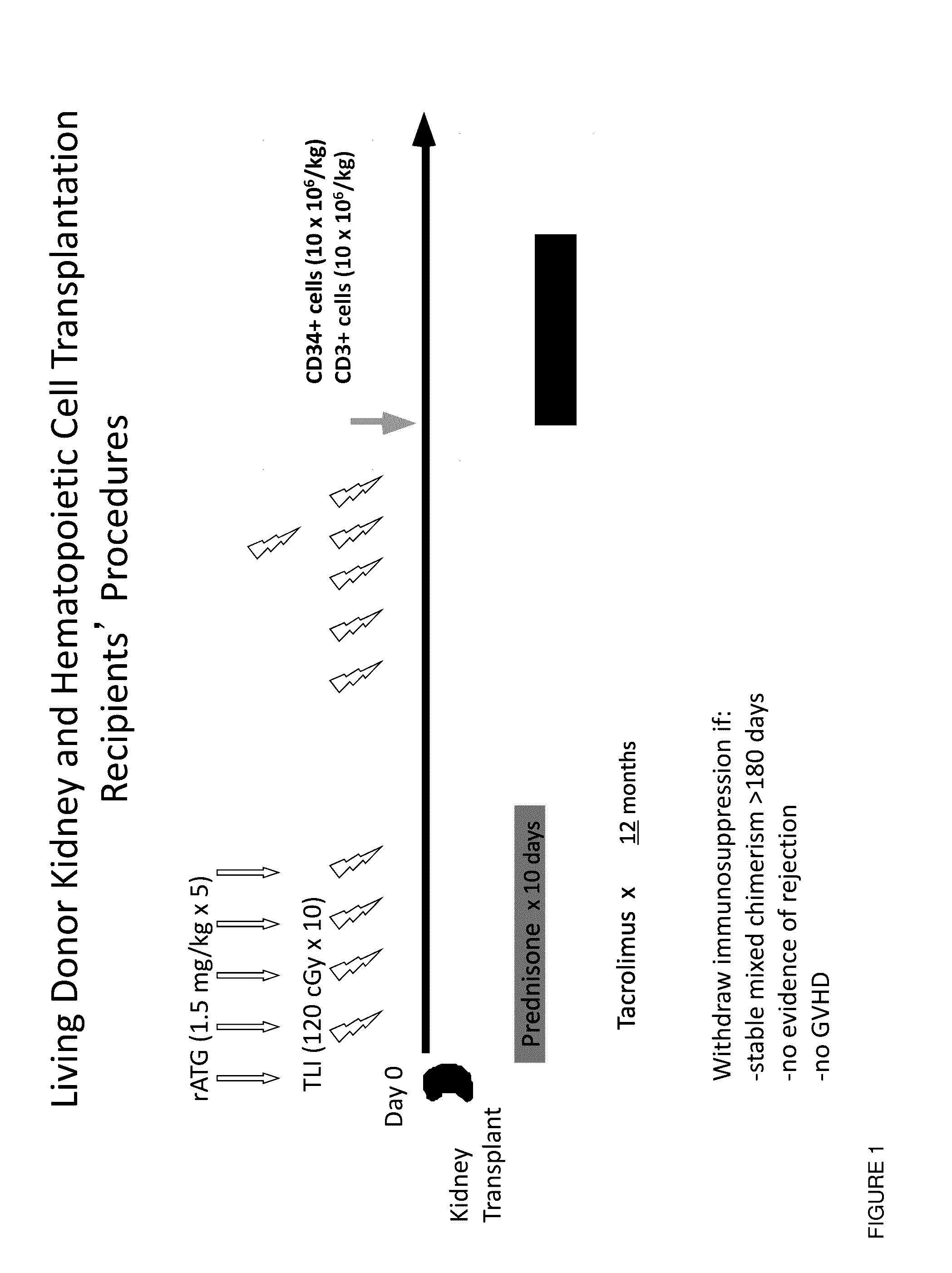
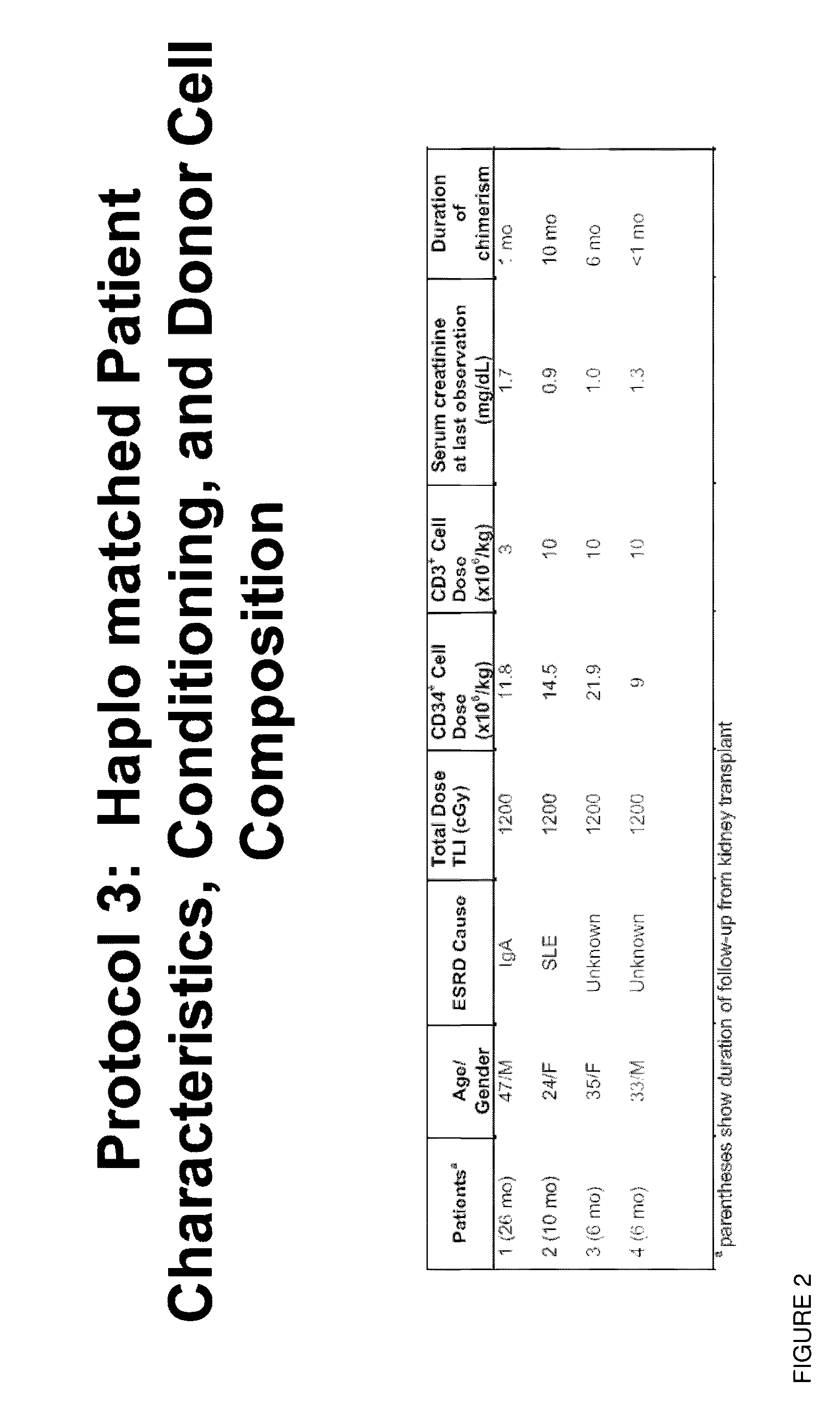
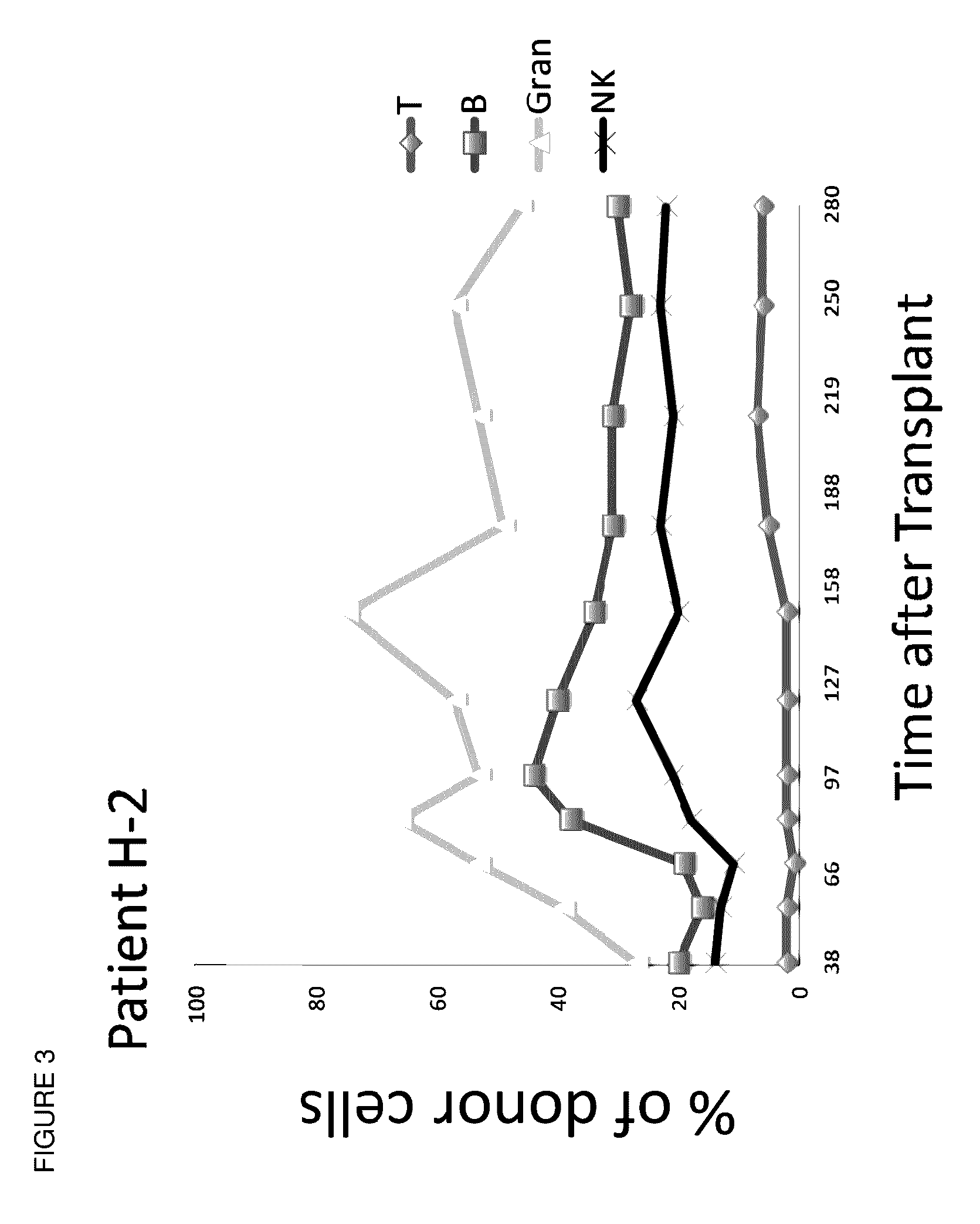
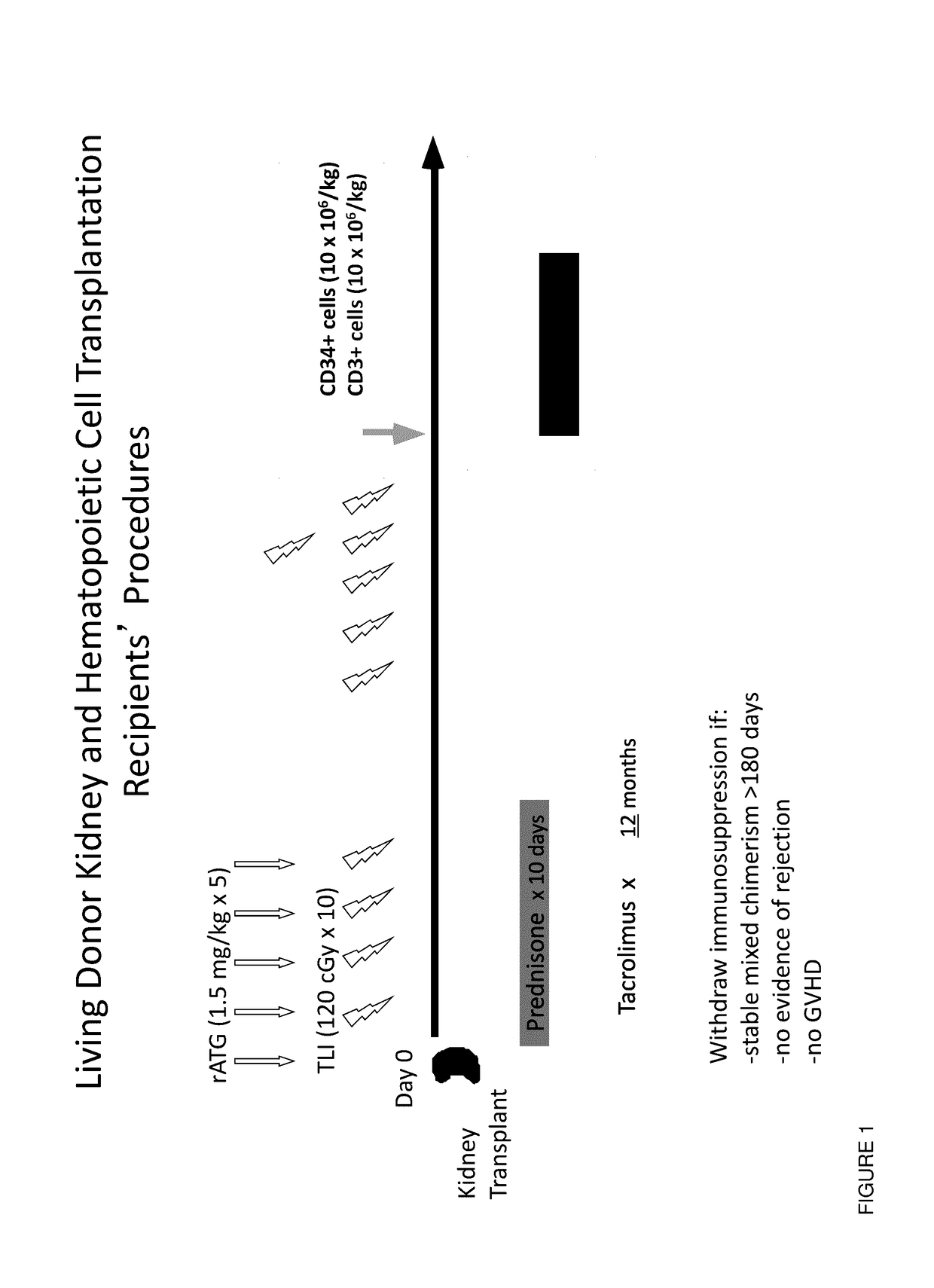
Methods and compositions are provided for combined transplantation of a solid organ and hematopoietic cells to an HLA mismatched recipient, where tolerance to the graft is established through development of a persistent mixed chimerism. An individual with persistent mixed chimerism, usually for a period of at least six months, is able to withdraw from the use of immunosuppressive drugs after a period of time sufficient to establish tolerance.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Combined organ and hematopoietic cells for transplantation tolerance of HLA mismatched grafts
ActiveUS20170106086A1Organic active ingredientsEnergy modified materialsImmunosuppressive drugHematopoietic cell
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Tumor vaccination in combination with hematopoietic cell transplantation for cancer therapy
ActiveUS20110129503A1Eliminate side effectsBiocideEnergy modified materialsAbnormal tissue growthVaccination
In one aspect, the present invention provides a method for treating cancer comprising tumor cell vaccination in combination with hematopoietic and immune cell transplantation. In some embodiments, the method involves autologous tumor cell vaccination prior to autologous hematopoietic and immune cell transplantation. In another aspect, the present invention provides a method of purifying tumor cells from a subject in preparation for vaccination.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods of using Flt3-Ligand in hematopoietic cell transplantation procedures incorporating nonmyeloablative conditioning regimens
InactiveUS20050058622A1BiocidePeptide/protein ingredientsHematopoietic cellHaematopoietic cell transplantation
The invention is directed to methods of using Flt3-Ligand in hematopoietic cell transplantation procedures using nonmyeloablative conditioning regimens. This abstract is provided for the sole purpose of enabling the reader to quickly ascertain the subject matter of the technical disclosure and is not intended to be used to interpret or limit the scope or meaning of the claims.
