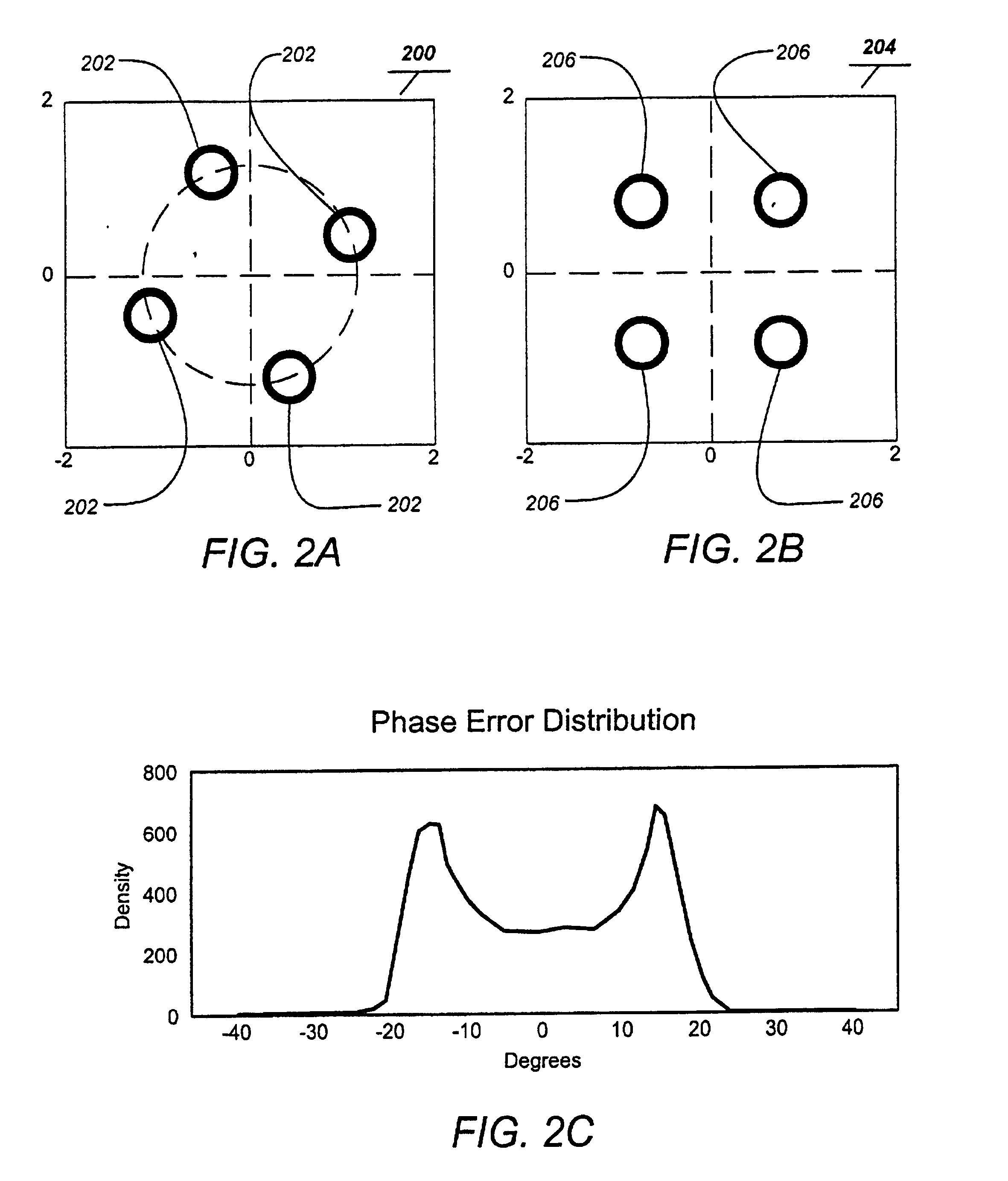
Patents
Literature
496results about How to "Maintain compatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
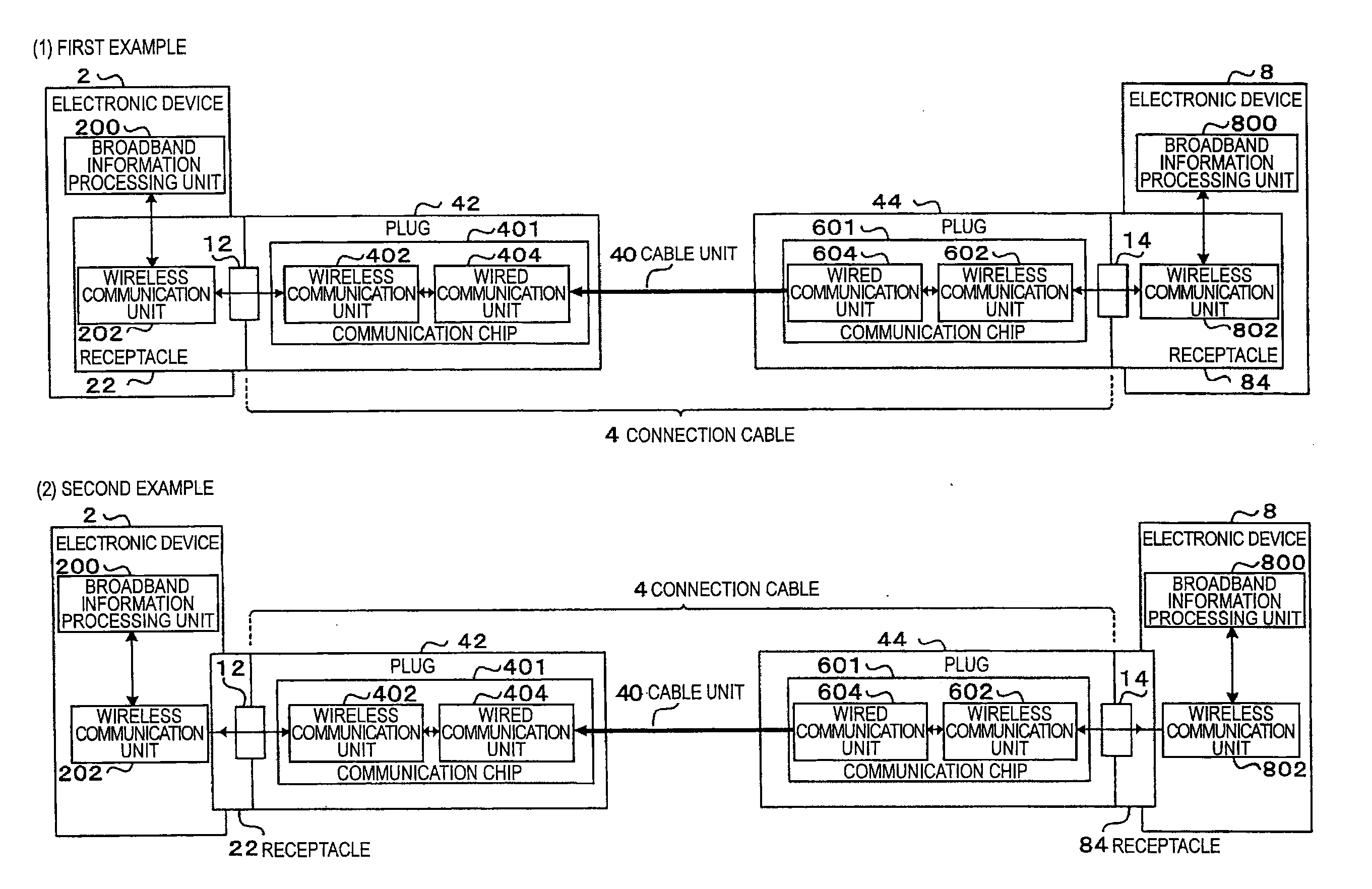
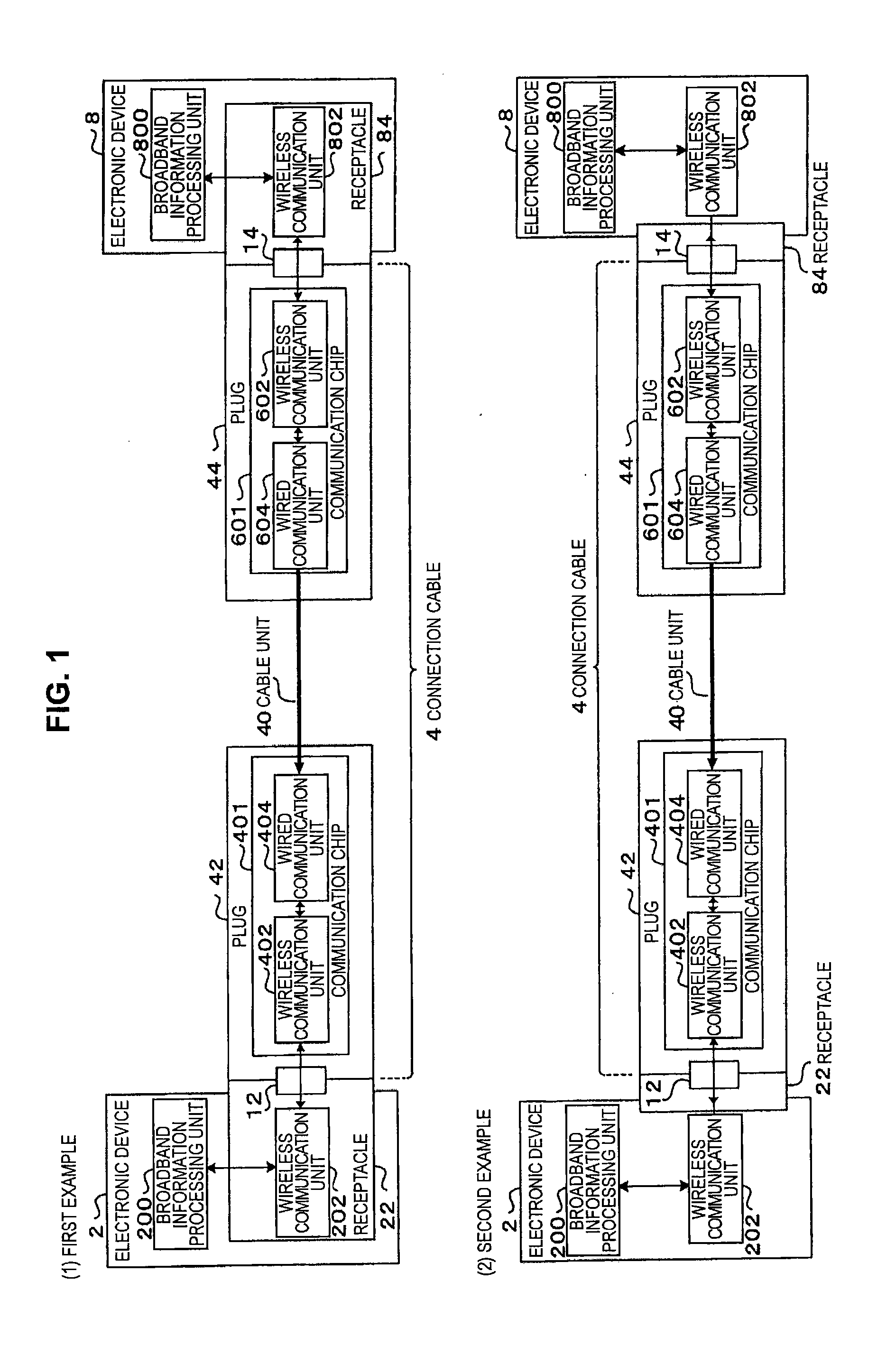
