Human-derived CD-19 chimeric antigen receptor T lymphocyte vector and application thereof
A chimeric antigen receptor, CD-19 technology, applied in the application, animal cells, vertebrate cells, etc., can solve the problems of reducing the recurrence rate and the recurrence rate of patients from time to time, so as to increase the transformation efficiency and breakthrough inhibition Restriction of the microenvironment, optimization of the effect of the carrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
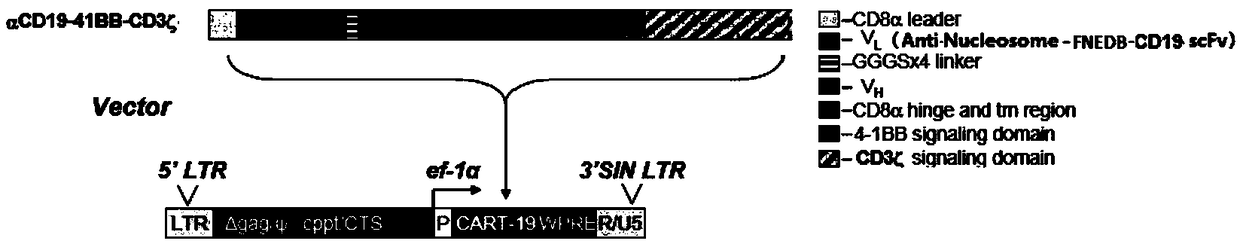
[0033] Construction of anti-Nucleosome-FNEDB-CD19 CAR gene
[0034](1) Design of anti-Nucleosome-FNEDB-CD19 CAR gene: the sequence consists of the ScFv gene of CD19 (SEQ ID NO 1), the ScFv gene of FNEDB (SEQ ID NO 3), and the nucleosome (SEQ ID NO 2) ScFv base, cytoplasmic region 4-1BB (ENST00000374478), CD3zeta gene (ENST00000392122), and CAR structural gene CD8 sequence (ENST00000283635) are connected. The two gene sequences are connected by 40-50NT nucleotide sequences, and HindⅢ and EcoRI restriction enzyme cutting sites were respectively introduced at both ends of the sequence.
[0035] (2) The designed anti-Nucleosome-FNEDB-CD19 CAR gene was synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the designed gene was on the plasmid HF392 provided by the company.
[0036] (3) According to the gene sequence in the genebank library, primers for the anti-Nucleosome-FNEDB-CD19 CAR gene were designed, the upstream primer P1 (5′-CTTAAGCCTATGCAGGTCCAACT...
Embodiment 2
[0038] Construction of anti-Nucleosome-FNEDB-CD19 CAR lentiviral expression vector
[0039] (1) Construction of anti-Nucleosome-FNEDB-CD19 CAR FNEDB lentiviral expression vector:
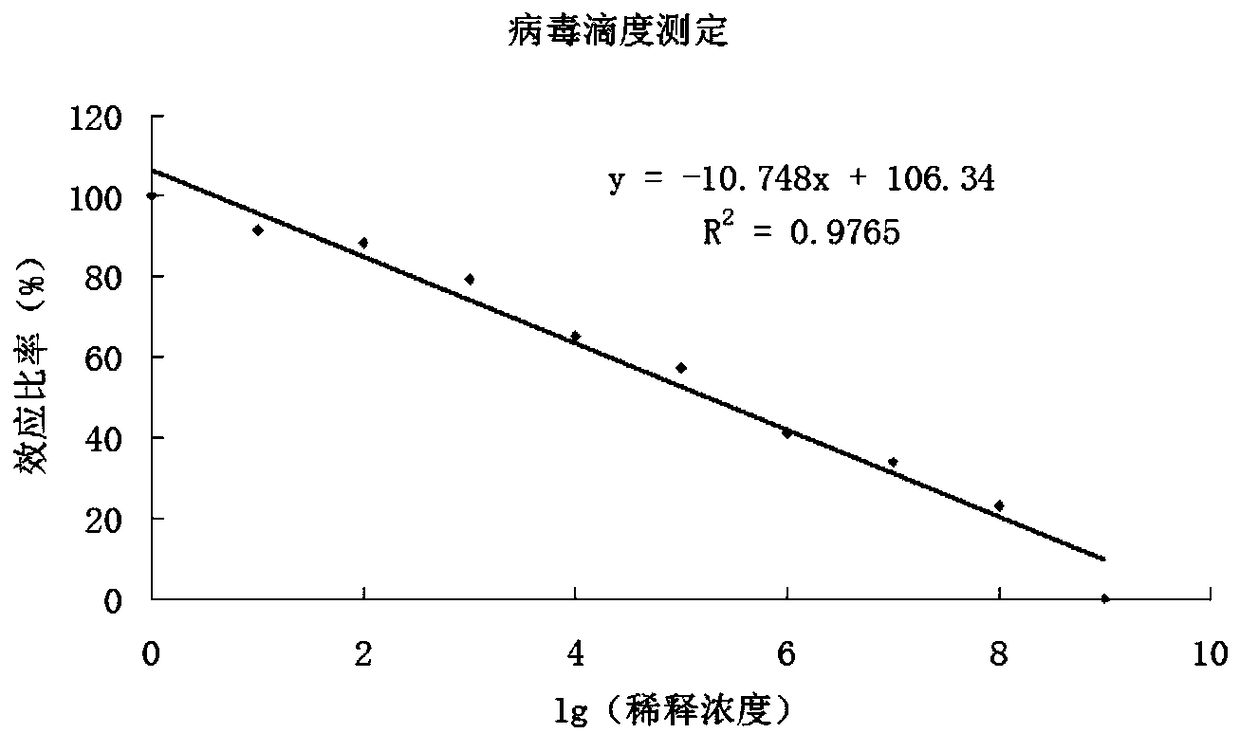
[0040] Cloning the anti-Nucleosome-FNEDB-CD19 CAR gene fragment into the lentiviral vector pc DNA TM For 3.1(+), the selected insertion site is Nhe I / Bam HI. 293T cells were cultured with RPMI1640+10% FBS. After the cells were 90% confluent, use lipo2000 to mix the transfection plasmids and transfect 293T cells. The transfection method is operated according to the standard of the Invitrogen manufacturer. The plasmids include: expression plasmids, pMDLg / pRRE, pRSV-Rev, and pMD2.0G, and the plasmids are mixed according to the molar ratio. Harvest the lentiviral supernatant after 24-48 hours. Then add Clontech's Lenti-X concentrator, 1500g after mixing, 4°C. Centrifuge for 45 minutes, remove the supernatant, and obtain the lentiviral pellet, that is, the anti-Nucleosome-FNEDB-CD19 CAR lentiviral e...