Owner:IMMUNEX CORP
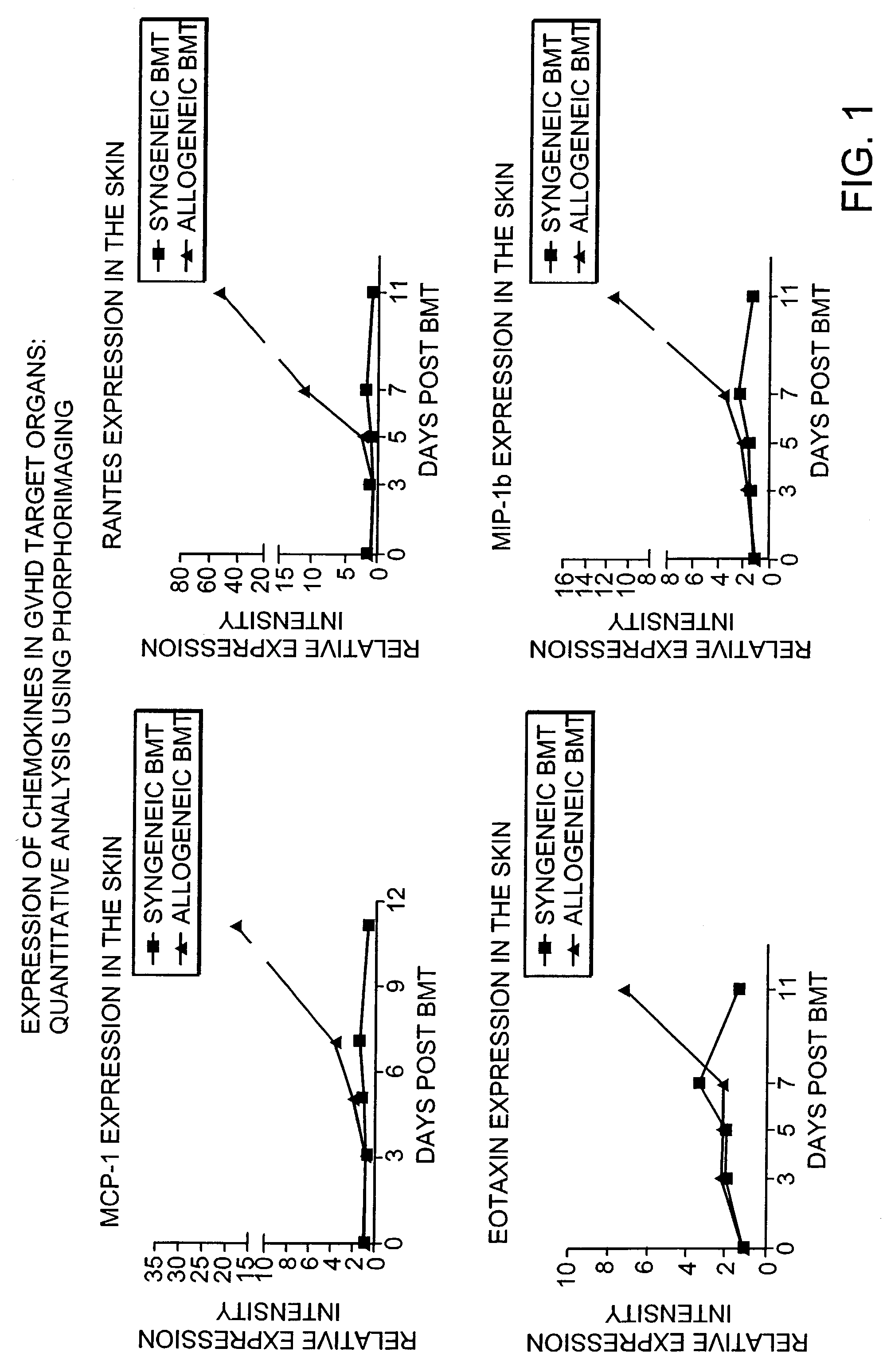
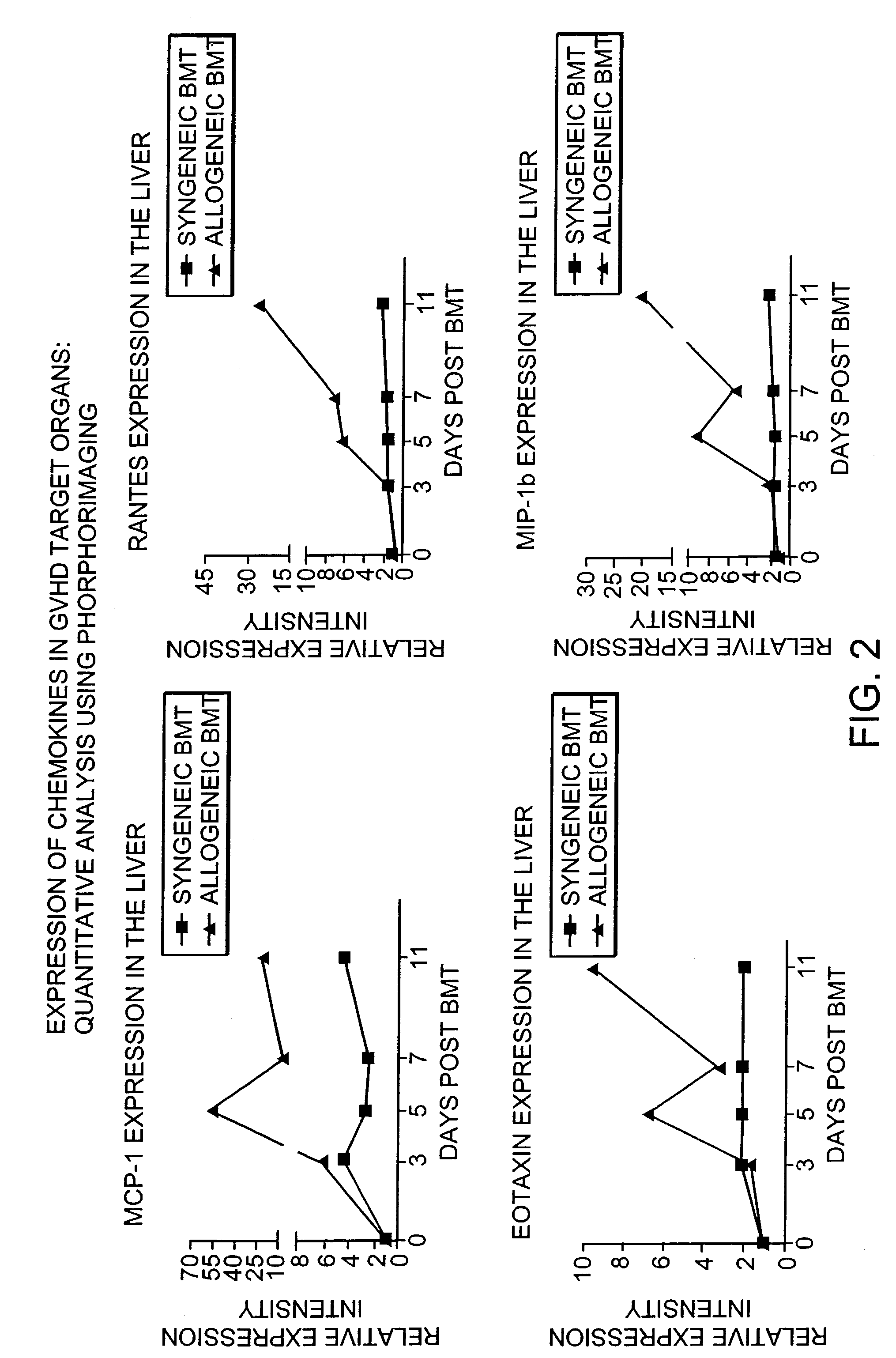
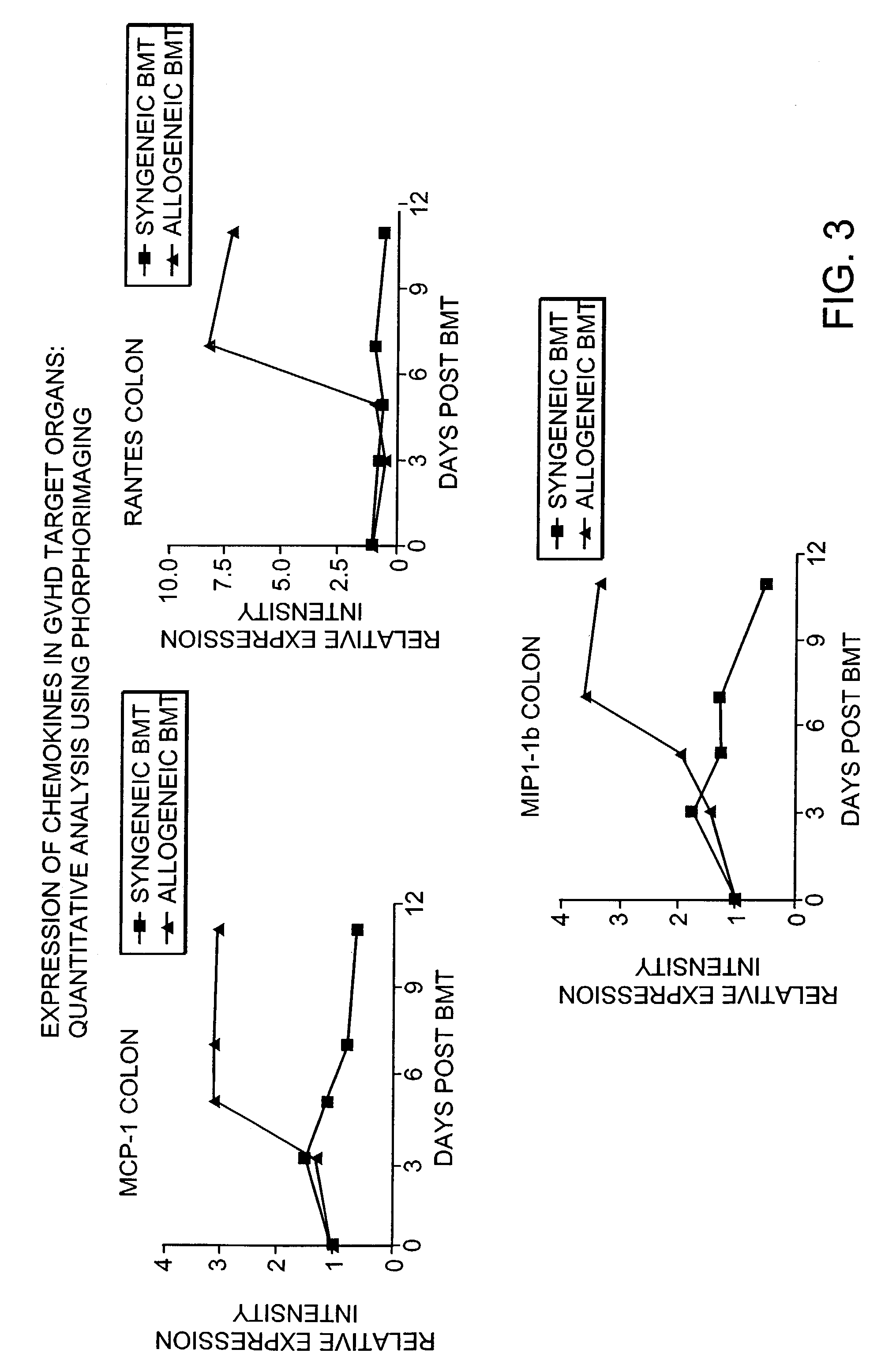
Blockade of T cell migration into epithelial GVHD target tissues as an approach to achieving anti-tumor effects against lymphohematopoietic malignancies without GVHD
InactiveUS7498023B2BiocideMammal material medical ingredientsHematopoietic cellHaematopoietic cell transplantation
Antagonists of T cell migration are used to reduce GVHD in recipients of hematopoietic cell grafts. The administration of antagonists of T cell migration can be used in combination with conventional methods of bone marrow transplantation and in combination with the administration of donor leukocytes.
Owner:THE GENERAL HOSPITAL CORP
Method of long-term treatment of graft-versus-host disease using topical active corticosteroids
InactiveUS20100184732A1Reduce severityFast metabolismOrganic active ingredientsBiocideHaematopoietic cell transplantationTissue damage
A method of long-term therapy using corticosteroids to treat tissue damage associated with graft-versus-host disease in a patient having undergone hematopoietic cell transplantation, and host-versus-graft disease in a patient having undergone organ allograft transplantation. The method includes orally administering to the patient a therapeutically effective amount of a topically active corticosteroids, such as beclomethasone dipropionate, from the 29th day until the 56th day following hematopoietic cell or organ allograft transplantation. Representative tissues include tissue of the intestine and liver, while representative tissue damage includes inflammation thereof.
Owner:MCDONALD GEORGE
Treatment of graft-versus-host disease and leukemia with beclomethasone dipropionate and prednisone
InactiveUS20060252735A1Reduce mortalityOrganic active ingredientsBiocideHematopoietic cellCo administration
A method for reducing mortality associated with GVHD by treating the patent with an oral BDP regimen that involves co-administration of: 1) a high dose of prednisone (about 1-2 mg / kg / day) for about 10 days, which is then tapered rapidly over the following 7 days to a physiological replacement dose of about 0.0625 mg / kg / day for the remainder of the treatment, and 2) about 4-12 mg oral BDP q.i.d. for about 50 days, where the BDP is administered in both immediate release and enteric coated preparations. Another method is for treating leukemia by performing hematopoietic cell transplantation followed by said regimen. A significant reduction in patient mortality is observed 200 days after the start of these treatments
Owner:SOLIGENIX INC
Method of predicting graft versus host disease
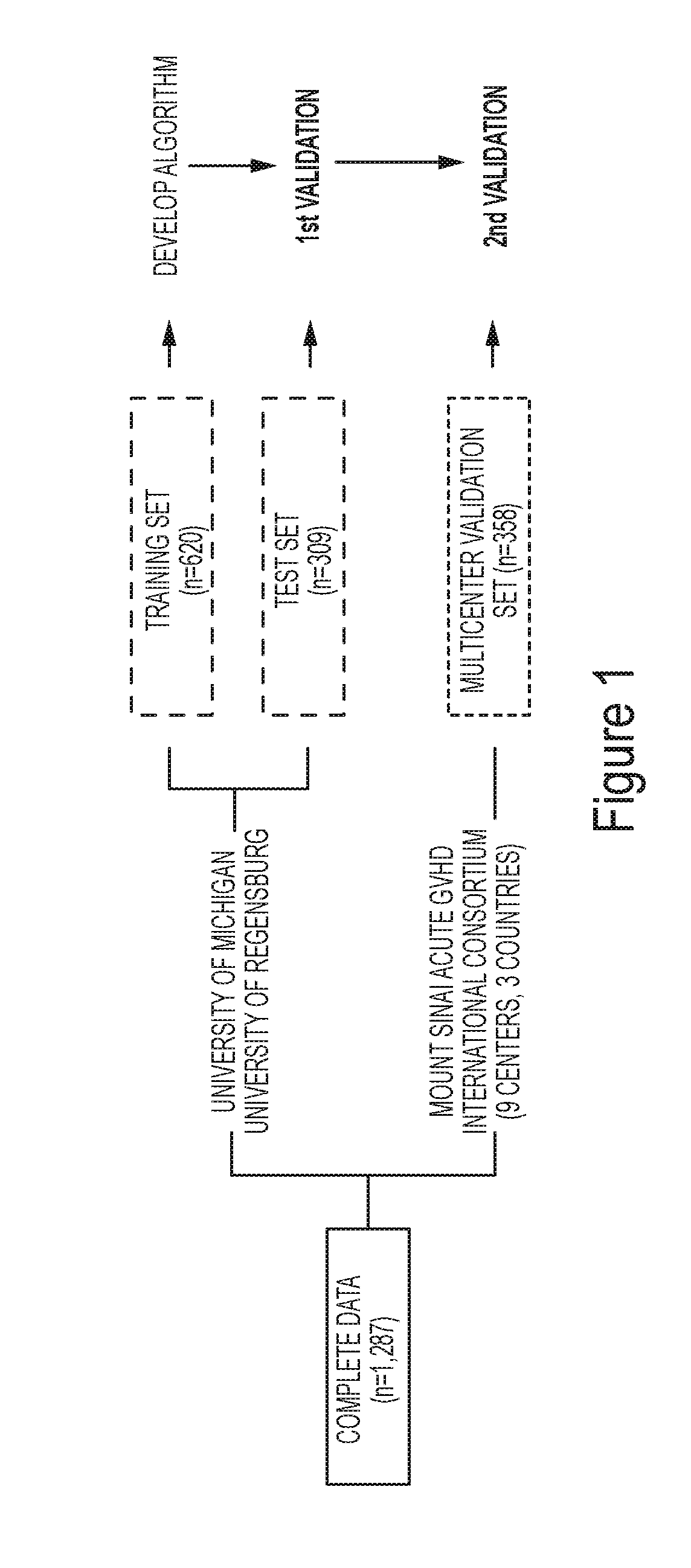
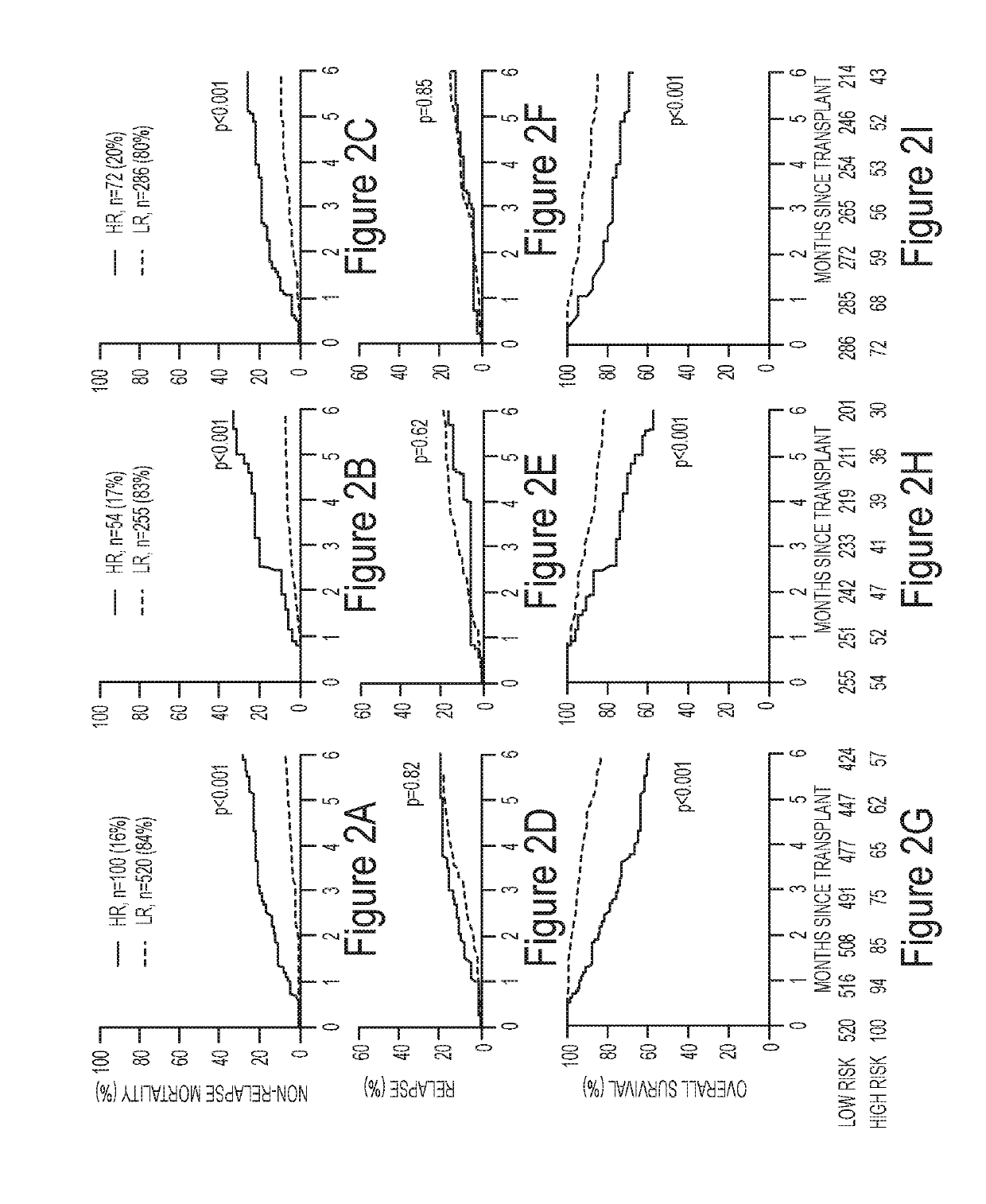
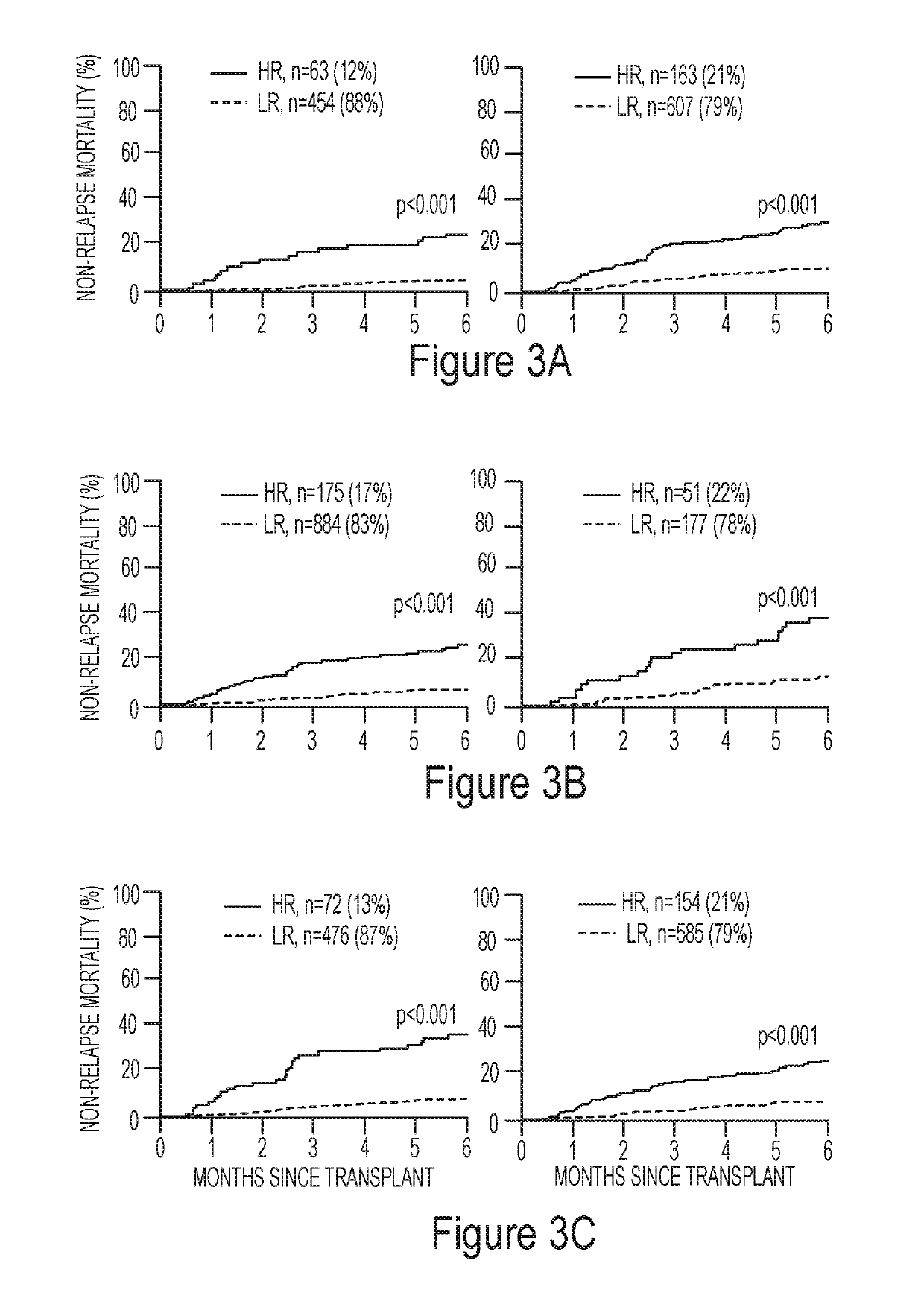
ActiveUS20190259467A1Satisfies needOrganic active ingredientsPeptide/protein ingredientsHaematopoietic cell transplantationMedicine
Embodiments of the invention relate generally to methods of diagnosing, classifying, and / or identifying a patient's risk of developing graft versus host disease, including severe or lethal graft versus host disease, after receiving hematopoietic cellular transplantation, a transfusion or a transplantation, but before the onset of clinical symptoms.
