Lysis/resealing process and device for incorporating an active ingredient in erythrocytes
A technology of red blood cells and active ingredients, which is applied in the field of cracking/resealing and devices for incorporating active ingredients into red blood cells, and can solve problems such as lack of industrialization methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Example 1: Device
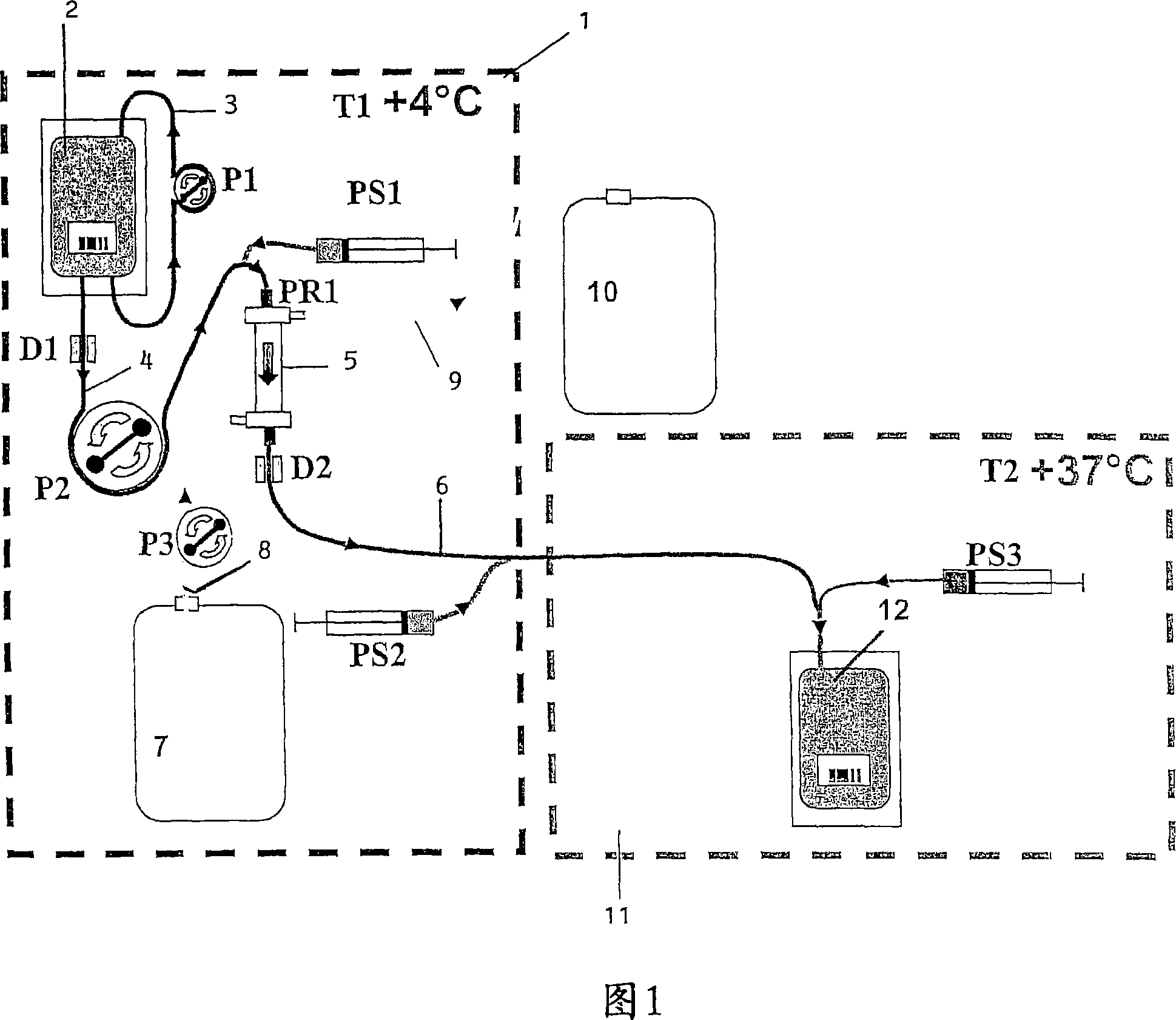
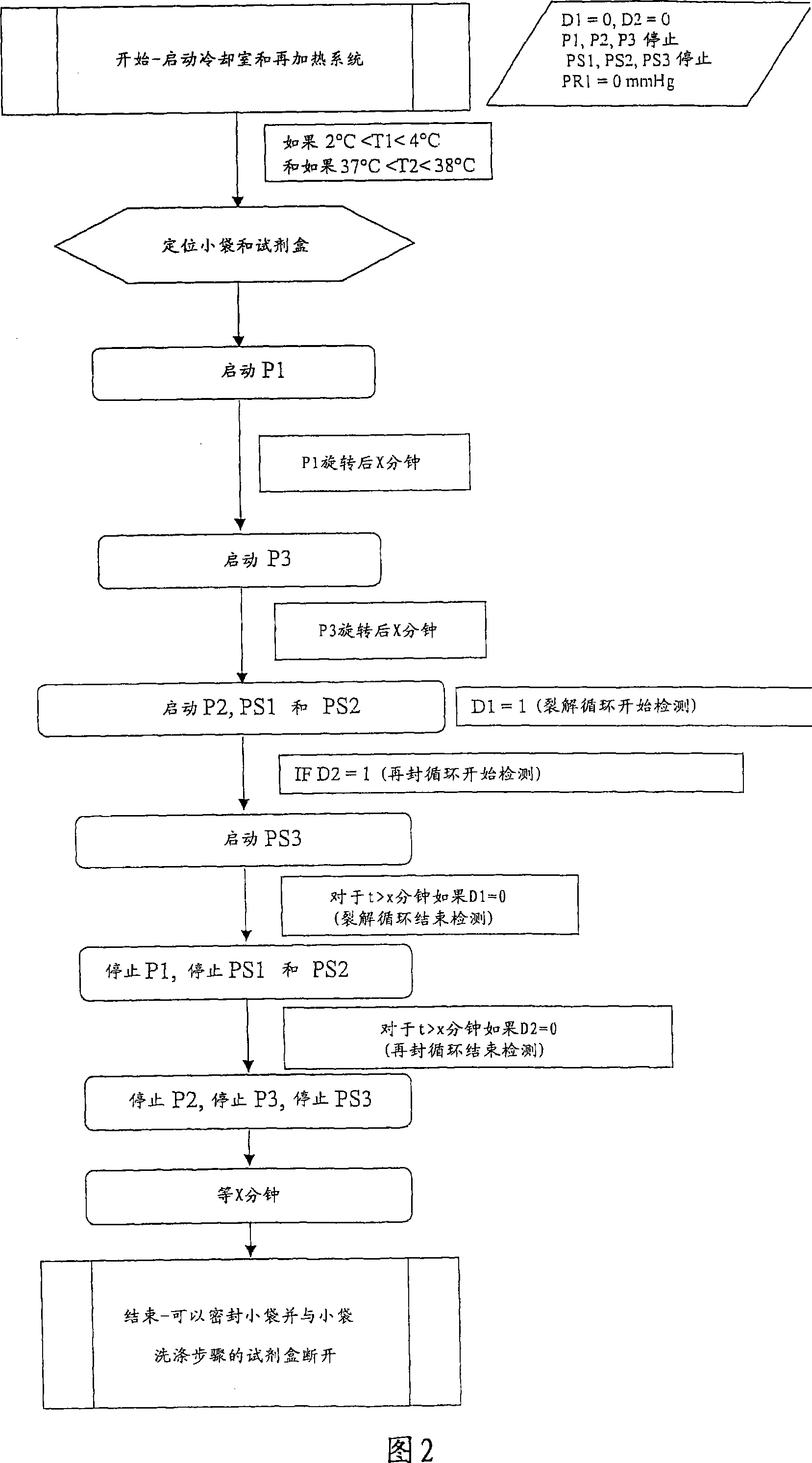
[0120] Refer first to Figure 1. The first dashed box indicates the first module 1 , generally parallelepiped-shaped, comprising a glass front (which is not shown), which can be opened and closed. Peristaltic pumps P1 , P2 and P3 are located at the bottom of the module as well as receivers (not labeled) for removable components which will now be described. Pumps P1 and P3 have constant, preset flow rates. Pump 2 is controlled to vary the flow rate.
[0121] The removable component comprises a flexible pouch 2 containing a suspension of red blood cells to be lysed. The bag 2 is accompanied by a flexible tube 3, ring-shaped, which cooperates with the pump 1 to circulate the pouch to keep the red blood cells in suspension. The bottom of the bag is further connected to a flexible tube 4 which is connected to the inlet of the "blood" compartment of the dialysis cartridge 5 . Tube 4 cooperates with pump 2 to circulate the suspension from the bag to the...