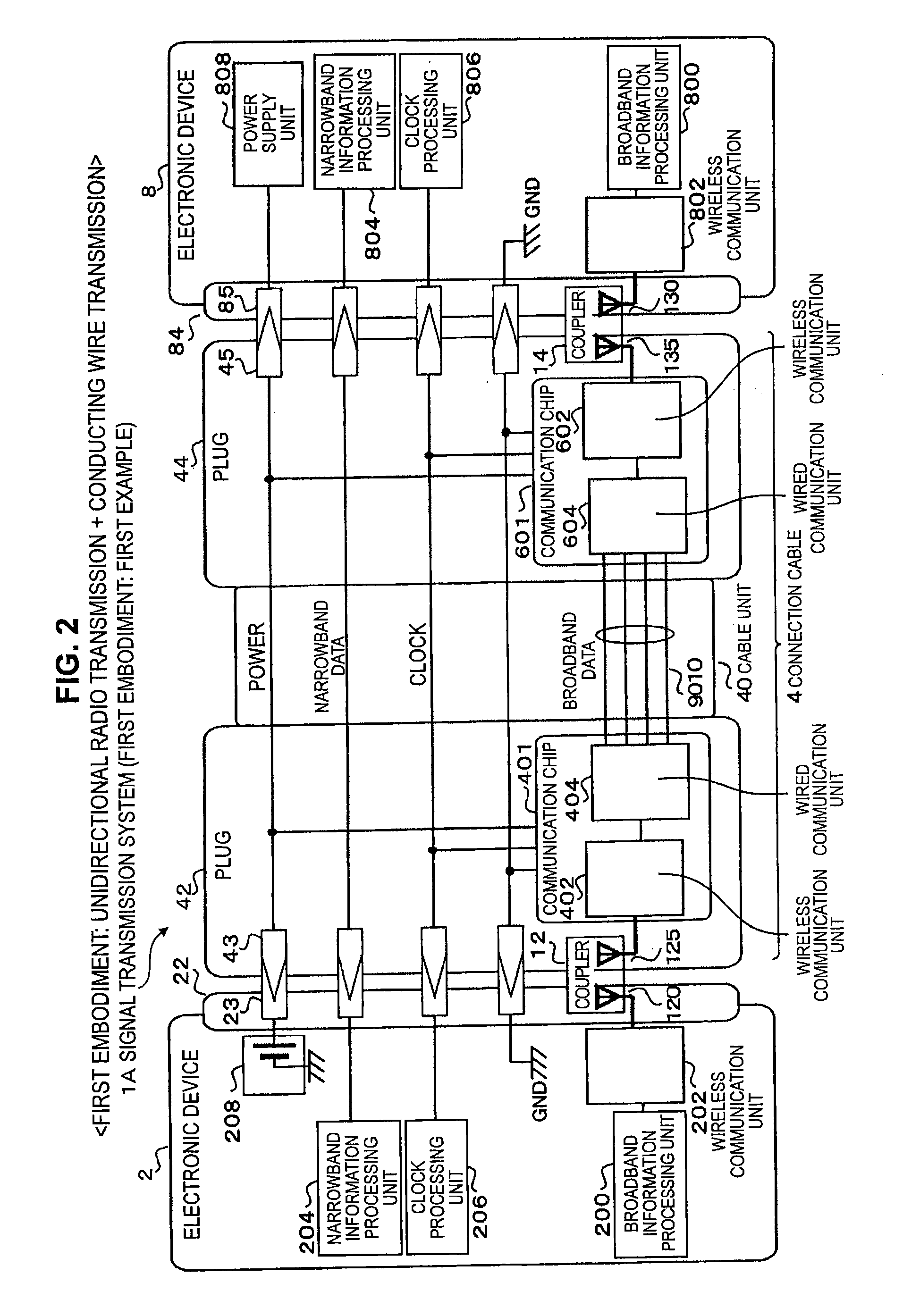
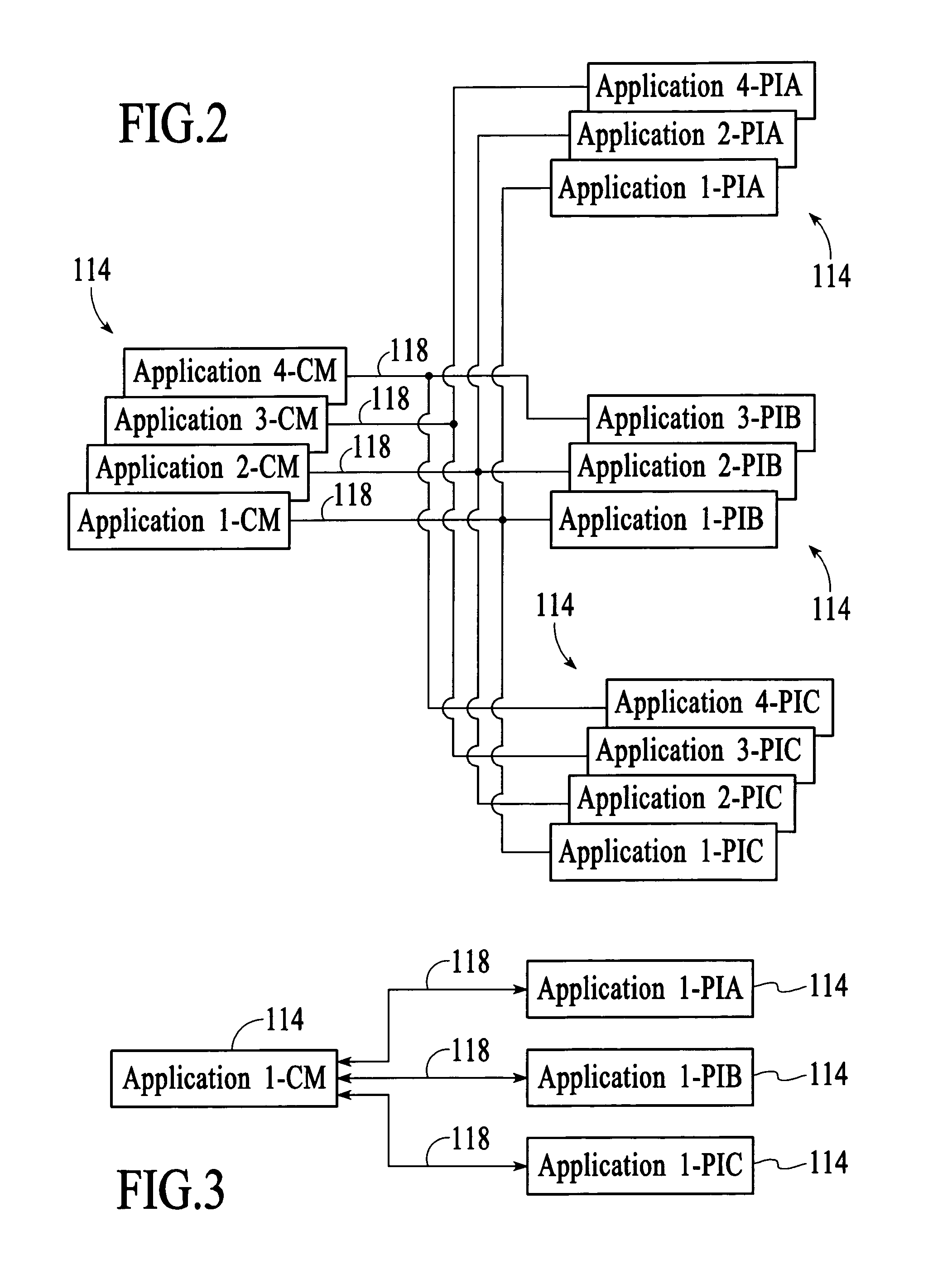
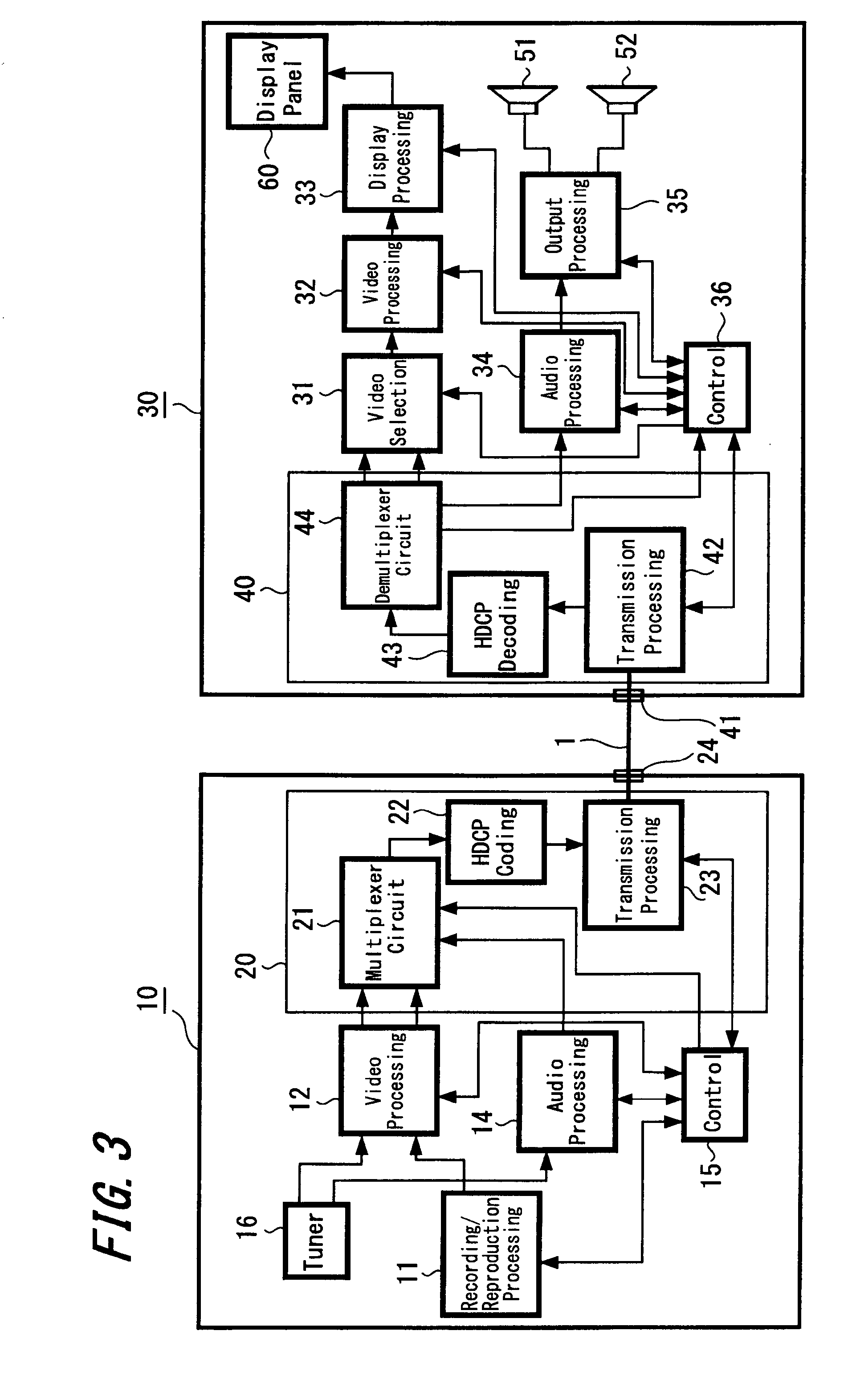
Signal transmission system, connector apparatus, electronic device, and signal transmission method