Owner:MT SINAI SCHOOL OF MEDICINE
Method and compositions for improving allogeneic hematopoietic cell transplantation
InactiveCN1333688ABioreactor/fermenter combinationsBiological substance pretreatmentsProgenitorDendritic cell
Methods of reducing or preventing graft versus host disease in an allogeneic hematopoietic system reconstituting cells transplant recipient comprising administering to the recipient allogeneic hematopoietic system reconstituting cells in which the number of dendritic cells has been effectively reduced, thereby reducing or preventing graft versus host disease, are provided. Also provided are methods of increasing the chance of survival for an allogeneic hematopoietic system reconstituting cells transplant recipient comprising administering to the recipient allogeneic hematopoietic system reconstituting cells in which the number of dendritic cells has been effectively reduced, thereby improving the chance for survival of the recipient. Columns for preparing hematopoietic system reconstituting cells prior to their transplantation are disclosed that provide for the selection of CD34+ cells and the removal of dendritic cell progenitors.
Owner:EMORY UNIVERSITY
Immune-tolerance inducer
InactiveCN105120877AEfficient inductionReduce receptor burdenOrganic active ingredientsAntibody ingredientsHematopoietic cellHaematopoietic cell transplantation
Owner:REGIMMUNE CORP
Methods of using flt3-ligand in hematopoietic cell transplantation
InactiveUS7294331B2Enhance immune responseImprove the quality of lifeBiocidePeptide/protein ingredientsProgenitorHematopoietic cell
Ligands for flt3 receptors capable of transducing self-renewal signals to regulate the growth, proliferation or differentiation of progenitor cells and stem cells are disclosed. The invention is directed to Flt3-ligand as an isolated protein, the DNA encoding the Flt3-ligand, host cells transfected with cDNAs encoding Flt3-ligand, compositions comprising Flt3-ligand and methods of using Flt3-ligand in hematopoietic cell transplantation.
Owner:CELLDEX THERAPEUTICS INC
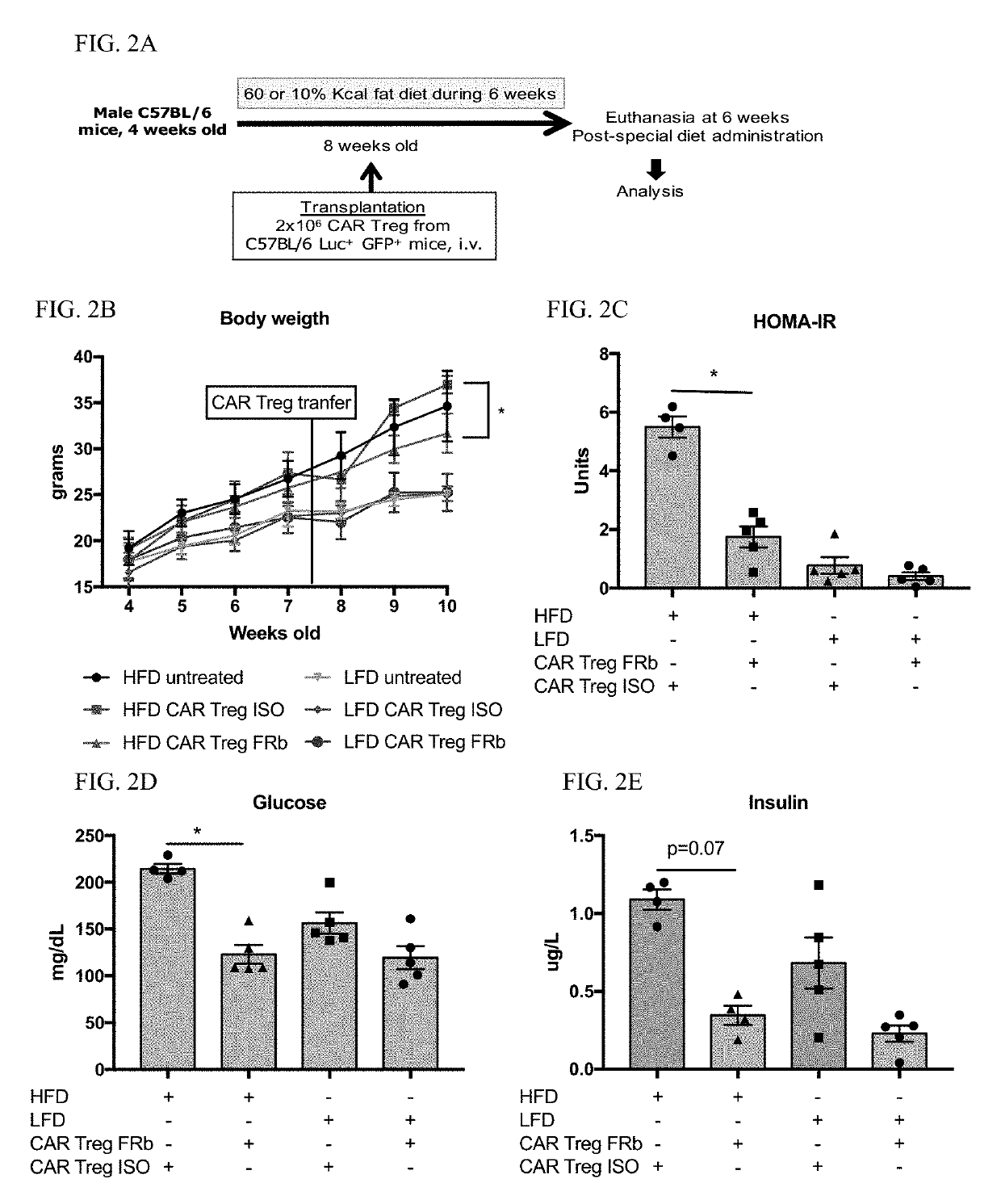
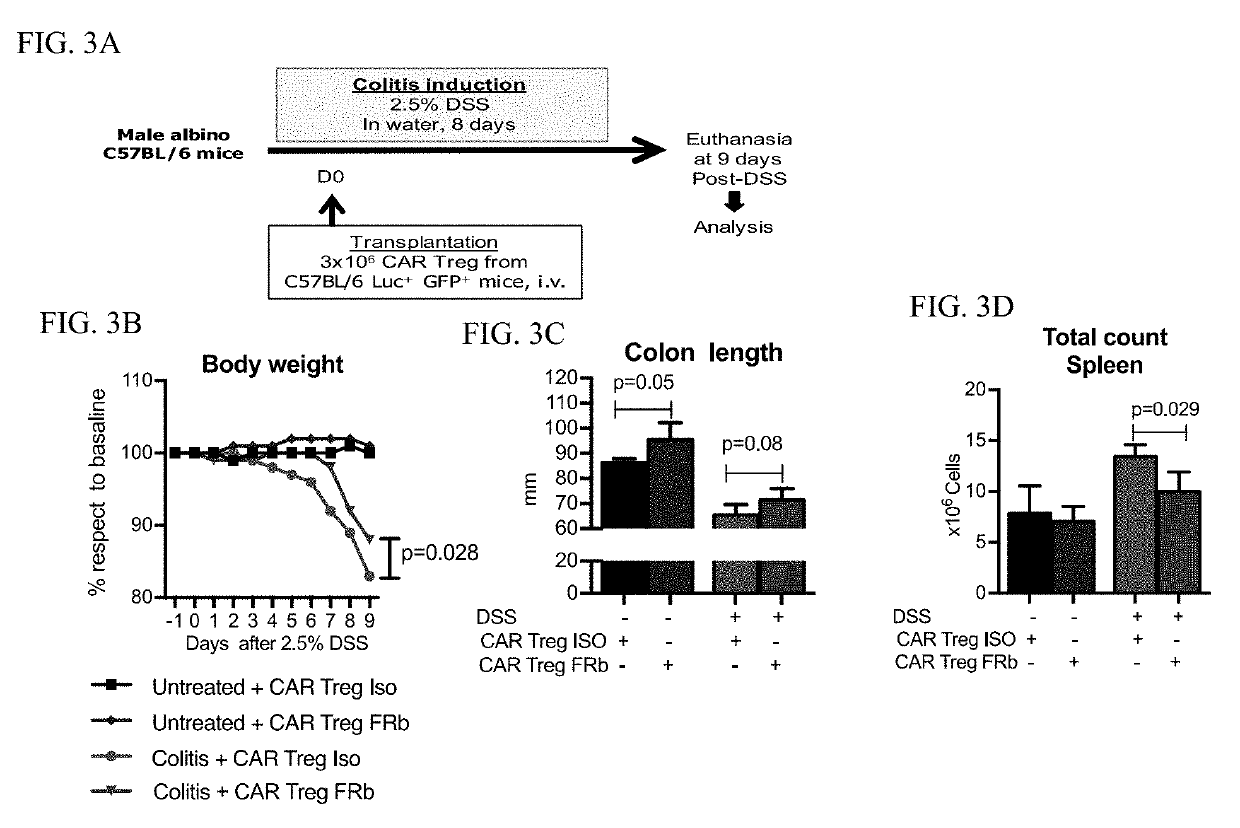
Regulatory t cells targeted with chimeric antigen receptors
InactiveUS20190307795A1Easy to transplantIncrease chimerismPeptide/protein ingredientsAntipyreticAntigenRegulatory T cell
Regulatory T cells (Treg) are engineered to express a chimeric antigen receptor (CAR), that specifically binds folate receptor beta; and are administered to an individual for treatment of inflammation at sites characterized by the presence of activated myeloid cells. Also provided are methods for utilized engineered T regulatory cells to enhance hematopoietic cell transplantation.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods of improving hematopoietic grafts
PendingCN110997904AUnknown materialsBlood/immune system cellsHematopoietic cellHaematopoietic cell transplantation
The present invention relates to a method of preparing hematopoietic cell graft or enriching a population of cells for hematopoietic stem cells that are capable of long-term multilineage engraftment and self-renewal. It also relates to hematopoietic grafts comprising said hematopoietic stem cells as well as their uses in therapy.
