Methods of improving hematopoietic grafts
A technology of transplantation and hematopoietic cells, applied in the medical field, can solve the problems of poor implantation potential and the use of plasmids encoding oncogenes hindering clinical application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0146] All embodiments described above for the method of preparing a hematopoietic cell graft of the invention are also encompassed in this aspect.
[0147] Expression of cell surface antigens CD135 and / or CD110 and / or expression of apelin receptor (APLNR) can be performed by any method known to the skilled person such as FACS, MACS, immunohistochemistry, Western blot, protein or antibody array, RT -PCR or by transcriptome approach to assess.
[0148] Depending on the method used to assess the expression of CD135, CD110 or APLNR, steps b) and c) may be sequential or simultaneous. Detection and selection of said expression can be simultaneous, for example, using FACS or MACS.
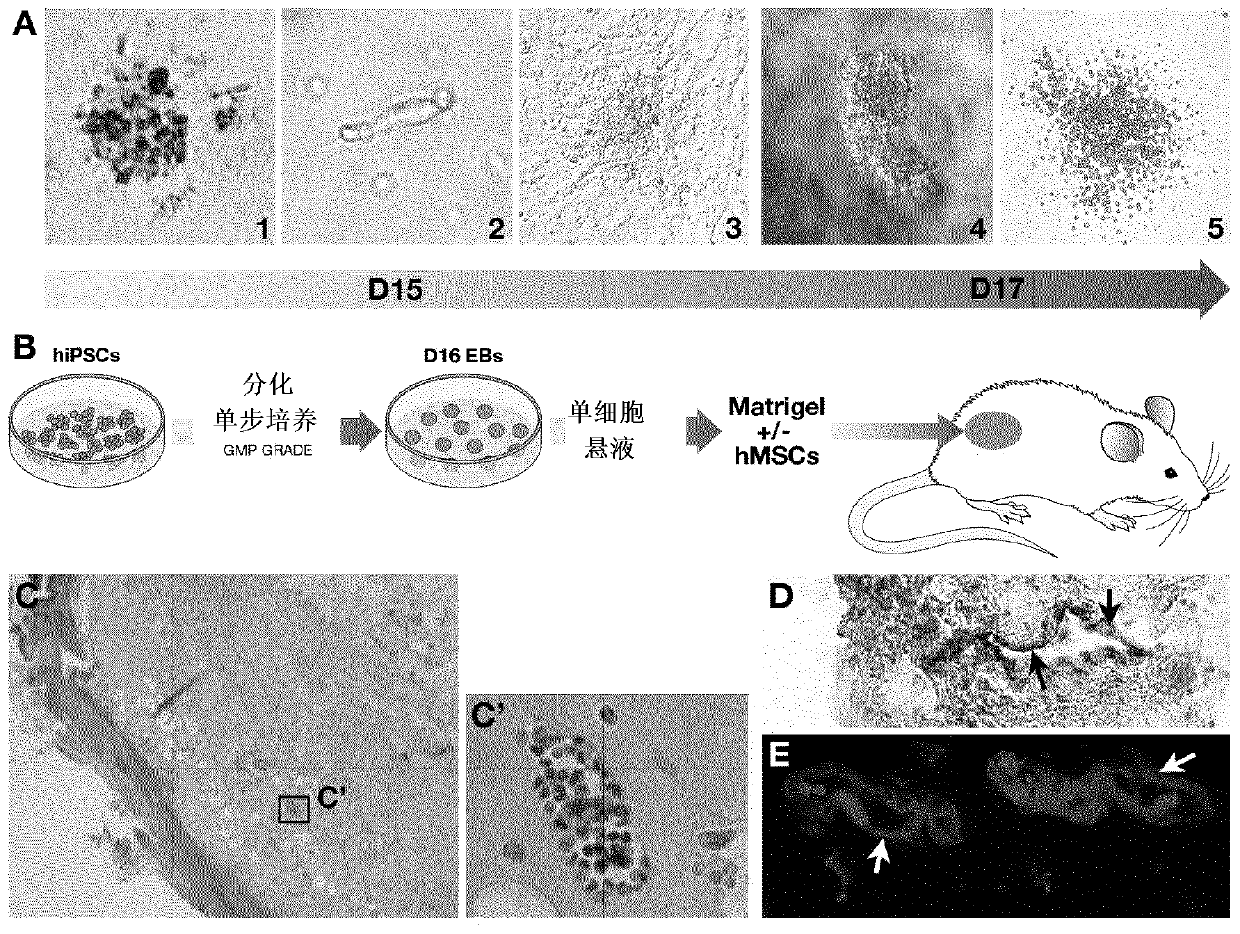
[0149] The identified and / or selected CD135+ and / or CD110+ and / or APLNR+ cells can be used as a hematopoietic graft, or can be included in or added to a hematopoietic graft (such as a bone marrow or cord blood graft) in order to enhance said transplantation effectiveness of the thing.
[0150] In anot...
