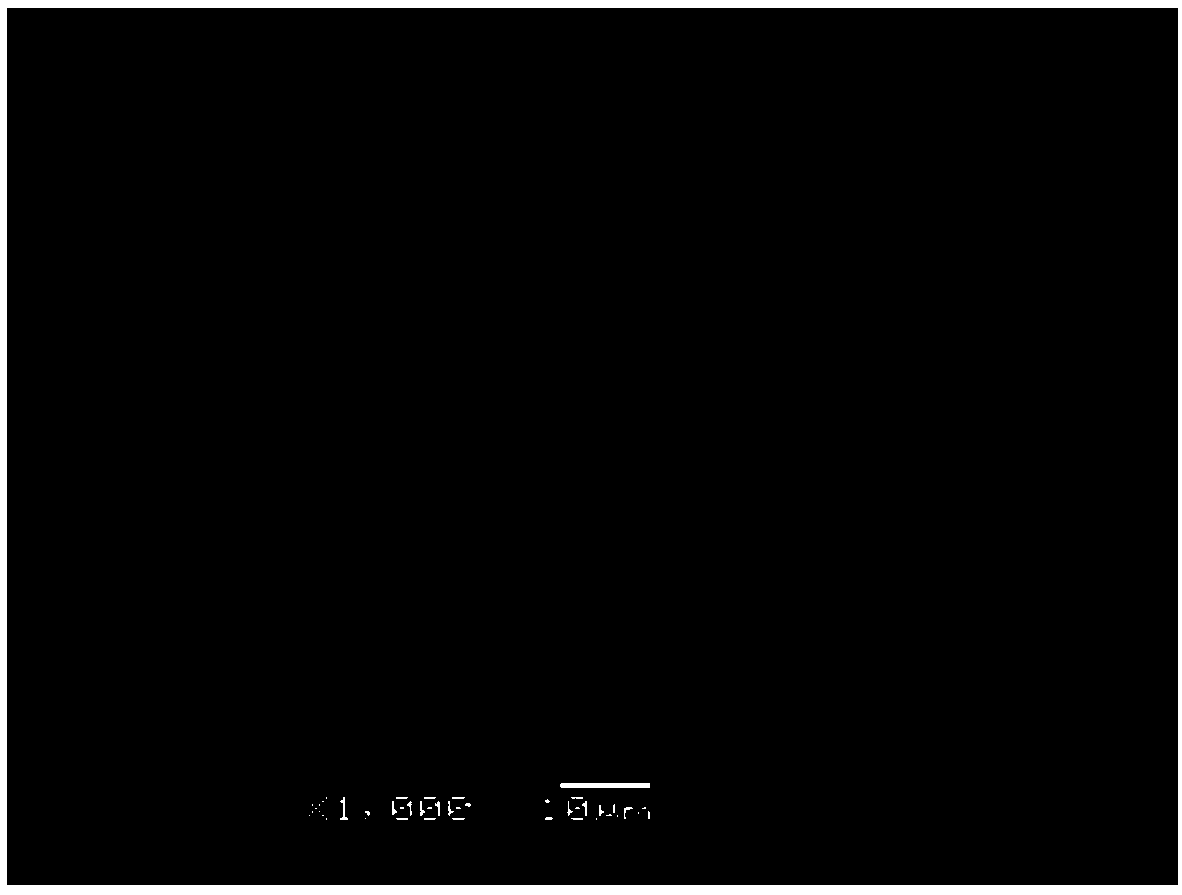Method for preparing decellularization cartilage matrix material
A decellularized cartilage and matrix material technology, applied in the field of biomaterial preparation, can solve problems such as long soaking time, failure to use the product, and high concentration of lye
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a preparation method of decellularized cartilage matrix material. In all embodiments of the present invention, all possess following common features: described preparation method comprises the following steps:
[0042] (1) placing human or animal cartilage tissue in a fixative for fixation;
[0043] (2) Wash the fixed cartilage tissue with a suitable solution, and the number of times of washing can be several times;
[0044] (3) placing the washed cartilage tissue in an alkaline solution for decellularization;
[0045] (4) extracting and cleaning the decellularized cartilage tissue;
[0046] The fixing agent is glutaraldehyde, formaldehyde or peracetic acid;
[0047] The suitable solution may be distilled water;
[0048] The alkaline solution is selected from NaOH or KOH solutions with a concentration ranging from 4% to 15%. Using this concentration of alkali solution can remove the cells of the treated decellularized cartilage matrix material w...
experiment example 1
[0061] Experimental example 1, concrete operation of the present invention
[0062] Cartilage obtained from human or animal tissues is removed, fat is removed, and fixed in a fixative. The fixative can be glutaraldehyde, formaldehyde or peracetic acid. The tissue is left in fixative for at least 2 hours, or until the tissue is fixed. After the tissue is fixed, it is washed with a suitable solution, the solution may be distilled water, and the number of times of washing may be several times.