Owner:ESTAB FR DU SANG +5
Immune-tolerance inducer
InactiveUS20150283235A1Stable maintenanceFacilitated DiffusionOrganic active ingredientsAntibody ingredientsTolerance inductionHematopoietic cell
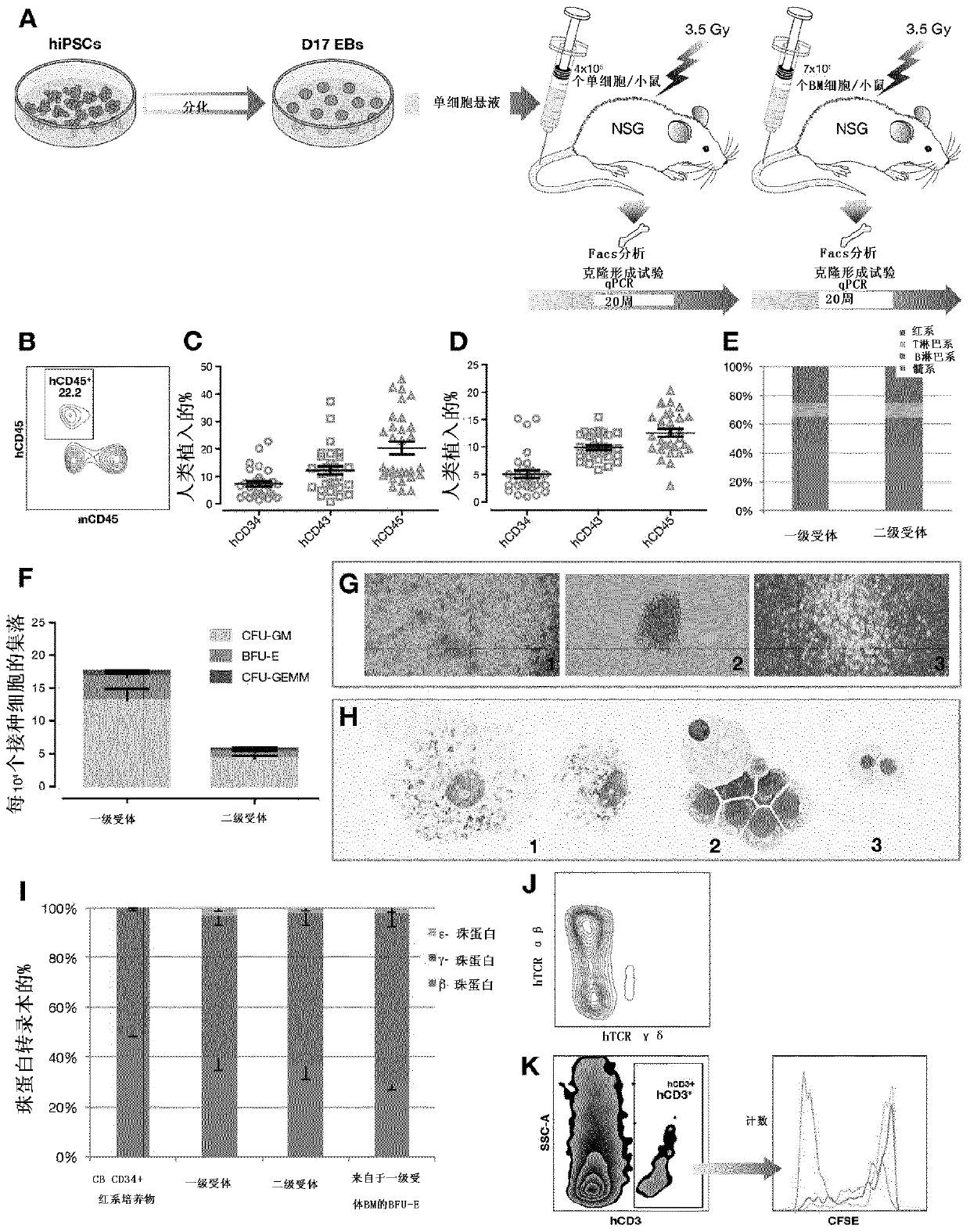
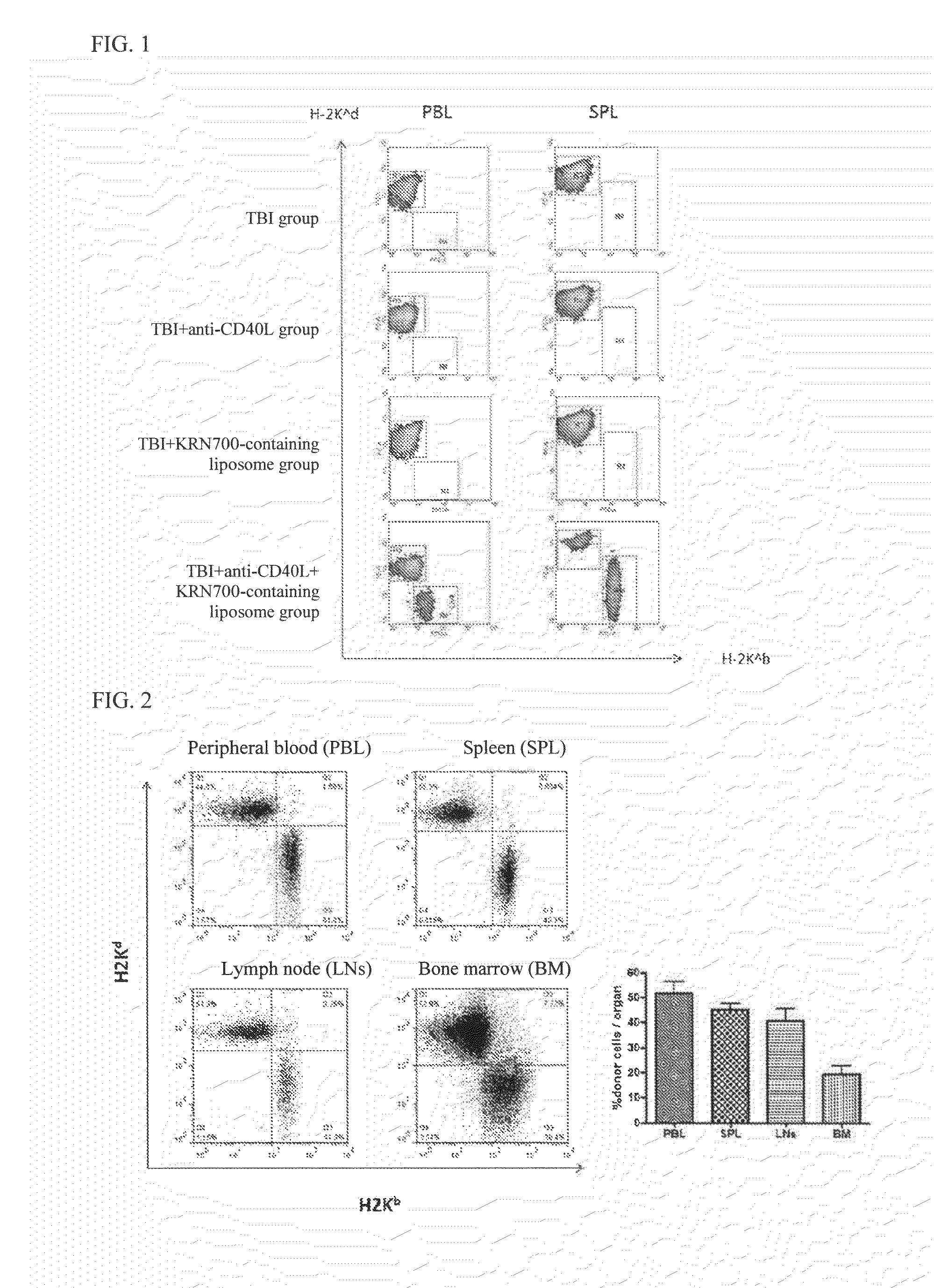
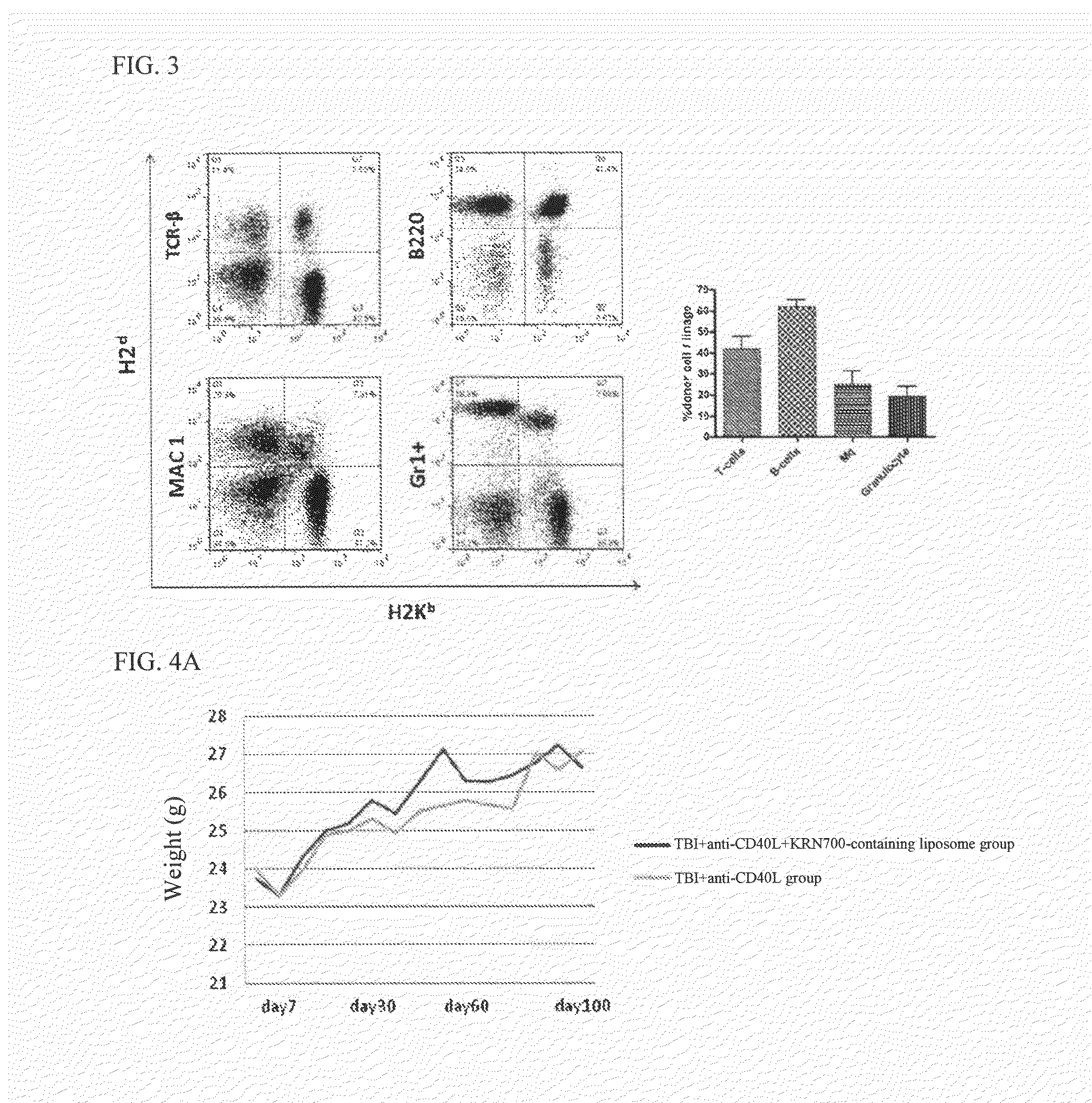
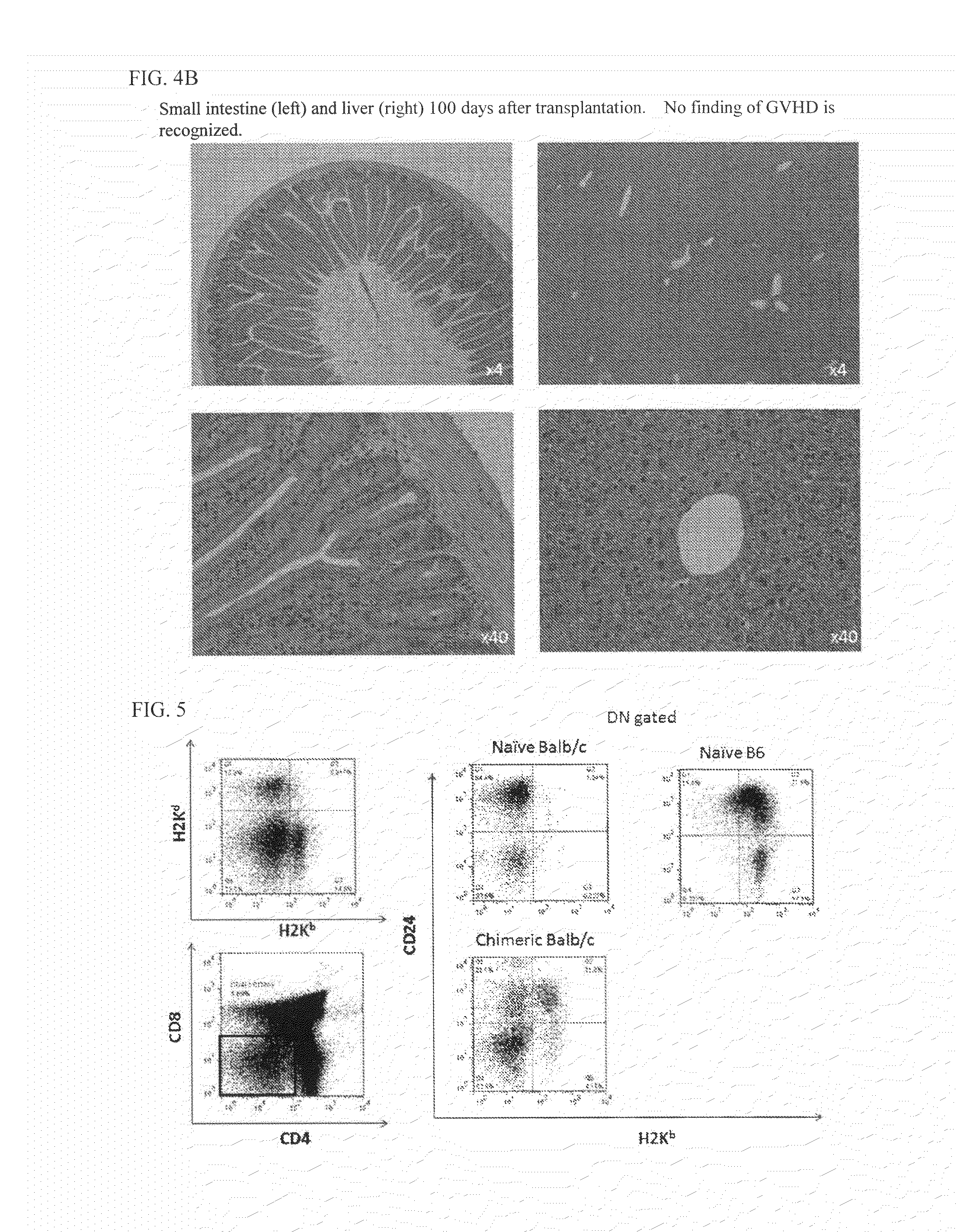
The purpose of the present invention is to create an immune tolerance-inducing agent used in therapy in which donor hematopoietic cells are transplanted into a recipient in order to induce immune tolerance in the recipient with respect to donor cells, tissue, or organs. By using an alpha-galactosylceramide-containing liposome in combination with a costimulatory-pathway-blocking substance, hematopoietic chimerism can be induced in the recipient by transplantation of donor hematopoietic cells, making it possible to induce immune tolerance in the recipient with respect to donor cells, tissue, or organs.
Owner:REGIMMUNE +1
Cell culture systems for producing il-33 induced t9 cells and methods of using the cells
InactiveUS20180139937A1Mammal material medical ingredientsBlood/immune system cellsHematopoietic cellCancer therapy
Cell culture systems for producing IL-33 induced T9 cells and methods of using the IL-33 induced T9 cells (T9IL-33 cells) in a cell therapy for increasing anti-tumoral activity following allogeneic hematopoietic cell transplantation (HCT) and / or treating graft-versus-host disease (GVHD) are disclosed herein. Further, methods of using the T9IL-33 cells, alone or in combination with allogeneic hematopoietic cell transplantation, are described herein for cancer treatment.
Owner:INDIANA UNIV RES & TECH CORP
Methods for reducing risk of onset of acute graft versus host disease after hematopoeitic cell transplantation
PendingUS20210008182A1Peptide/protein ingredientsMammal material medical ingredientsHematopoietic cellHaematopoietic cell transplantation
This disclosure relates to methods for preventing or reducing the risk of development of graft versus host disease (GVHD) in patients receiving hematopoietic cell transplantation (HCT) by particular methods of administering alpha-1 antitrypsin (A1AT or AAT) to patients both prior to and following and HCT procedure. The disclosure also relates to specific methods of treating acute GVHD (aGVHD) after HCT with A1AT.
Owner:CSL BEHRING LLC
Stem cell culture media and methods of enhancing cell survival
ActiveUS9943545B2Enhance cell viabilityIncreased proliferationElectrotherapyCulture processHematopoietic cellHaematopoietic cell transplantation
The invention provides improved methods for preparing hematopoietic cells for transplantation and the resulting improved hematopoietic cell compositions. The invention further relates to improved culture media and methods of culturing, processing, modulating, and expanding blood cell products for hematopoietic transplantation.
Owner:FATE THERAPEUTICS
Methods of improving hematopoietic grafts
PendingUS20200080058A1Easy to transplantUnknown materialsBlood/immune system cellsHematopoietic cellHaematopoietic cell transplantation
The present invention relates to a method of preparing hematopoietic cell graft or enriching a population of cells for hematopoietic stem cells that are capable of long-term multilineage engraftment and self-renewal. It also relates to hematopoietic grafts comprising said hematopoietic stem cells as well as their uses in therapy.