Embodiment 1
[0272] Materials and methods
[0273] hiPSC expansion
[0274] The studies were performed using three different hiPSC lines: FD136-25, reprogrammed with retroviral vectors and Thomson's combination (endogenous expression of Oct4, Sox2, Nanog and Lin28); Pci-1426 and Pci-1432 Line (Phenocell), programmed with additional body weight (Sox2, Oct4, KLF, cMyc). hiPSCs were maintained on CellStart (Invitrogen, Carlsbad, USA) in TESR2 medium (Stem Cell Technologies, Bergisch Gladbach, Germany) and cells were plated every 5 days on freshly coated plates using standard TRYple select (Invitrogen). The mass passage method was passaged at 1:6.
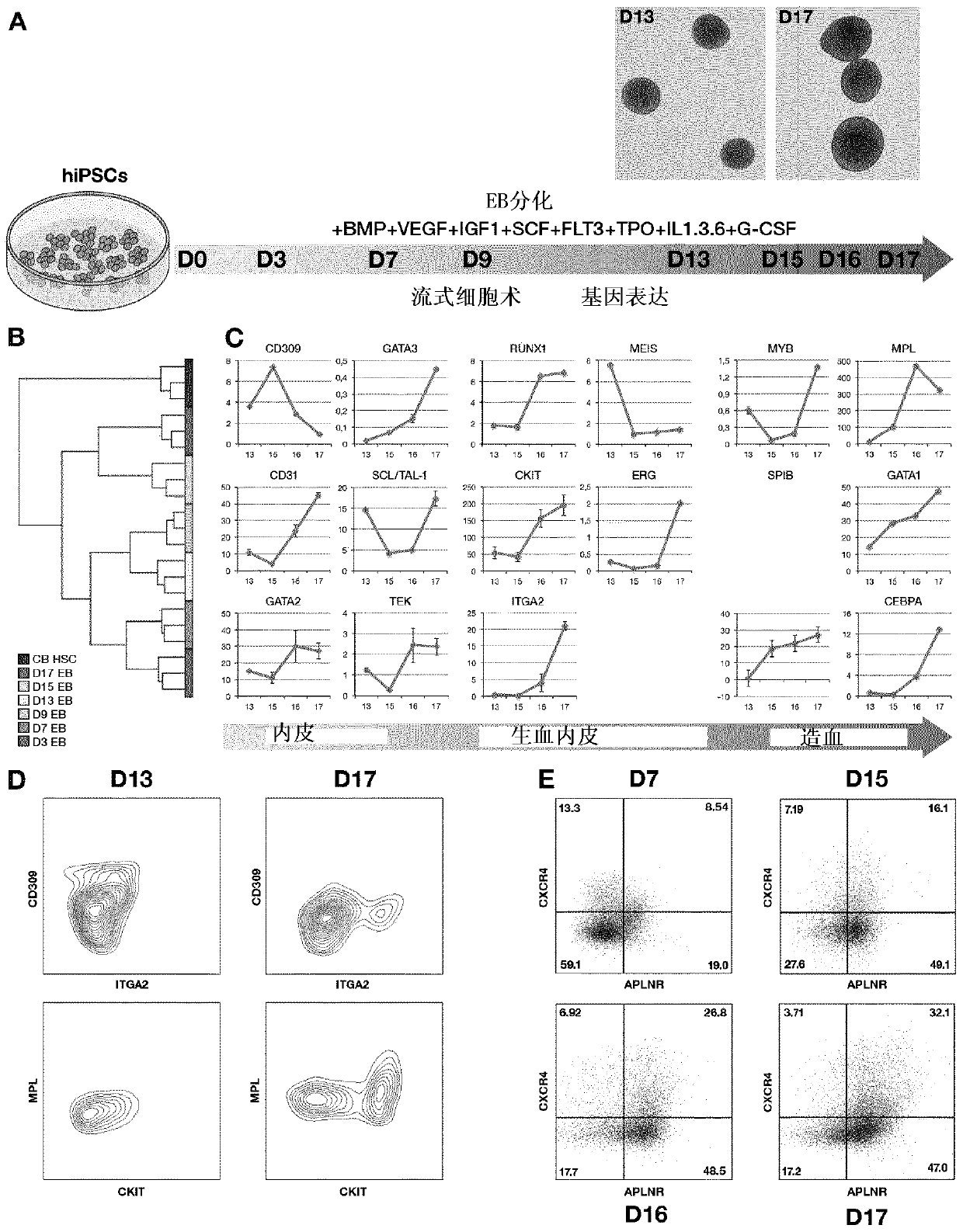
[0275] EB differentiation
[0276] After 24 h, the cells were transferred to differentiation medium containing 24 ng / mL of SCF, 21 ng / mL of TPO, 21 ng / mL of FLT3L, 194 ng / mL of BMP4, 200 ng / mL of VEGF, 50 ng / mL of mL of IL3, 50 ng / mL of IL6, 5 ng / mL of IL1, 100 ng / mL of GCSF, 5 ng / mL of IGF1 (PeproTech, Neuilly-sur-Seine, France). Medium w...