Embodiment 2
[0133] Example 2: Encapsulating Asparaginase
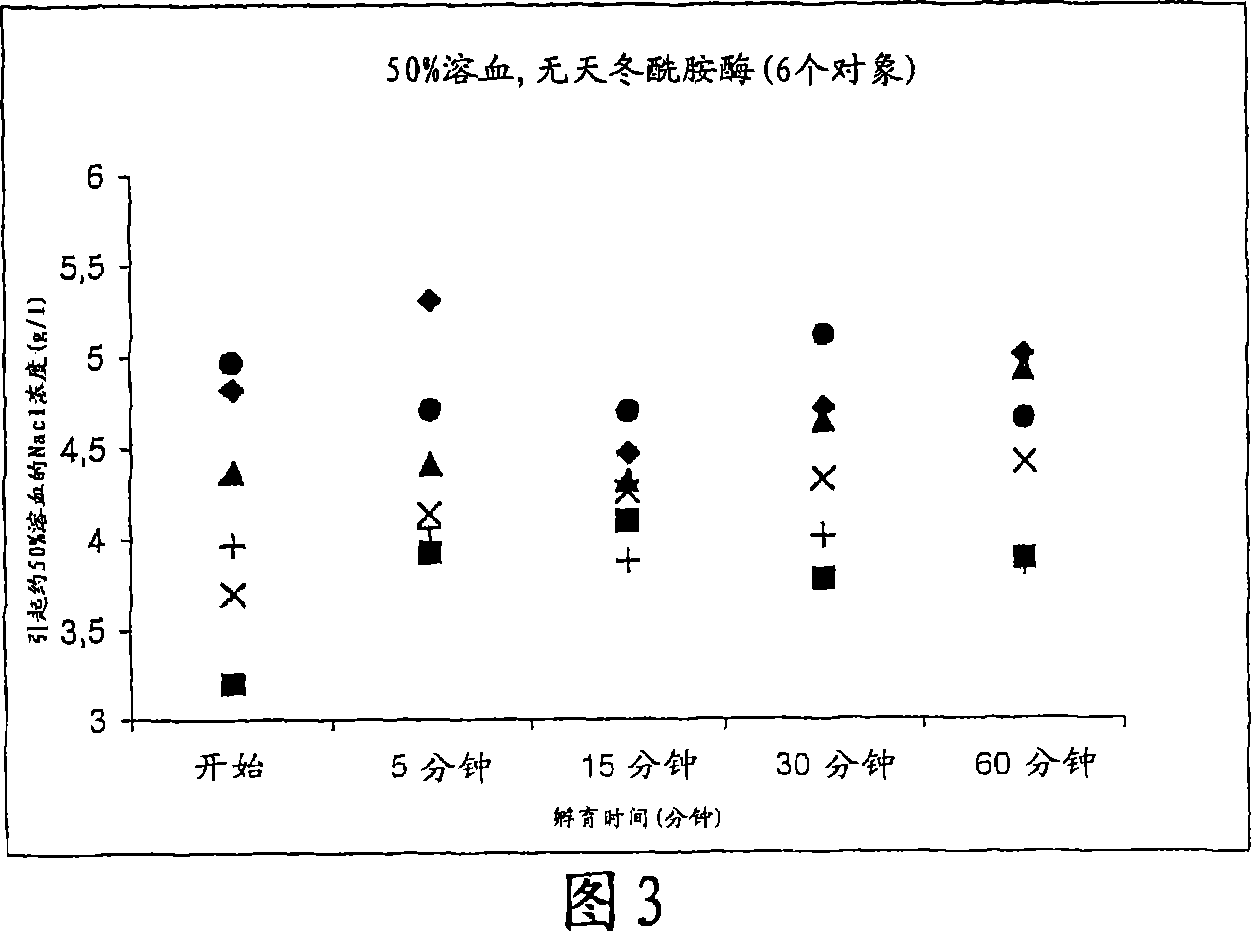
[0134] In this example, osmotic fragility is defined as the concentration of NaCl expressed in g / L that causes about 50% hemolysis.
[0135] 1) Effect of asparaginase on osmotic fragility:
[0136] a. Prepare asparaginase solution:
[0137] Inject 2.5 ml of 0.9% NaCl through the septum into the vial containing 10000 IU powdered asparaginase via syringe. The mixture was stirred until dissolved, thus obtaining a stock solution with a concentration of 4000 IU / ml. The contents were withdrawn with a syringe and placed in 5 ml of lysate. Prepare three solutions and store at +4°C: 0 IU / ml solution (constitutes 0.9% NaCl control), 3200 IU / ml solution (add 625 μl 0.9% NaCl to stock solution) and 1600 IU / ml solution (take 1 ml of 3200 IU / ml solution, Add 1 ml of 0.9% NaCl solution).
[0138] b. Wash red blood cells:
[0139] - starting from whole blood taken on dextrose citrate phosphate and centrifuged at 1000g for 20 minutes at +4°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com