ActiveUS20130109317A1Increase speedLarge capacityTwo pole connectionsPower distribution line transmissionElectromagnetic field couplingRadio signal
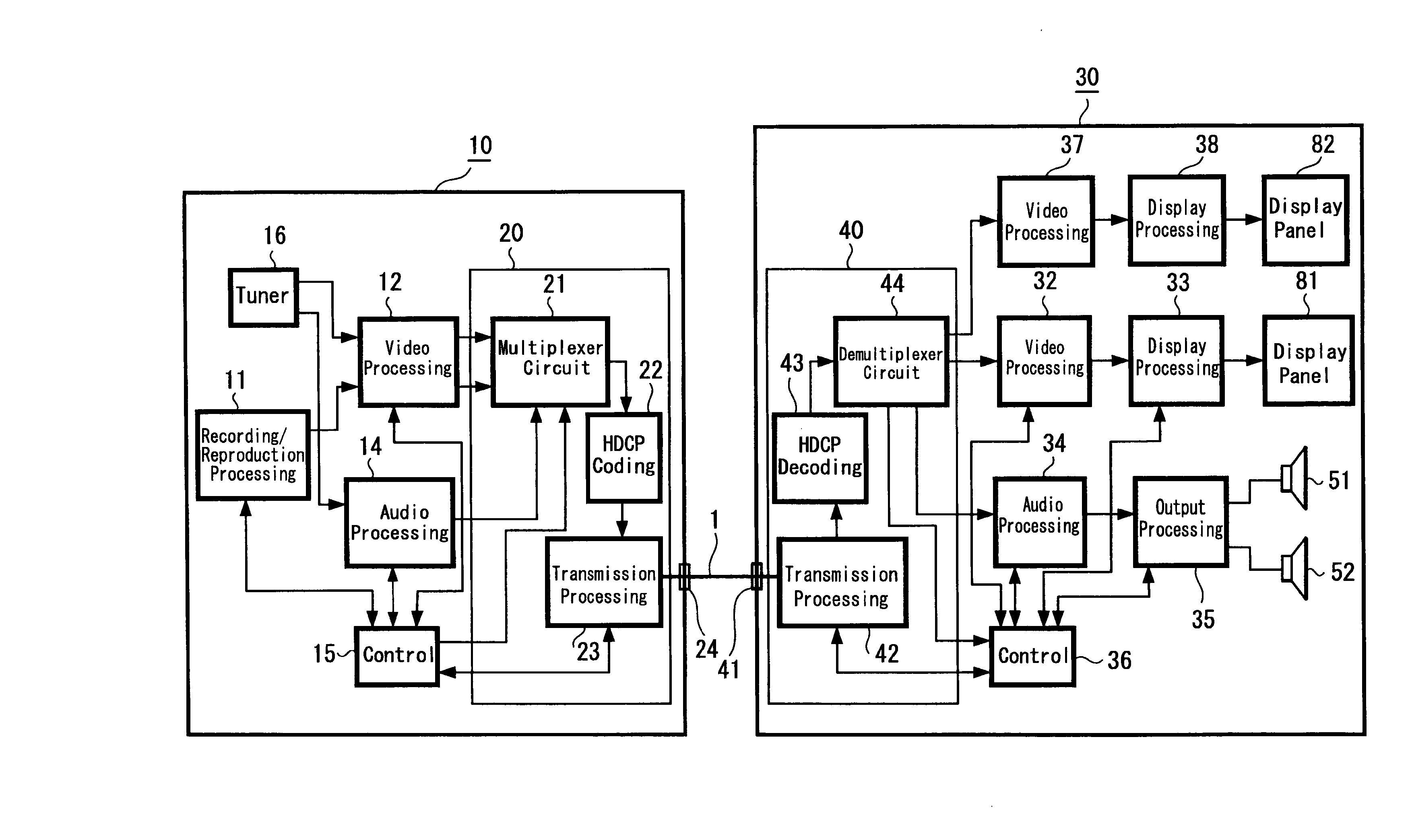
A signal transmission system including: a first connector apparatus, and a second connector apparatus that is coupled with the first connector apparatus. The first connector apparatus and the second connector apparatus are coupled together to form an electromagnetic field coupling unit, and a transmission object signal is converted into a radio signal, which is then transmitted through the electromagnetic field coupling unit, between the first connector apparatus and the second connector apparatus.