Owner:UNIV PARIS SACLAY +5
Use of veto cells for the treatment of sickle cell disease
PendingUS20220265726A1Mammal material medical ingredientsBlood/immune system cellsTolerance inductionHematopoietic cell
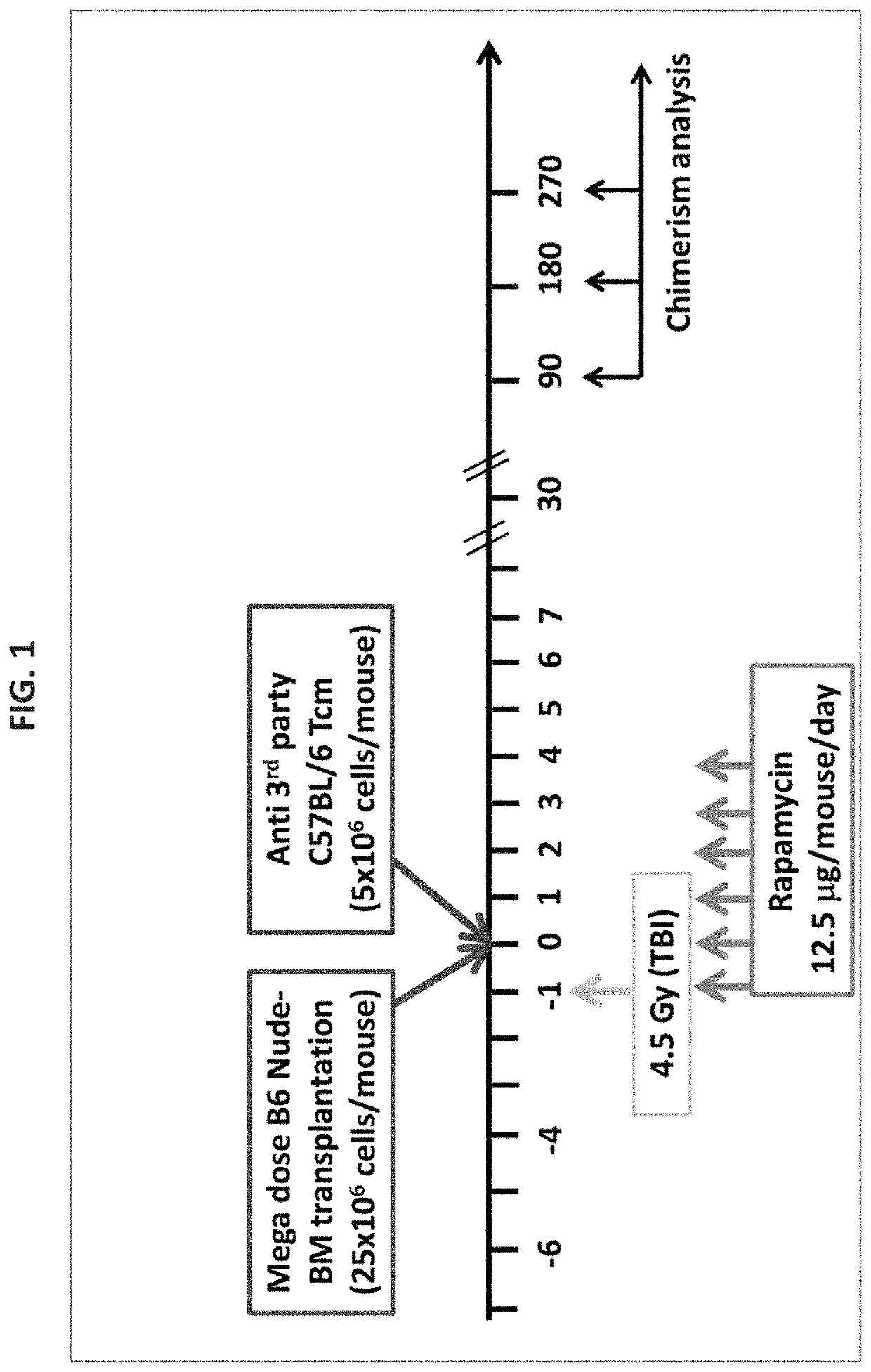
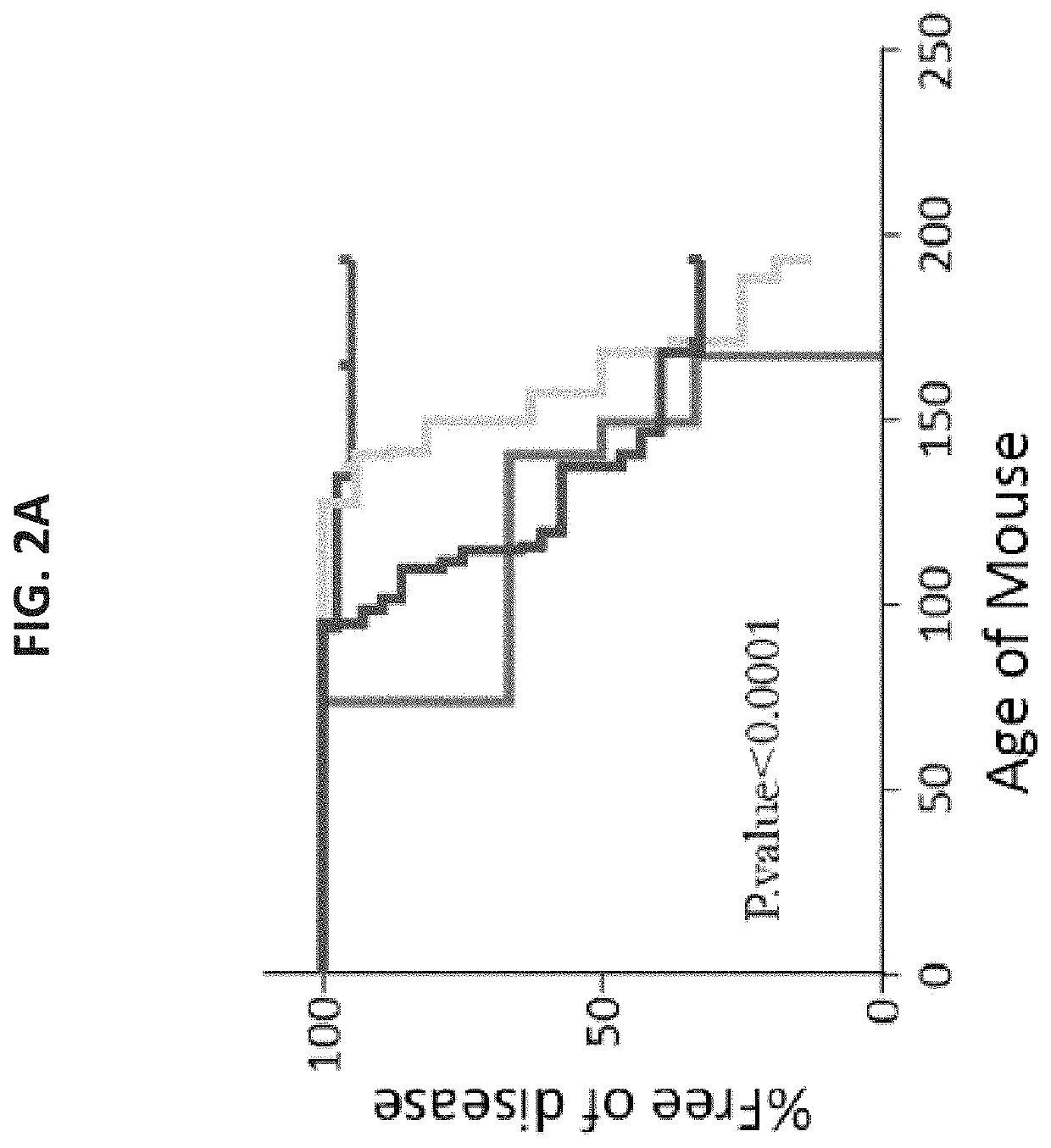
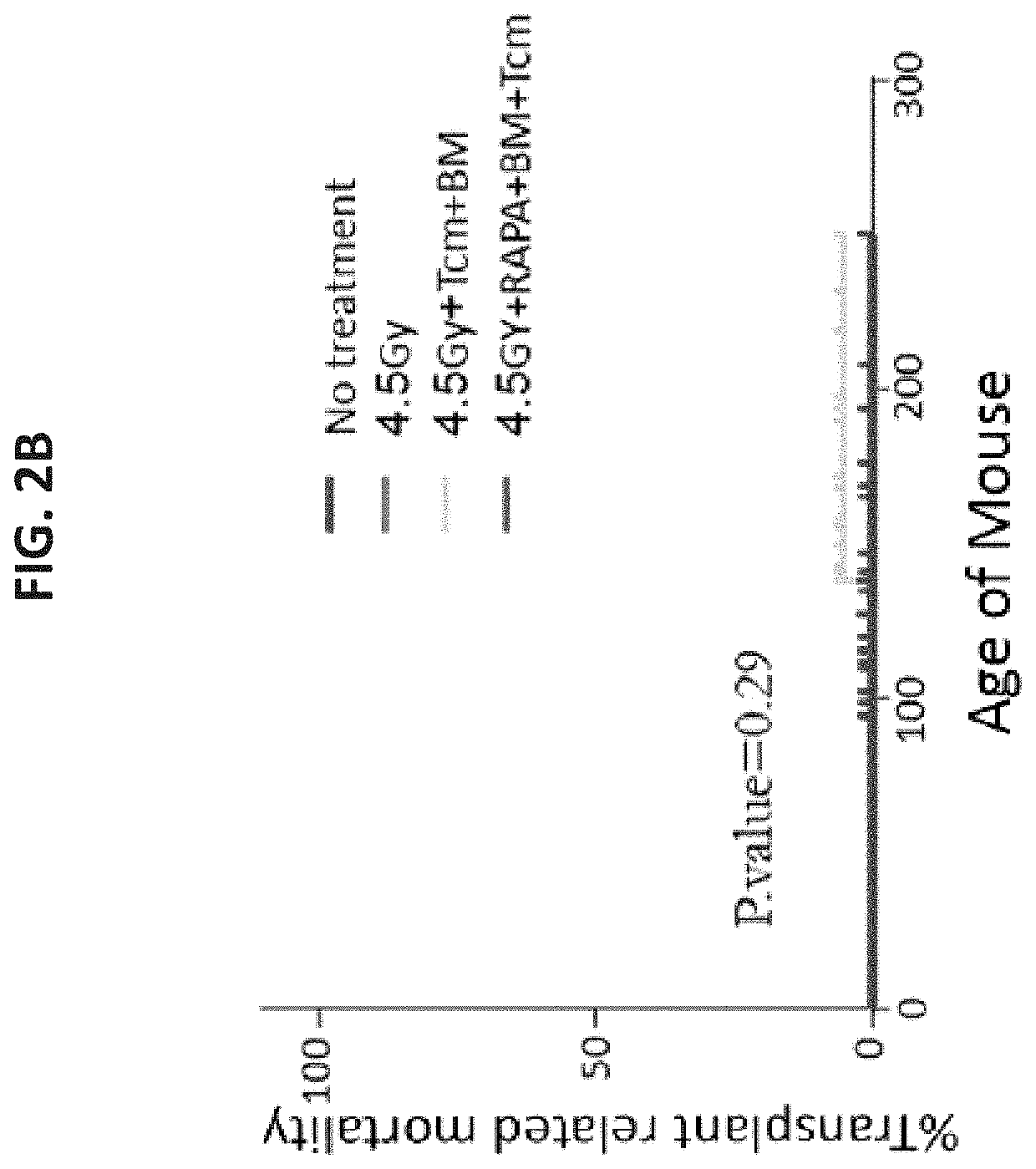
A method of treating or preventing a sickle cell disease in a subject in need thereof is disclosed. The method comprising: (a) transplanting immature hematopoietic cells into the subject; and (b) administering to the subject a therapeutically effective amount of an isolated population of non-GVHD inducing anti-third party cells comprising cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance inducing cells and capable of homing to the lymph nodes following transplantation.
Owner:YEDA RES & DEV CO LTD +1
Methods of using flt3-ligand in the treatment of cancer
InactiveUS20070207164A1Enhance immune responseImprove the quality of lifePeptide/protein ingredientsAntibody mimetics/scaffoldsProgenitorHematopoietic cell
Ligands for flt3 receptors capable of transducing self-renewal signals to regulate the growth, proliferation or differentiation of progenitor cells and stem cells are disclosed. The invention is directed to Flt3-ligand as an isolated protein, the DNA encoding the Flt3-ligand, host cells transfected with cDNAs encoding Flt3-ligand, compositions comprising Flt3-ligand and methods of using Flt3-ligand in hematopoietic cell transplantation.
Owner:CELLDEX THERAPEUTICS INC
Methods for enhancing hematopoietic stem/progenitor cell engraftment
ActiveUS20110293582A1Ease of obtaining and preparationBiocidePeptide/protein ingredientsProgenitor Cell EngraftmentMolecular biology
Described herein are methods for enhancing engraftment of hematopoietic stem and progenitor cells using farnesyl compounds identified using a zebrafish model of hematopoietic cell engraftment. The compounds can be used to treat hematopoietic stem cells ex vivo prior to transplantation of the cells. Alternatively, the compounds can be administered to an individual undergoing cell transplantation.
Owner:CHILDRENS MEDICAL CENT CORP
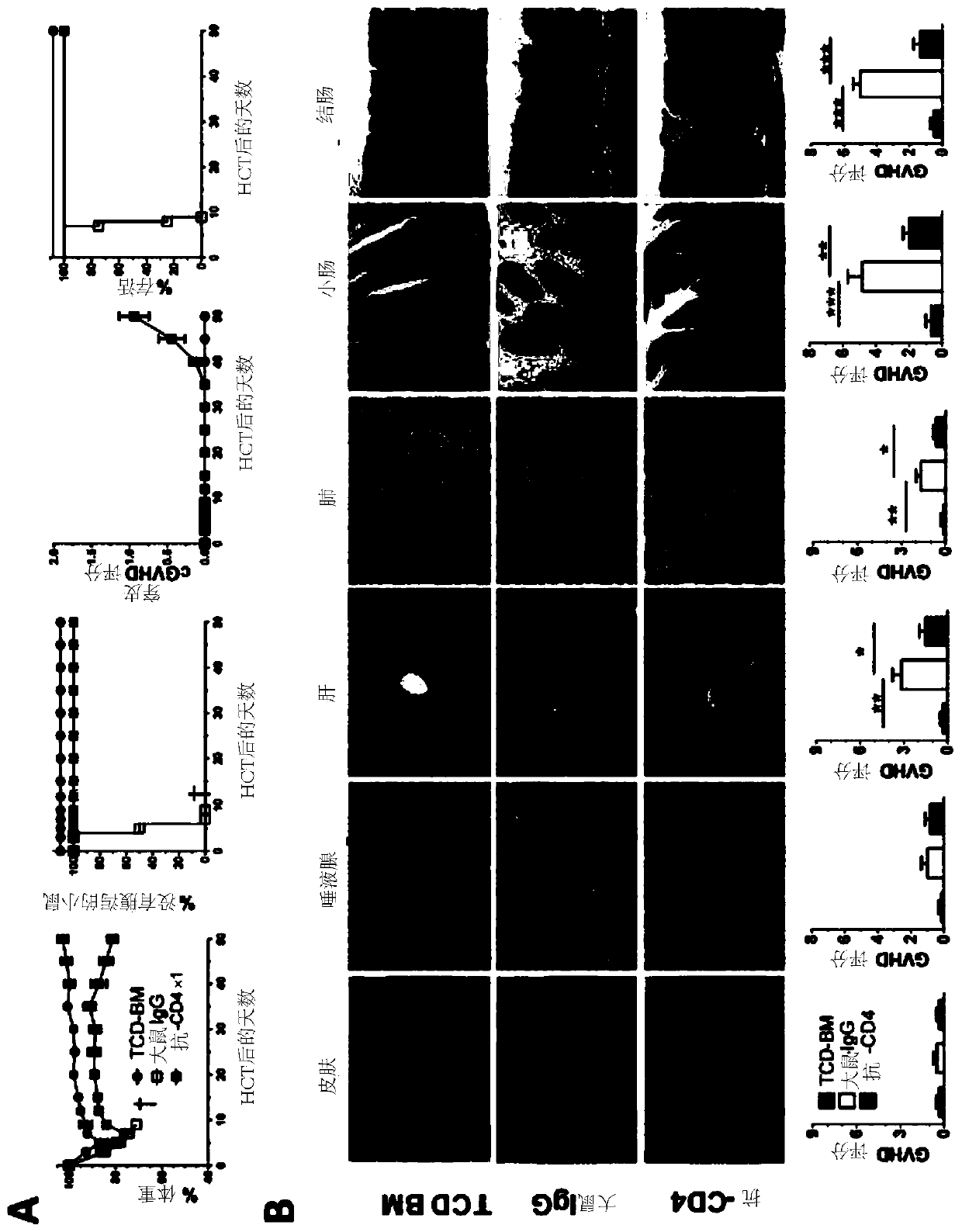
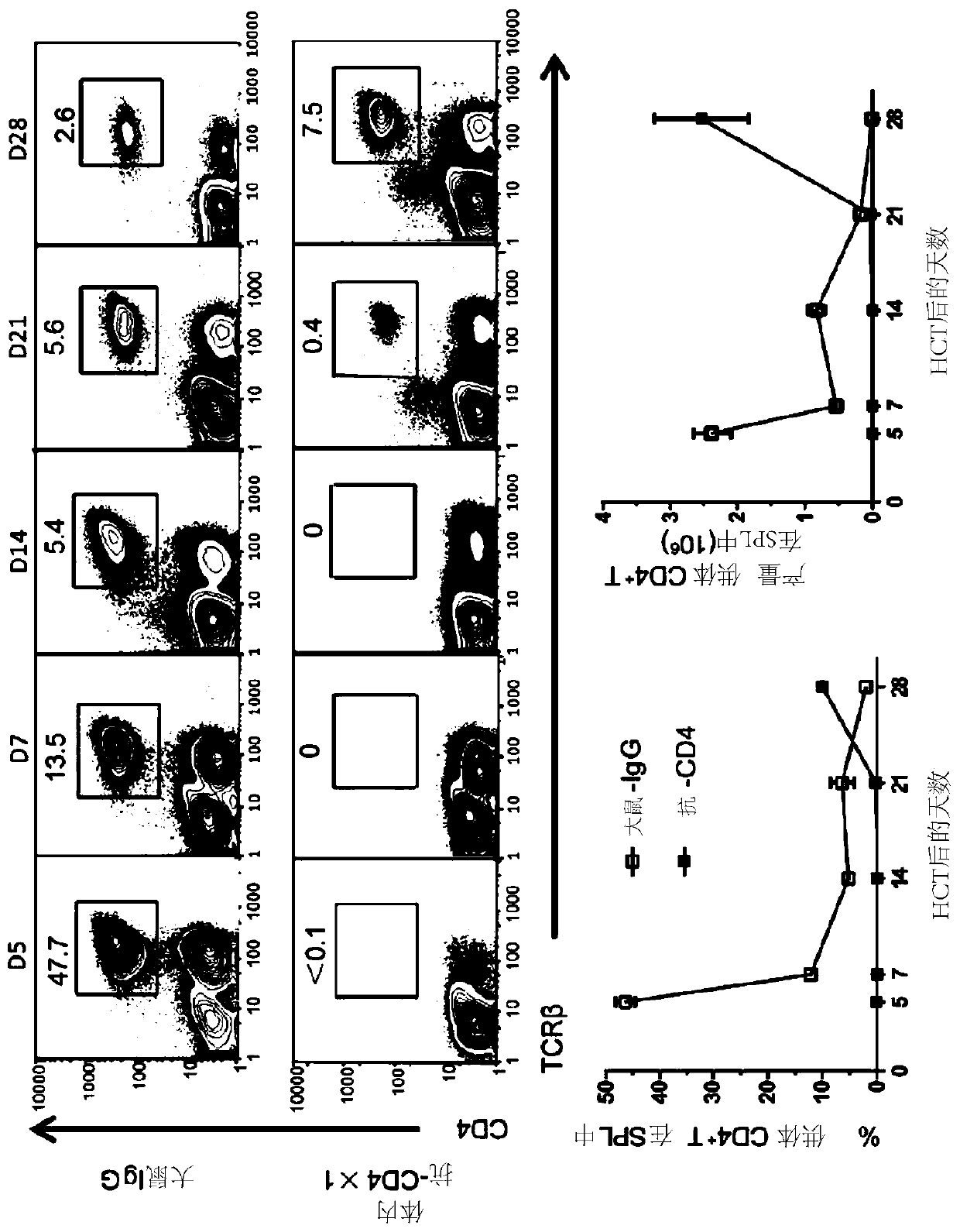
Methods for in vivo expansion of cd8+ t cells and prevention or treatment of gvhd
PendingCN110913873APeptide/protein ingredientsAntibody mimetics/scaffoldsHematopoietic cellAntiendomysial antibodies
The present invention discloses methods of preventing and treating acute GVHD and chronic GVHD after hematopoietic cell transplantation (HCT), as well as methods of in vivo augmenting expansion of donor CD8+ T cells in the lymphoid tissues in vivo after HCT and methods of augmenting recipient tissue expression of programmed death-ligand 1 (PD-L1, or B7H1) after HCT. The methods entail administering one or more doses of an effective amount of a therapeutic agent to a recipient simultaneously, immediately before, or immediately after HCT to temporarily deplete CD4+ T cells or to reduce serum IL-2. Some examples include an anti-CD4 antibody or an anti-CD4-meditope-immunotoxin, an anti-IL-2 antibody, an agent blocking IL-2R, and / or a PD-L1-Ig. One or more additional therapeutic agents such asIFN-y can be administered.