Embodiment 3
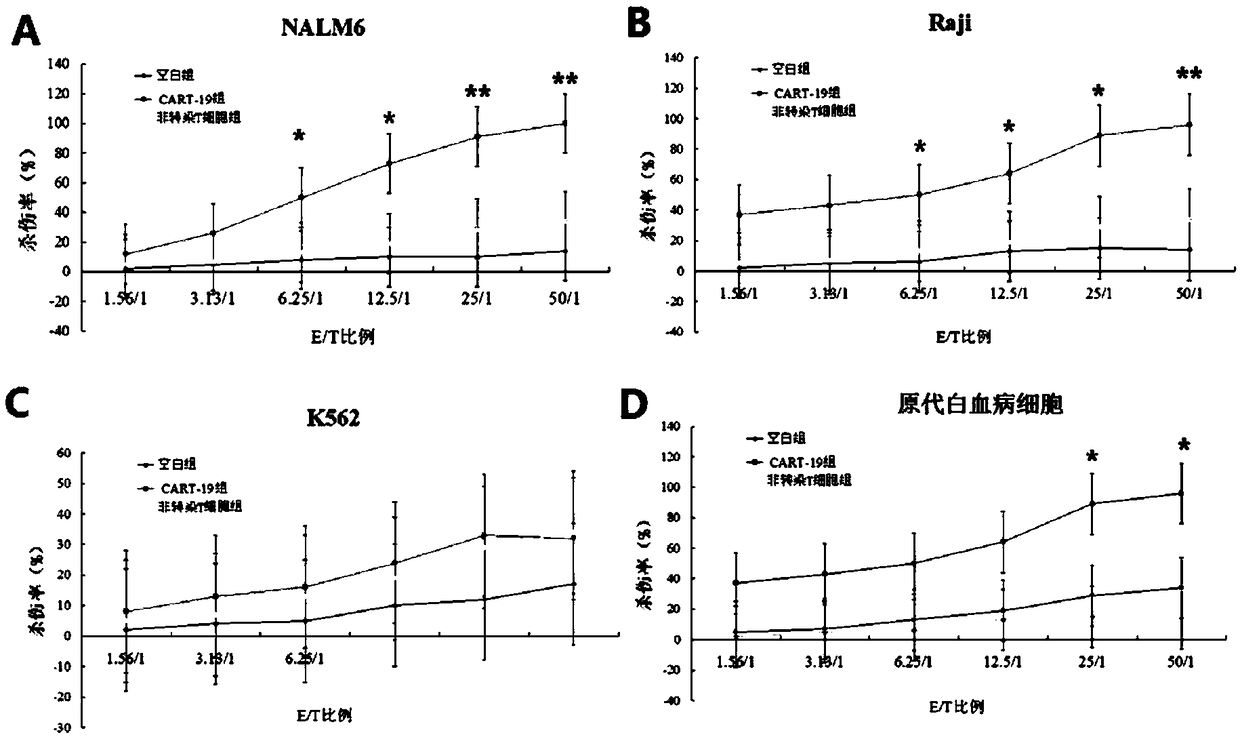
[0044] Cell killing experiment of anti-Nucleosome-FNEDB-CD19 CAR lentiviral expression vector:
[0045] (1) Preparation of CAR-T cells:
[0046] Collect 50-60ml of venous blood from the patient; centrifuge at 2000rpm for 10min, draw plasma, and bathe in water at 56°C for 30min; dilute the blood sediment by one time with PBS, add 20ml of lymphocyte layering solution, centrifuge at 2000rpm for 20min; collect buffy coat cells , washed 3 times with PBS, and then counted cells using trypan blue; resuspended cells in GT-551 culture medium, selected the culture flask coated with anti-CD3 monoclonal antibody, transferred the cells, and added 500U / ml rIL-2, placed in 37°C, 5% CO 2 cultured in an incubator. On the second day of culturing the cells, add 200 μL of the lentiviral vector obtained in Example 2 of the present invention and 2 μL of 1×10 -6 U protamine, after 12 hours of co-cultivation, change the medium, and add 500U / ml rIL-2; on the 4th day of culture, transfer to a cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com