Owner:SONY SEMICON SOLUTIONS CORP
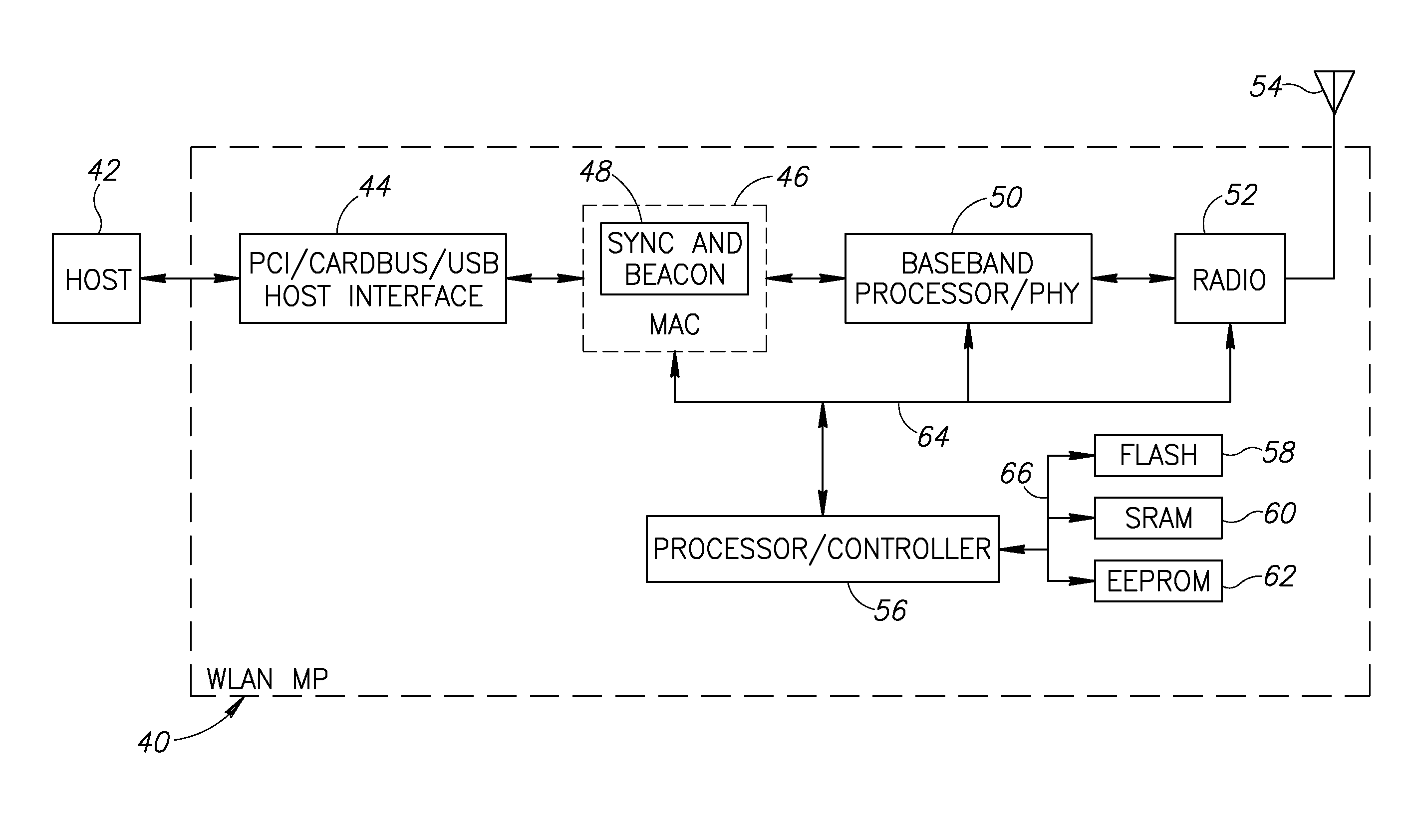