Owner:CITY OF HOPE
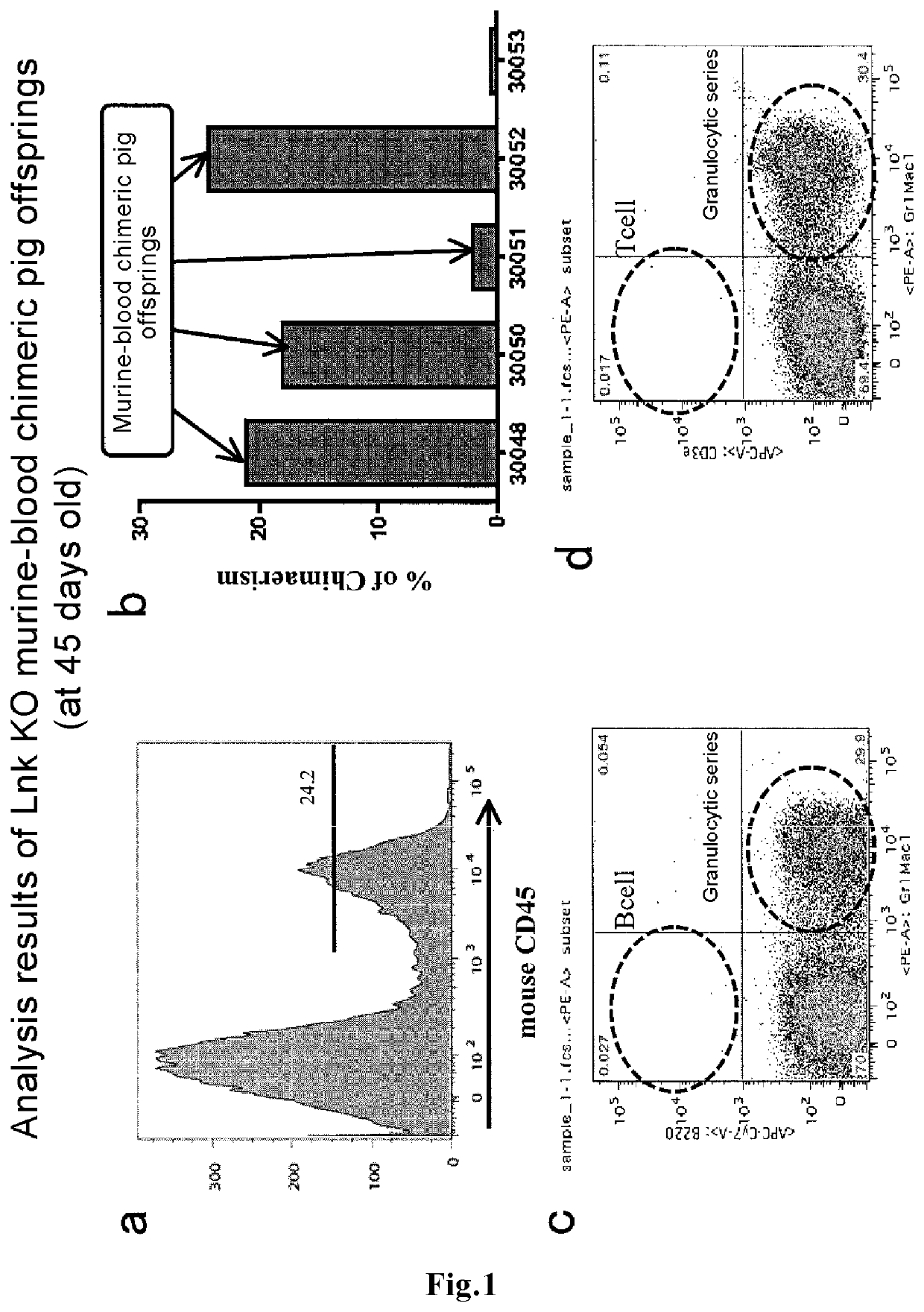
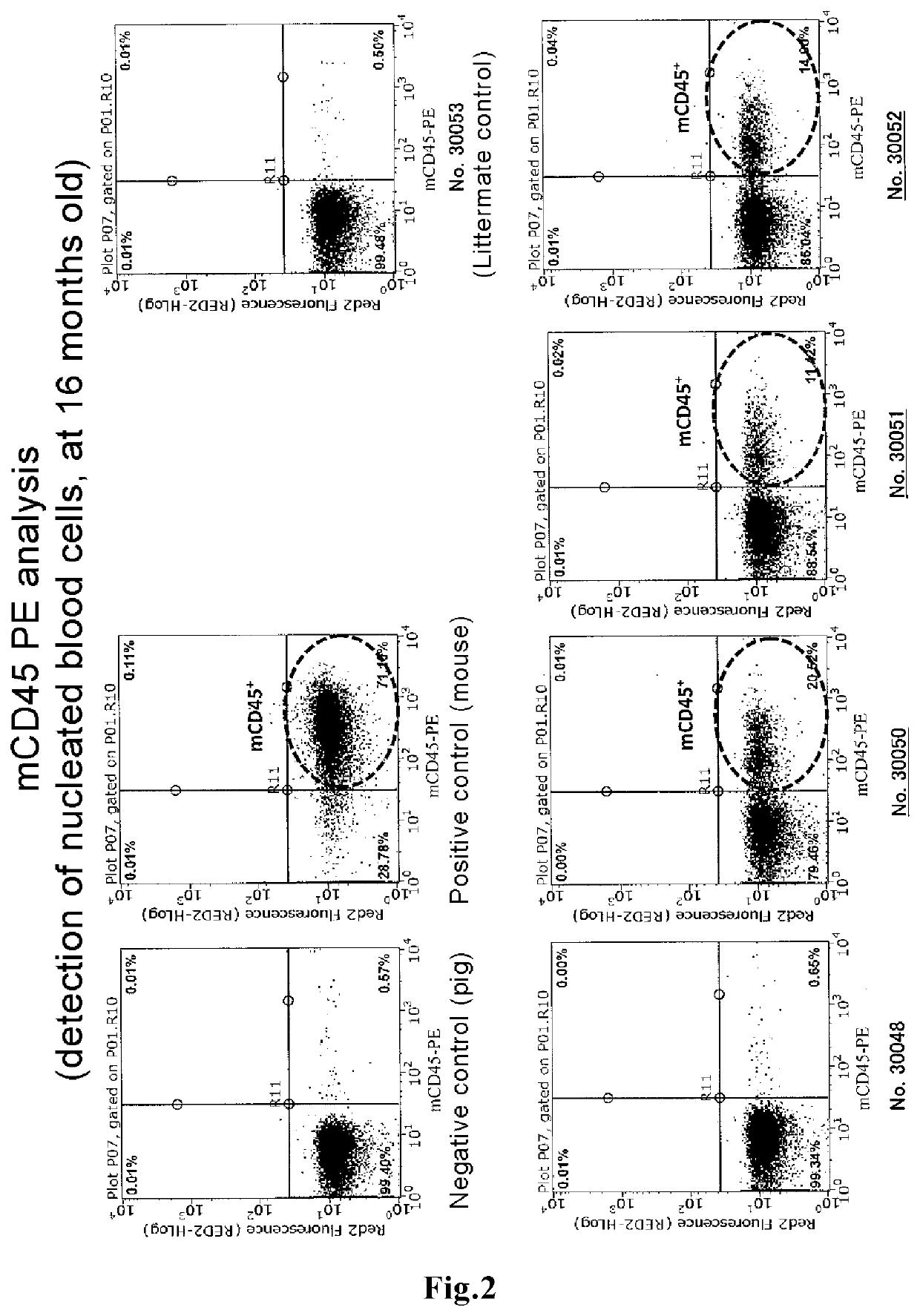
Method for producing blood chimeric animal
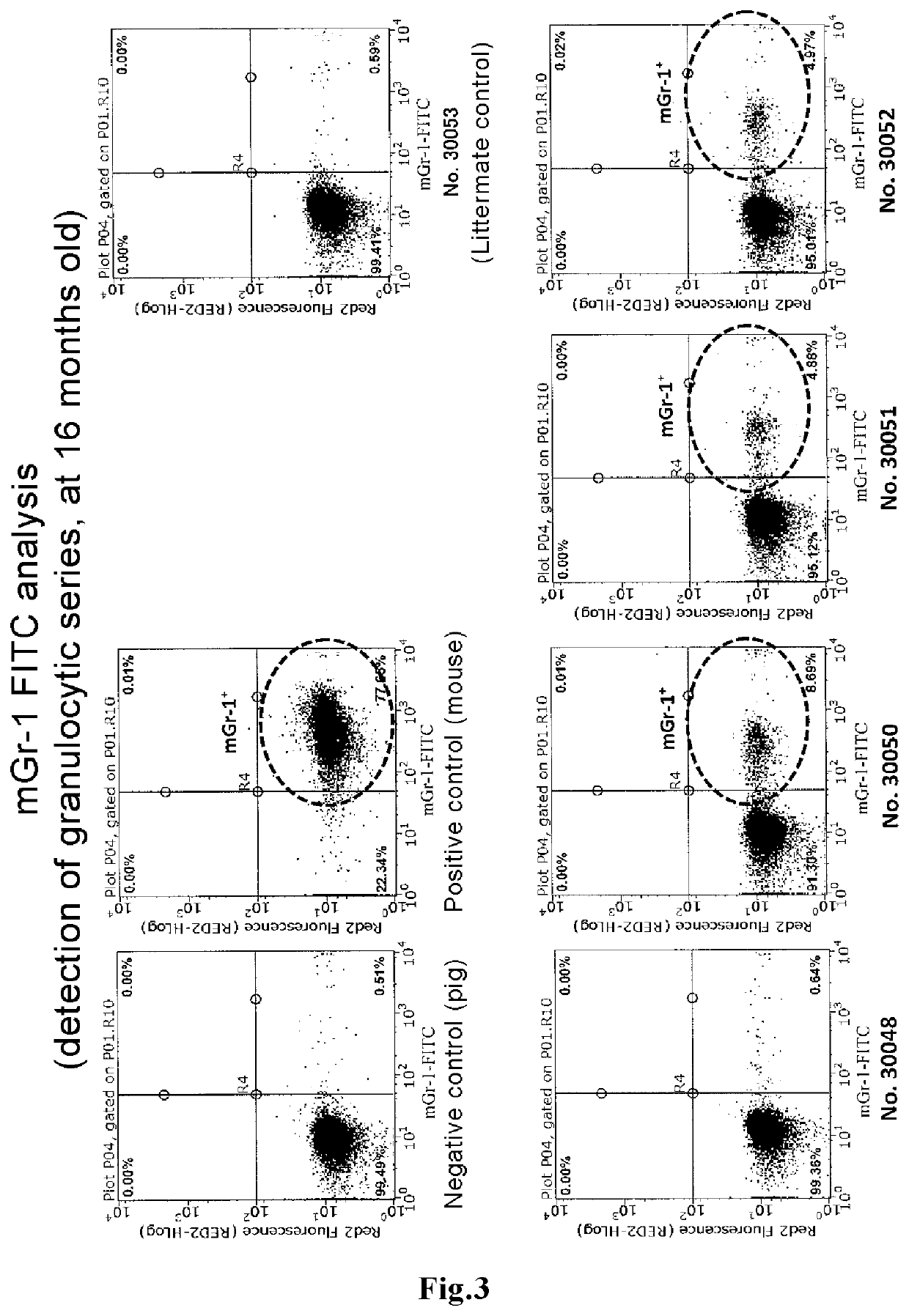
ActiveUS11432537B2Improve the level ofImprove survival rateNew breed animal cellsGenetically modified cellsHeterologousAnimal science
The present invention discloses a novel means capable of producing a blood chimeric animal in which a state of retaining blood cells originating in a heterologous animal at a high percentage is sustained for a long period of time. The method for producing a non-human animal that retains blood cells originating in a heterologous animal, according to the present invention, comprises transplanting hematopoietic cells of a heterologous animal into a non-human animal, in which hematopoietic cells the function of a gene that acts on the hematopoietic system is modified, The gene that acts on the hematopoietic system is, for example, Lnk gene, When a medium to large mammal is used as a recipient, the survival rate of hematopoietic cells originating in a heterologous animal is dramatically increased such that blood chimerism of 10% or more can be maintained even in a 16 month old animal.
Owner:THE UNIV OF TOKYO +1
Use of veto cells in treatment of t cell mediated autoimmune diseases
PendingUS20220265725A1Mammal material medical ingredientsBlood/immune system cellsTolerance inductionHematopoietic cell
A method of treating or preventing a T cell mediated autoimmune disease in a subject in need thereof is disclosed. The method comprising: (a) transplanting immature hematopoietic cells into the subject; and (b) administering to the subject a therapeutically effective amount of an isolated population of non-GVHD inducing anti-third party cells comprising cells having a central memory T-lymphocyte (Tcm) phenotype, the cells being tolerance inducing cells and capable of homing to the lymph nodes following transplantation.
Owner:YEDA RES & DEV CO LTD
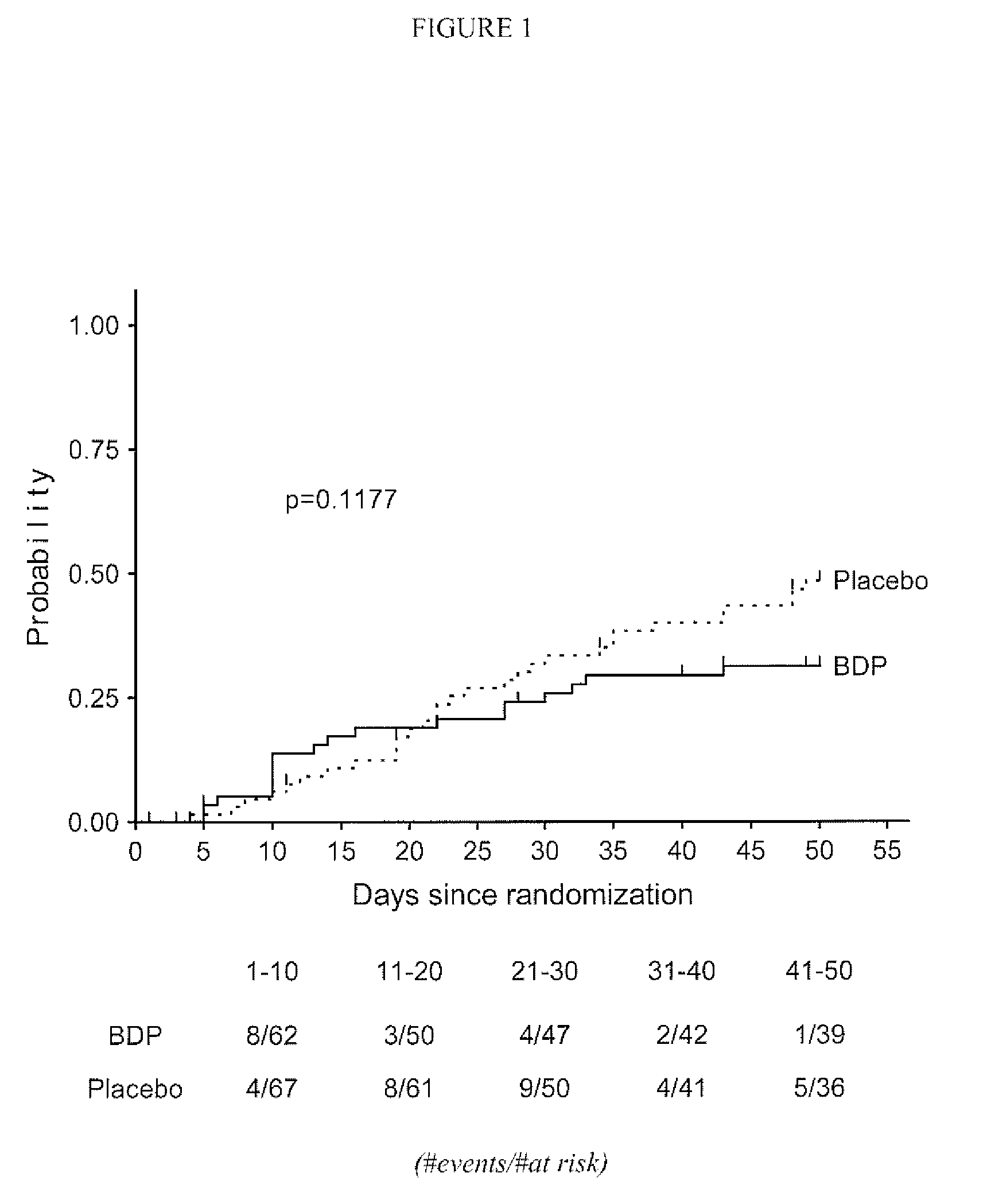
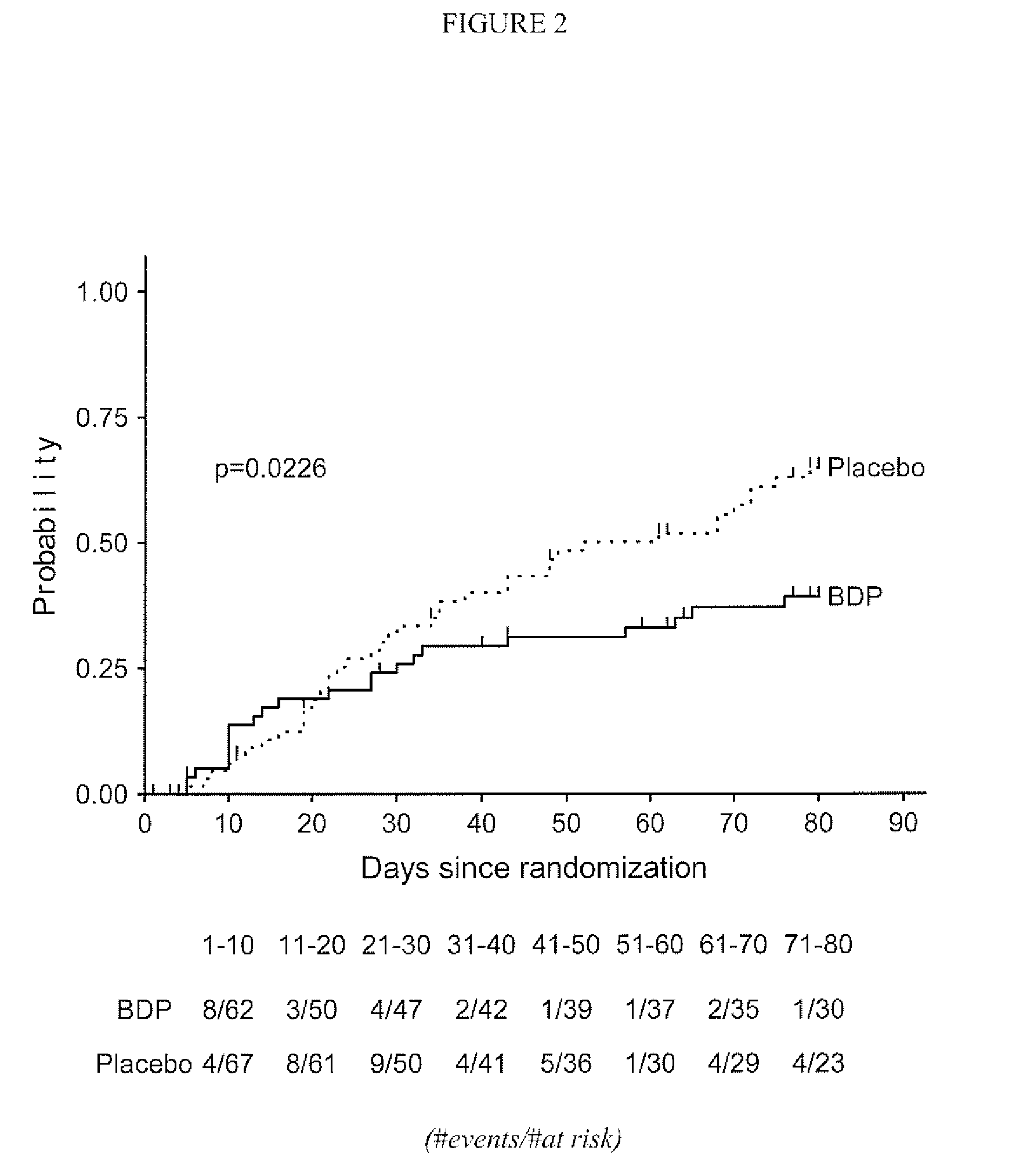
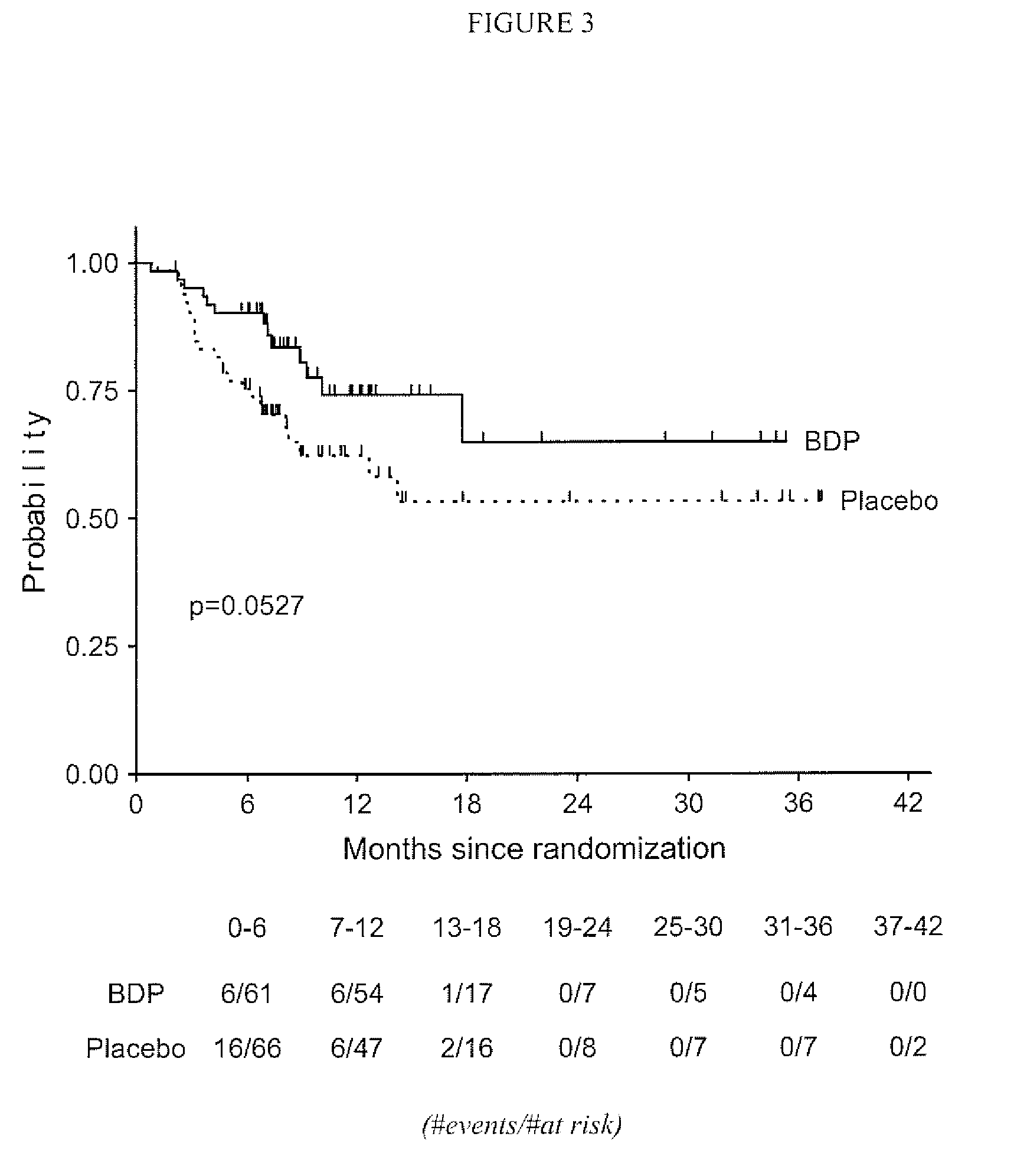
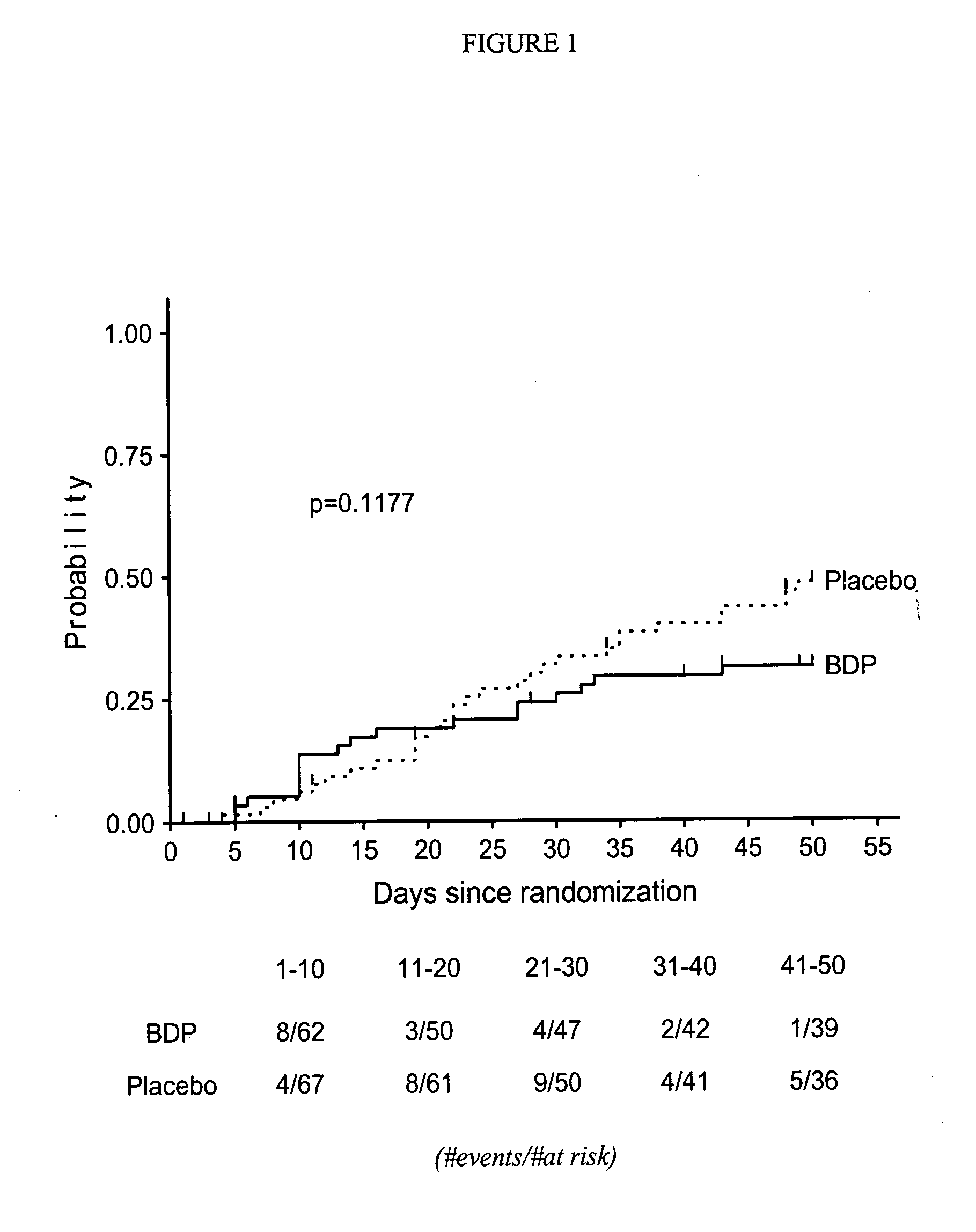
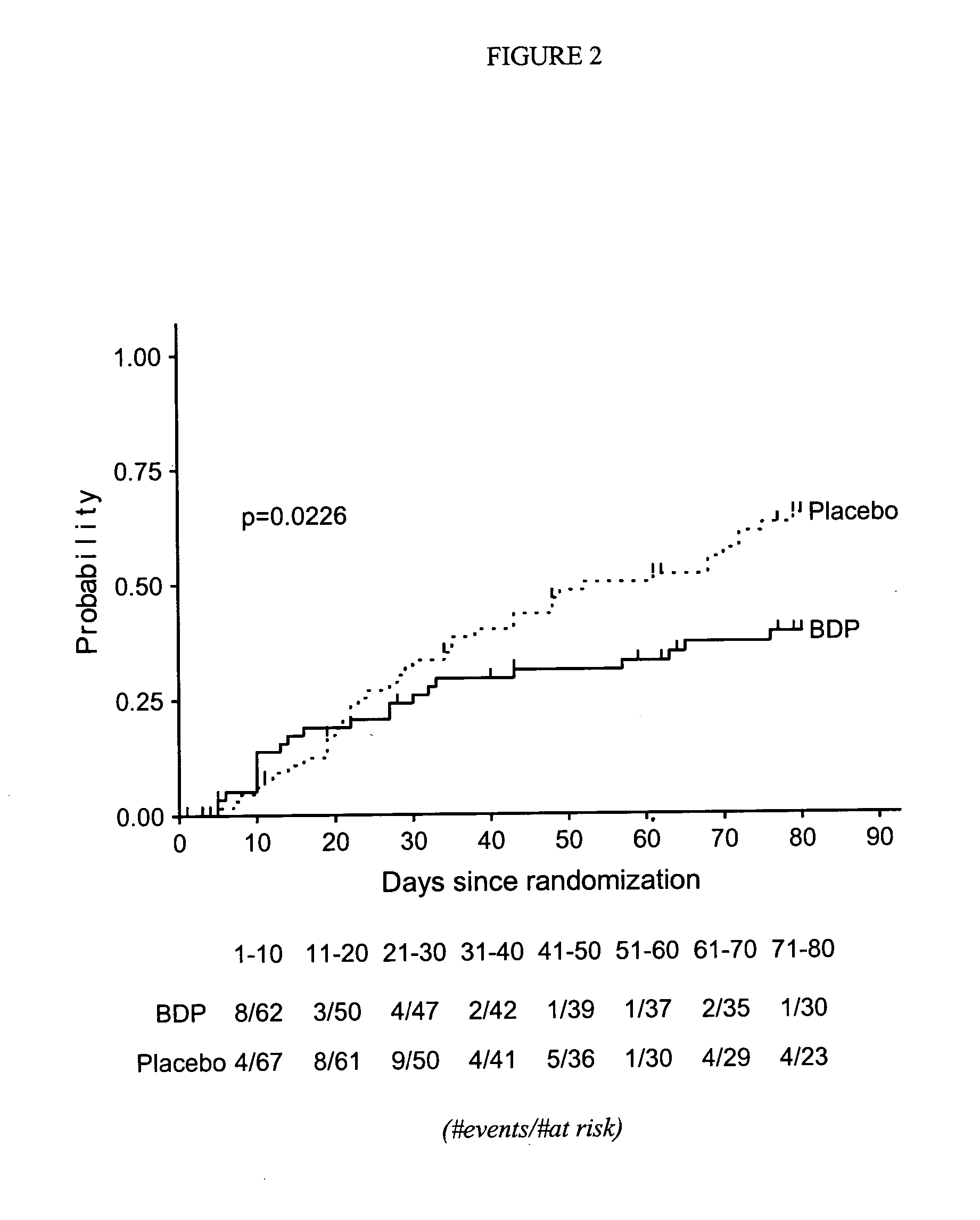
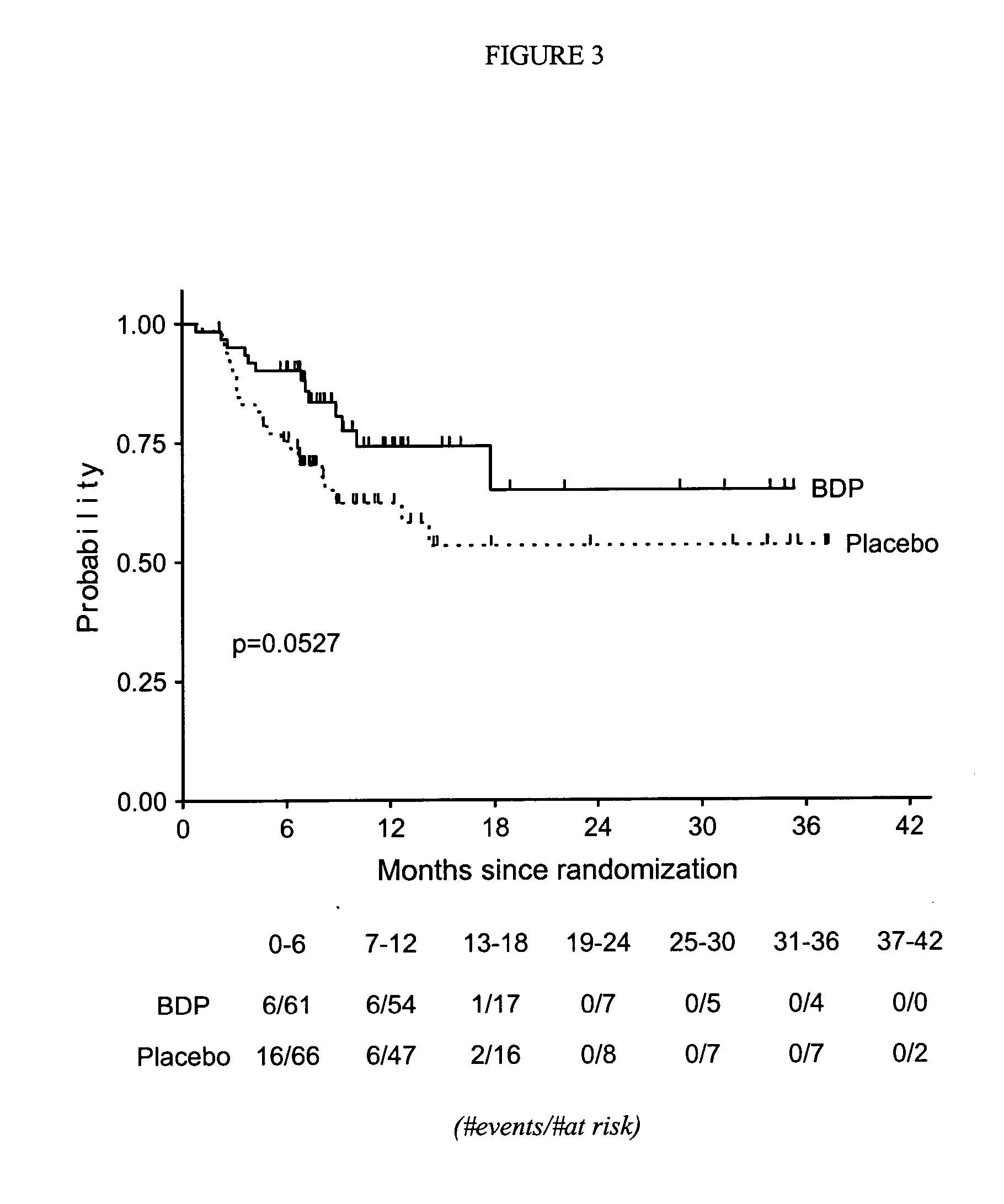
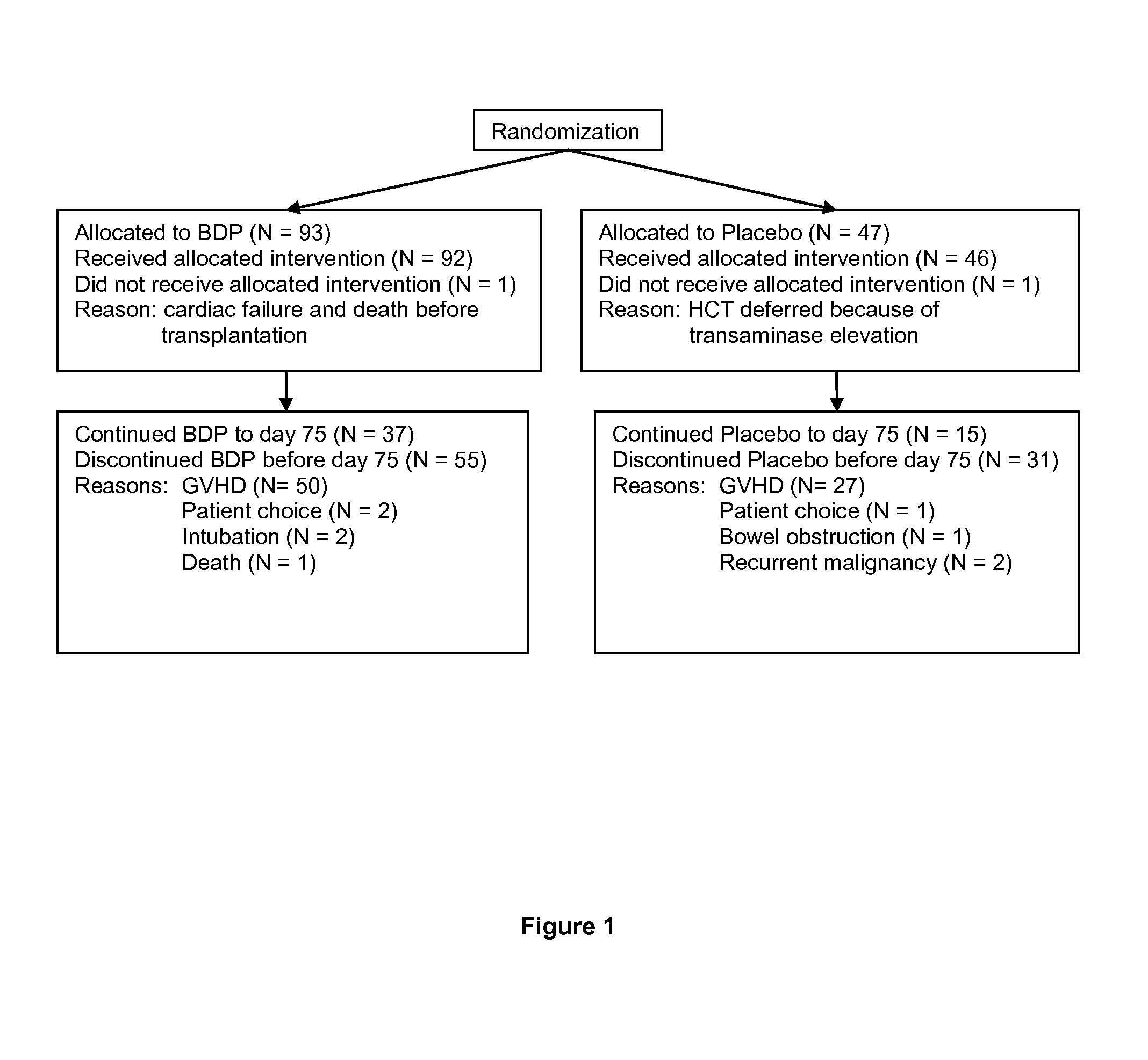
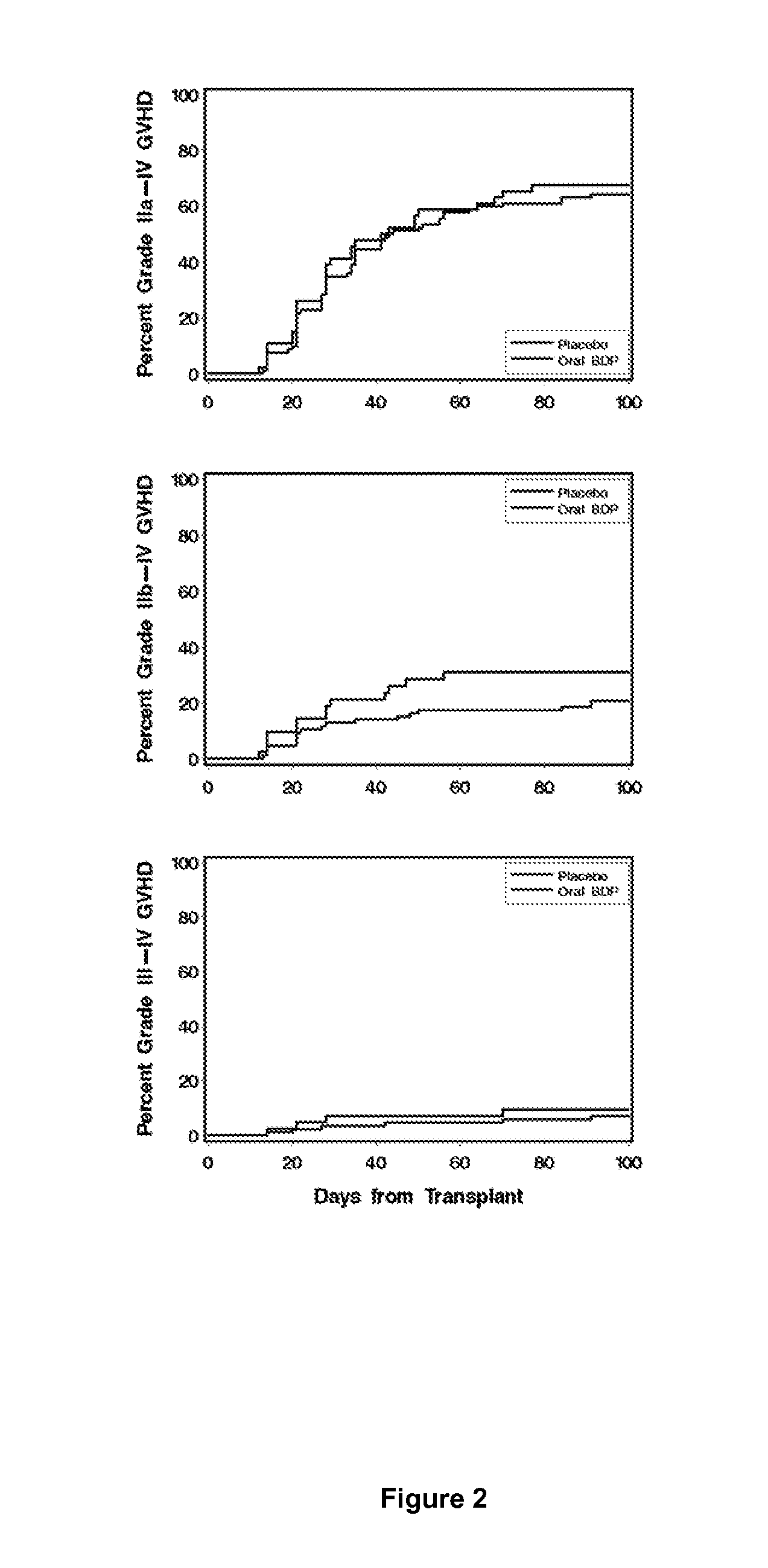
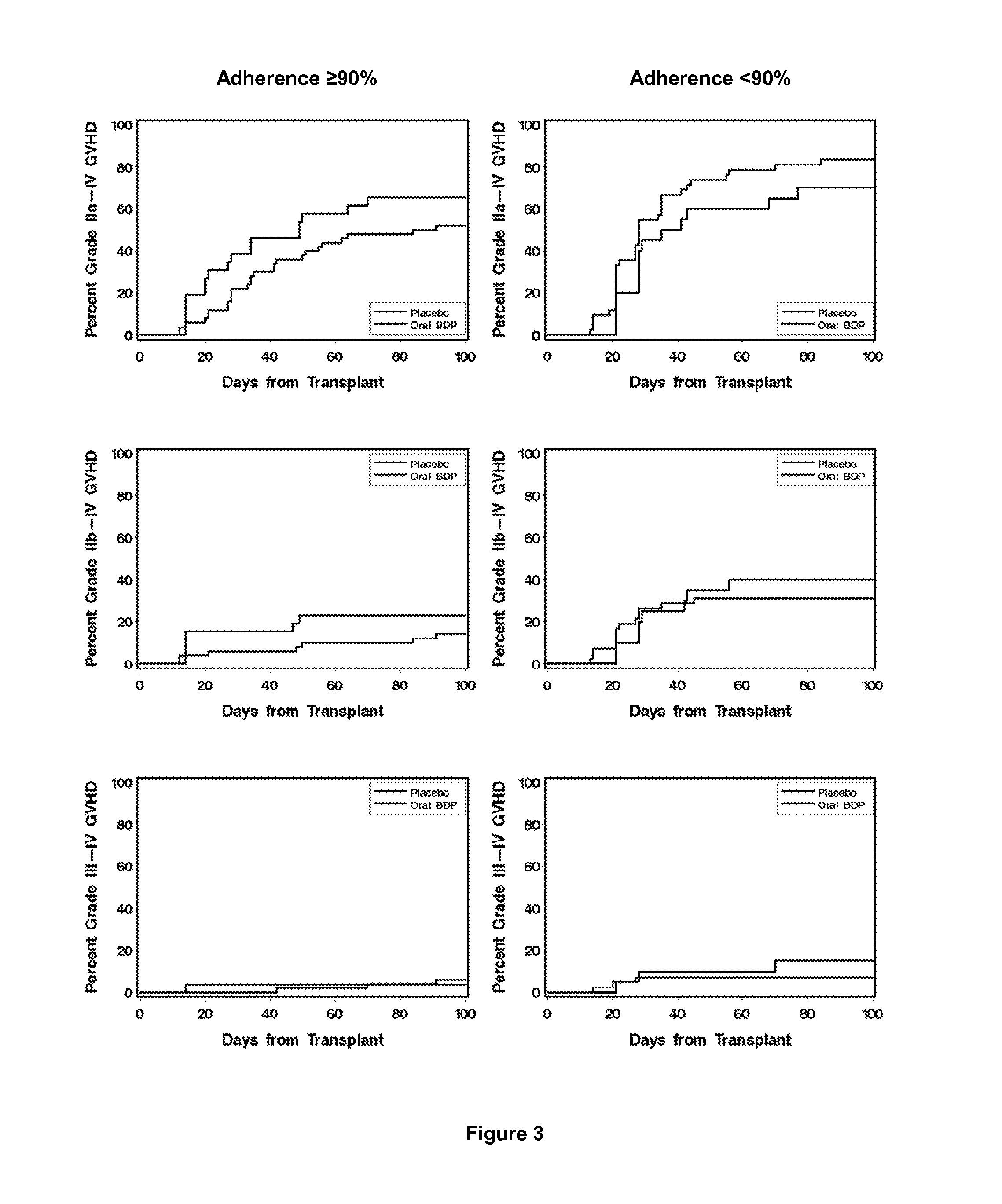
Method of Preventing Acute Graft-Versus-Host Disease using Oral Beclomethasone Dipropionate