Embodiment 2
[0332] Materials and methods
[0333] hiPSC expansion, EB differentiation, assessment of human cell engraftment, T cell maturation and functional assays, quantitative PCR, were performed as described above.
[0334] cell sorting
[0335] Dissociated EB cells were stained with antibody CD110-PE (MPL) or CD135-PE (FLT3), then re-stained with PE-MicroBeads (Miltenyi), and finally used Cell separation device for sorting.
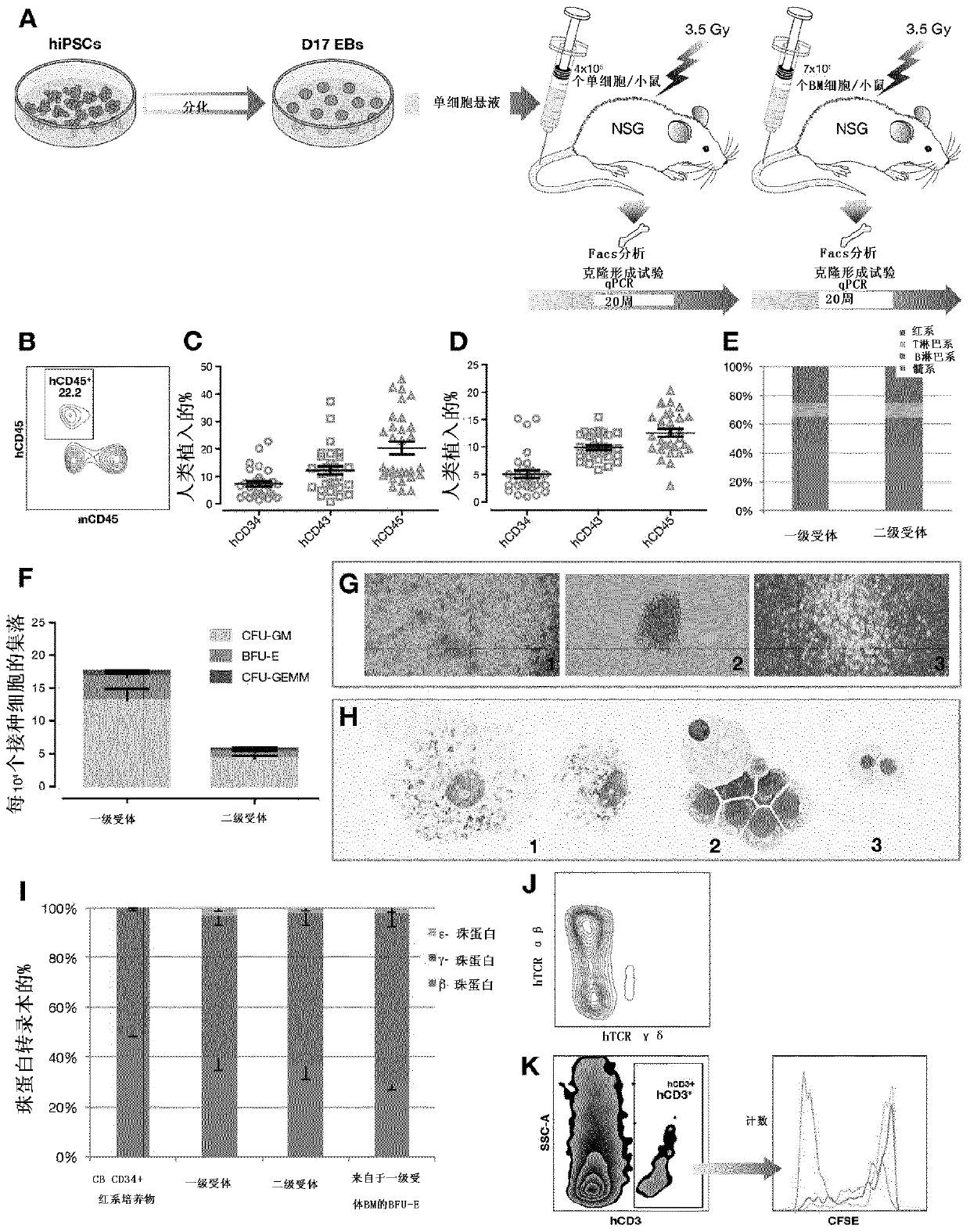
[0336] mouse transplantation
[0337] NOD.Cg-Prkdc scid Il2rg tm1Wjl / SzJ (NSG) (Charles River, L'Abresle, France) was housed at the IRSN animal care facility. All experiments and procedures were carried out in accordance with the regulations of the French Ministry of Agriculture on animal experiments and were approved by the local ethics committee.
[0338] Twenty-four hours before cell injection, mice aged 6-8 weeks and housed under sterile conditions were sublethally irradiated with 3.5 Gy from a 137Cs source (2.115 Gy / min). To ensure consistency ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com