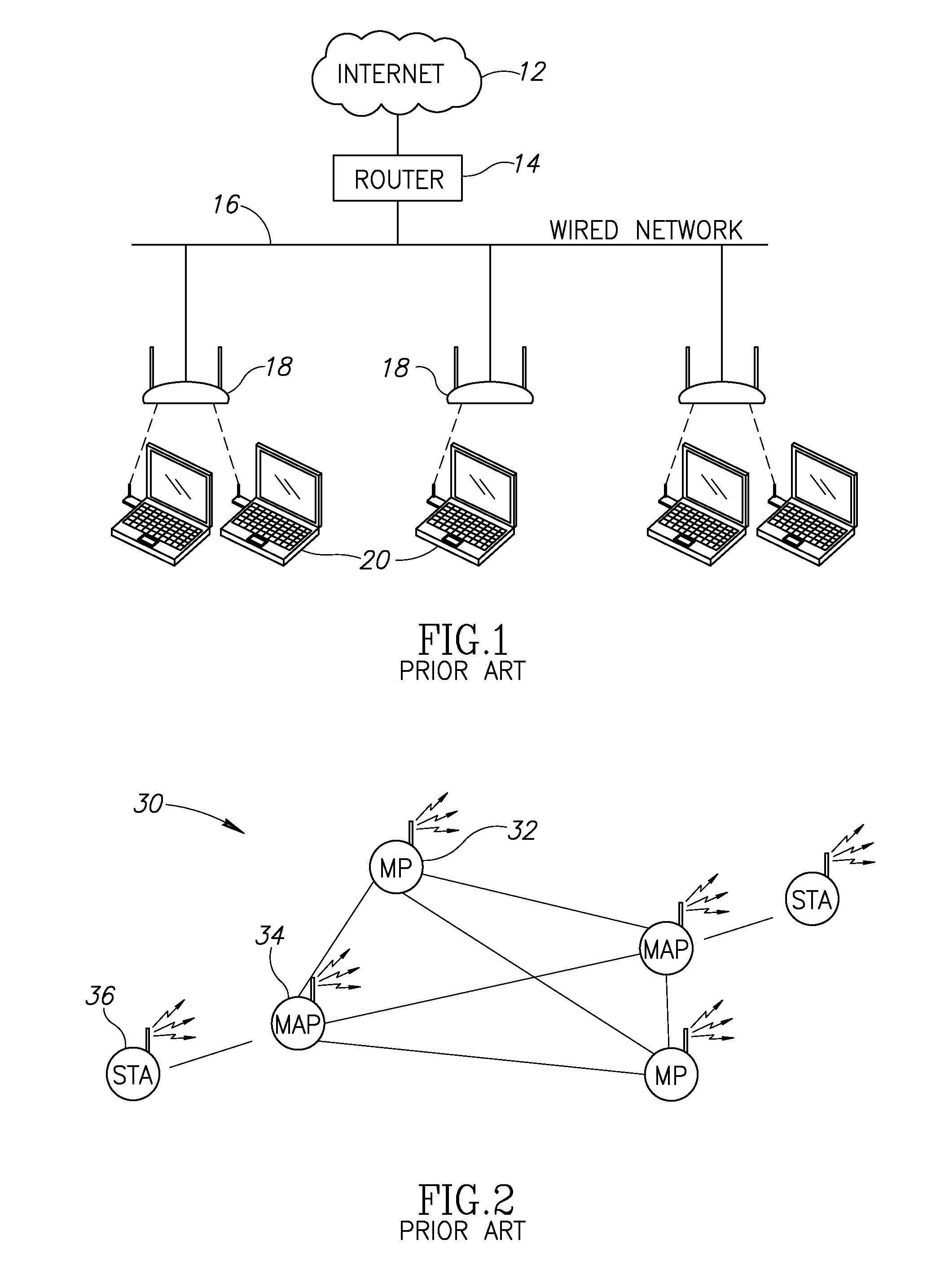