InactiveUS20140328929A1Avoid it happening againReduce severityPowder deliveryOrganic active ingredientsDouble blindHaematopoietic cell transplantation
Results from two randomized trials have shown that oral beclomethasone dipropionate (BDP) is effective for treatment of acute gastrointestinal graft-versus-host disease (GVHD). Here, we report results of a double-blind, randomized placebo-controlled phase II study designed to test the hypothesis that acute GVHD could be prevented by administration of oral BDP, beginning before hematopoietic cell transplantation (HCT) and continuing until day 75 after HCT. Study drug (BDP or placebo) was administered as 1 mg immediate-release formulation plus 1 mg delayed-release formulation orally four times daily. According to the primary endpoint, systemic glucocorticoid treatment for GVHD was given to 60 of the 92 participants (65%) in the BDP arm, versus 31 of 46 participants (67%) in the placebo arm. The secondary efficacy endpoints showed no statistically significant differences between the two arms. The proportion of participants who took at least 90% of the prescribed study drug during the first 4 weeks after HCT was 54% overall. Lower severity of mucositis strongly correlated with higher adherence to the schedule of study drug administration. Inconsistent adherence related to mucositis during recovery after myeloablative conditioning may have obscured a beneficial therapeutic effect in the current study.
Owner:SOLIGENIX INC
Reduced intensity conditioning with melphalan
PendingUS20210380946A1High feasibilityImprove securityOrganic active ingredientsPeptide/protein ingredientsAlkylating antineoplastic agentNitrogen mustard
A method of conditioning a subject for hematopoietic cell transplantation, wherein the method involves the use of a nitrogen mustard alkylating agent such as melphalan in an amount to achieve reduced-intensity conditioning.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
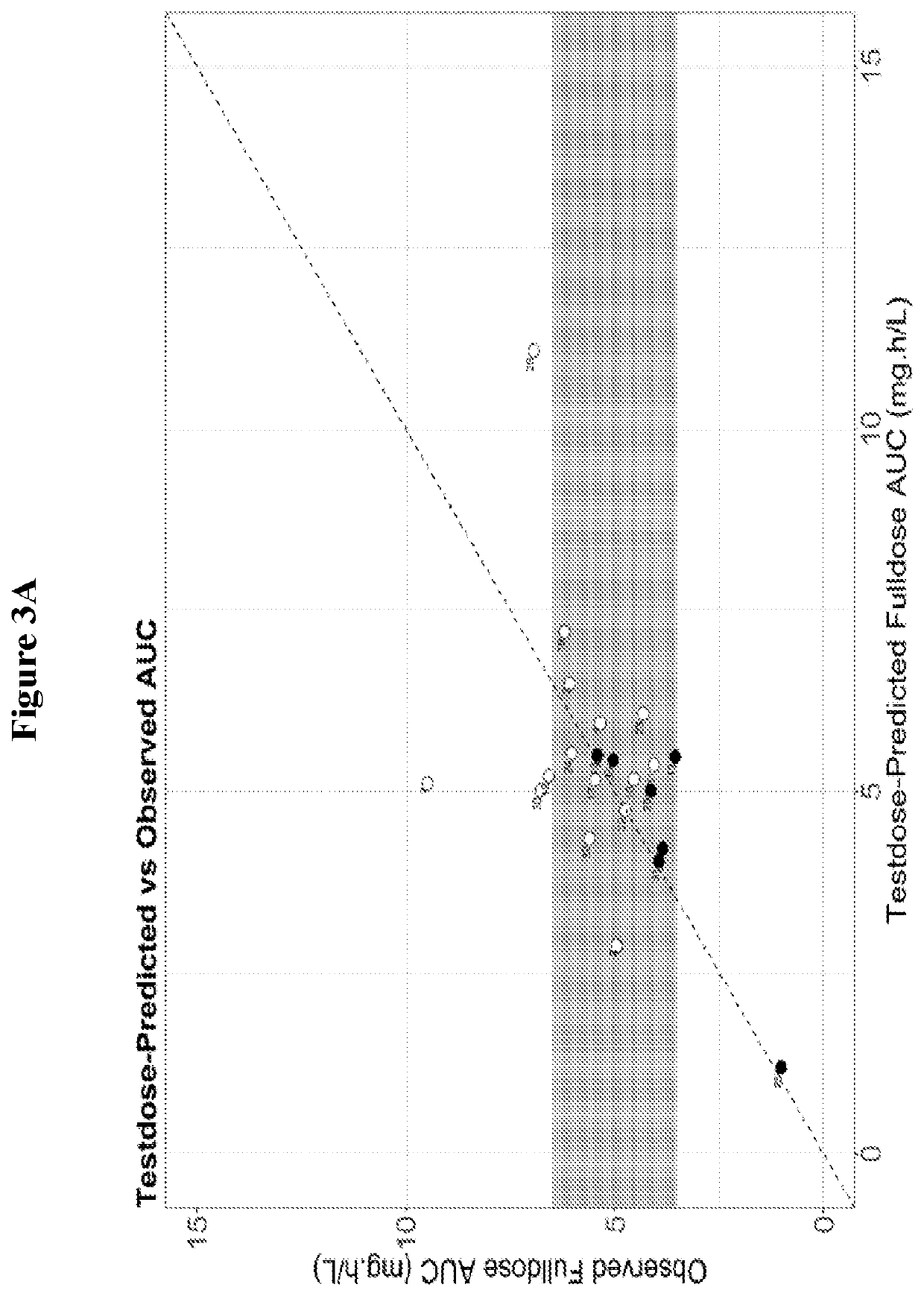
Methods for determining personalized full dose of melphalan in reduced intensity regimen prior to hematopoietic cell transplantation
PendingUS20220205982A1Minimize ToxicityDestroying bone marrow cellsOrganic active ingredientsUnknown materialsHematopoietic cellReduced Intensity Conditioning
A method for determining a personalized full dose of a melphalan compound (e.g., melphalan) in a reduced intensity conditioning regimen (RIC) prior to hematopoietic cell transplantation for a subject based on pharmacokinetic features of the melphalan compound administered to the subject at a test dose.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Methods for enhancing hematopoietic progenitor cell engraftment