[0063] The tissue is then decellularized in an alkaline solution, which may be NaOH or KOH solution. The standing time is 15-30 hours, the temperature is 15-35°C, and the concentration of lye is 4%-15%.
[0064] After the tissue is decellularized, it is extracted and cleaned with physiological saline, and the soaking time is 5-7 hours to ensure the biological safety of the tissue. In practice, it has been found that if there is no immersion cleaning, the cytotoxicity of the tissue is n...
experiment example 2
[0066] Experimental example 2, the performance effect verification of the cartilage matrix material prepared by the method of the present invention
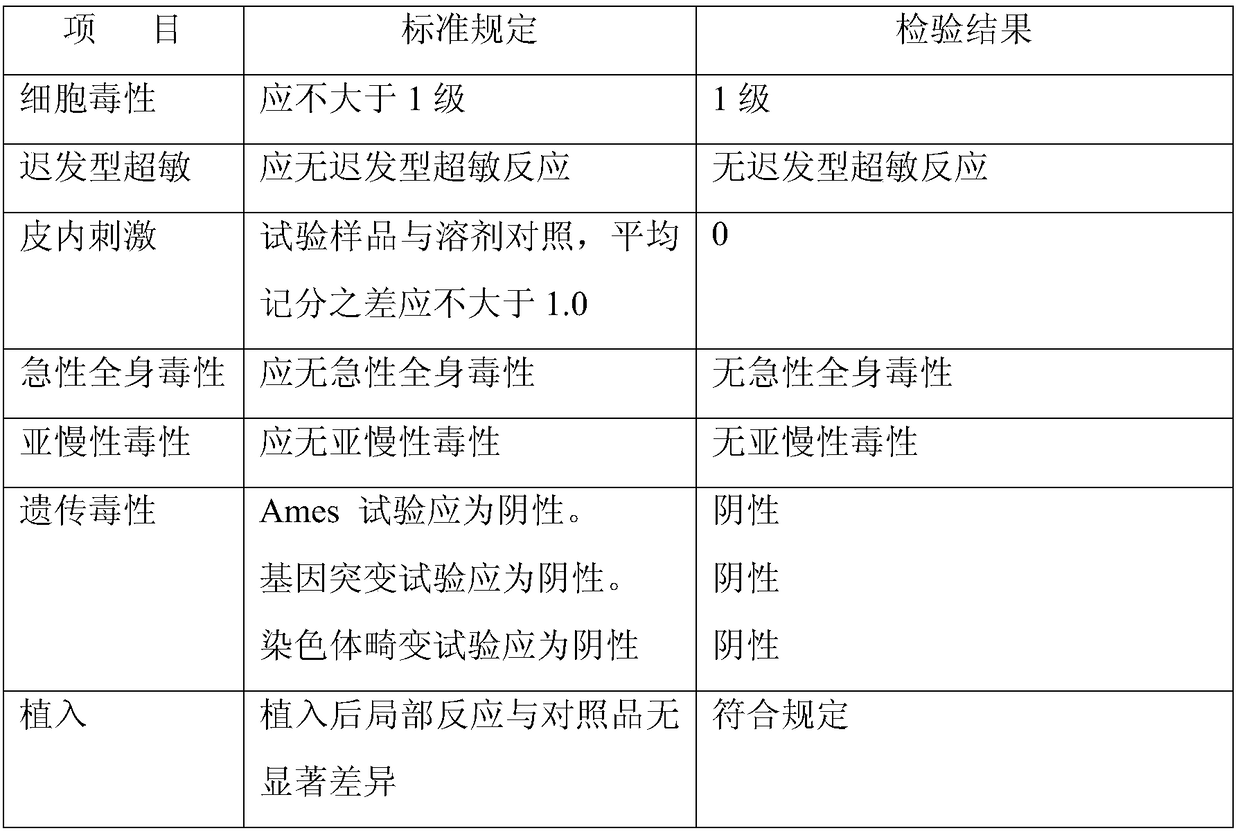
[0067] The cartilage matrix material obtained by the preparation method of the present invention is subjected to conventional detection methods in the field, such as the detection method described in the invention patent application 201711056849.0, to detect the following performance indicators, and obtain the following results:
[0068] The cytotoxicity test result of the cartilage matrix material obtained by the preparation method of the present invention: good cytocompatibility;
[0069]
[0070] Detection of immunogenic components: no immunogen, the number of residual cells is 0;
[0071] Decellularization effect data: DNA residue figure 1 Shown, cell residue: 0;
[0072] Such as figure 2 As shown, the porosity data of the acellular cartilage matrix material obtained by the preparation method of the present invention ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com