ActiveUS8546382B2Easy to implantIncreased proliferationBiocidePeptide/protein ingredientsHematopoietic cellAdipogenesis
Described herein are methods for improving engraftment of hematopoietic cells in an individual following hematopoietic progenitor cell transplantation (e.g., via bone marrow or cord blood transplantation). Methods for increasing hematopoietic progenitor cell proliferation in individuals with bone marrow aplasia are also described. The methods involve administering an agent that inhibits adipogenesis, adipocyte growth, adipocyte differentiation and / or adipocyte proliferation.
Owner:CHILDRENS MEDICAL CENT CORP
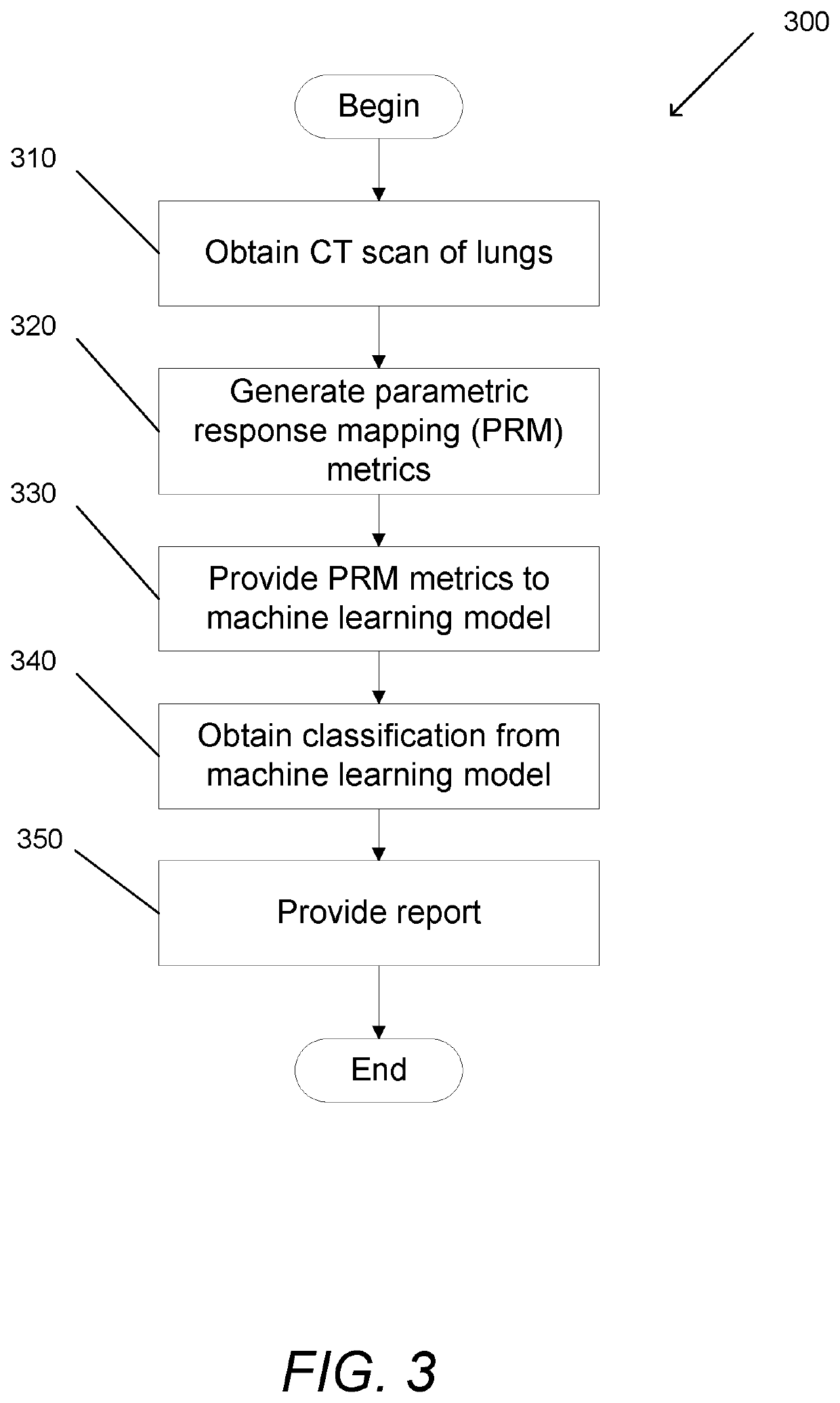
Systems and Methods for Identification of Pulmonary Conditions
PendingUS20220039770A1Medical data miningRadiation diagnostic clinical applicationsHematopoietic cellHaematopoietic cell transplantation
Systems and methods for identification of pulmonary conditions accordance with embodiments of the invention are illustrated. One embodiment includes a method for identifying pulmonary conditions in hematopoietic cell transplantation patients, include obtaining a computed tomography (CT) scan of a patient's lungs, calculating a plurality of parametric response mapping (PRM) metrics, providing the plurality of PRM metrics to a machine learning model, obtaining a classification of the CT scan as indicating whether or not the patient's lungs present with a pulmonary condition, and providing a report comprising the classification.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Ligands to gm-csf or gm-csf-receptor for use in treatment of a haematologic malignancy in a patient having undergone allo-hct
PendingUS20210070851A1Immunoglobulins against cytokines/lymphokines/interferonsImmunoglobulins against cell receptors/antigens/surface-determinantsHematopoietic cellAntiendomysial antibodies
Described herein is the use of a non-agonist ligand, particularly an antibody, specifically binding to GM-CSF or one of CD116, CD131 and the GM-CSF receptor composed of CD116 and CD131 for use in treatment of leukemia in a patient having undergone allo-HCT or in treatment of other complications arising as a consequence of hematopoietic cell transplantation from an immunologically non-identical donor.
Owner:UNIV ZURICH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com