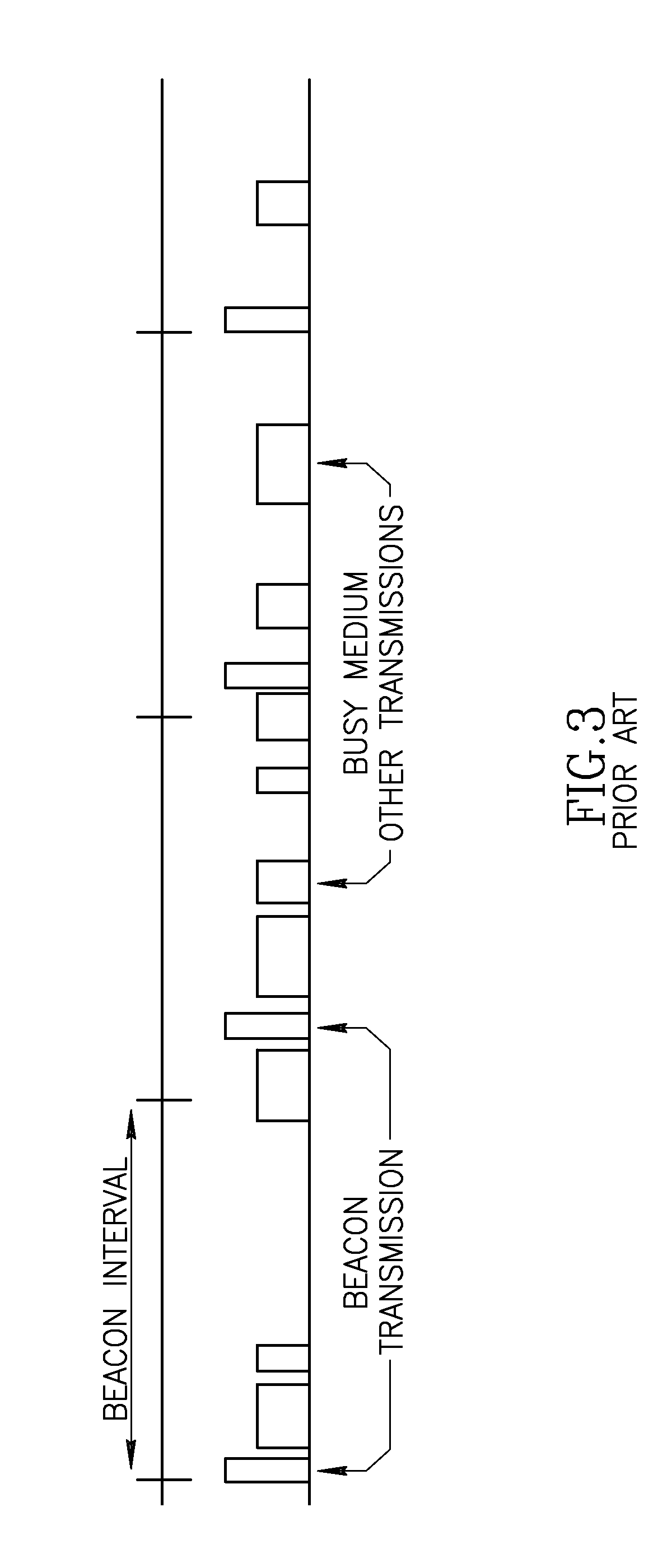
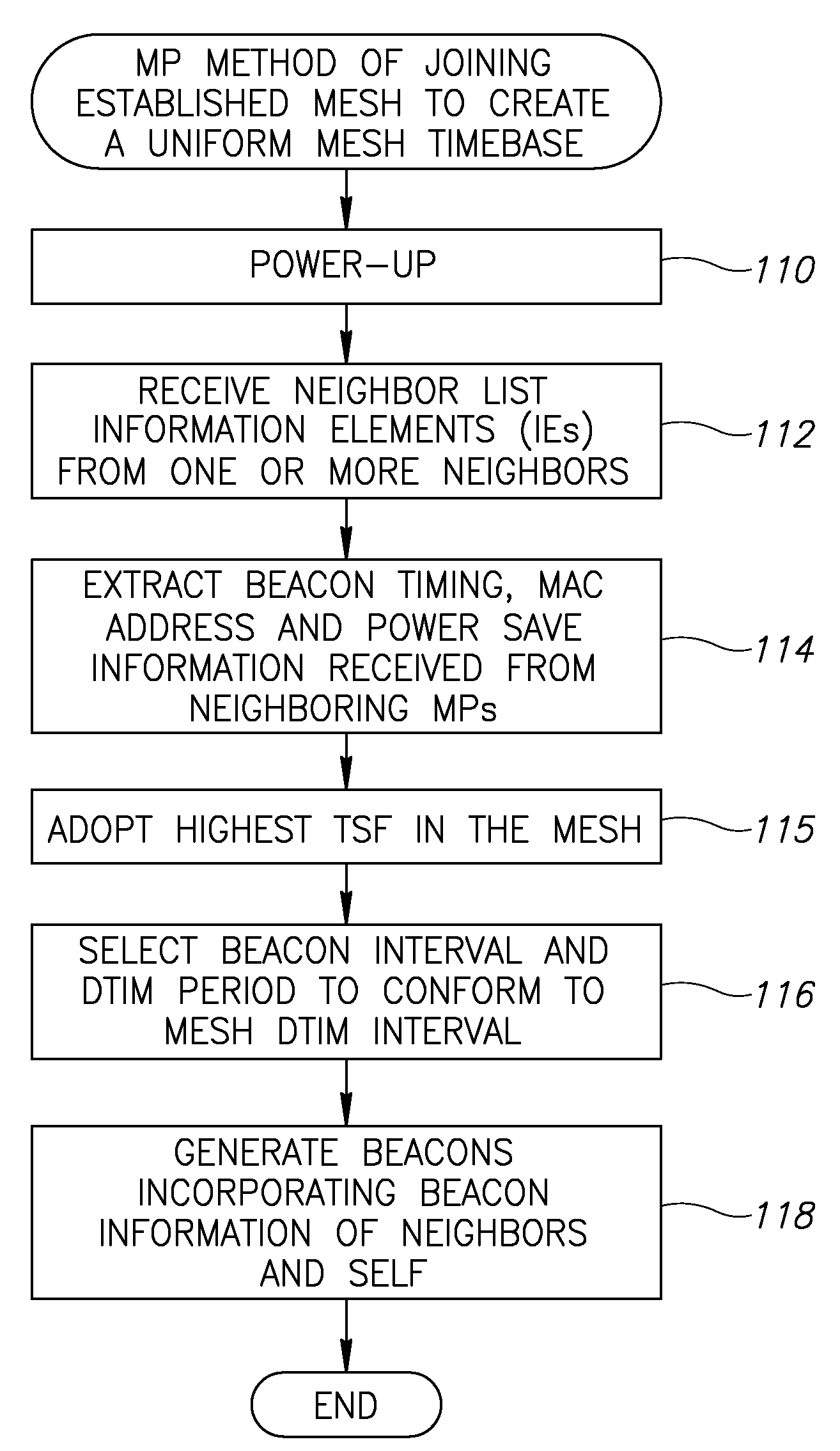
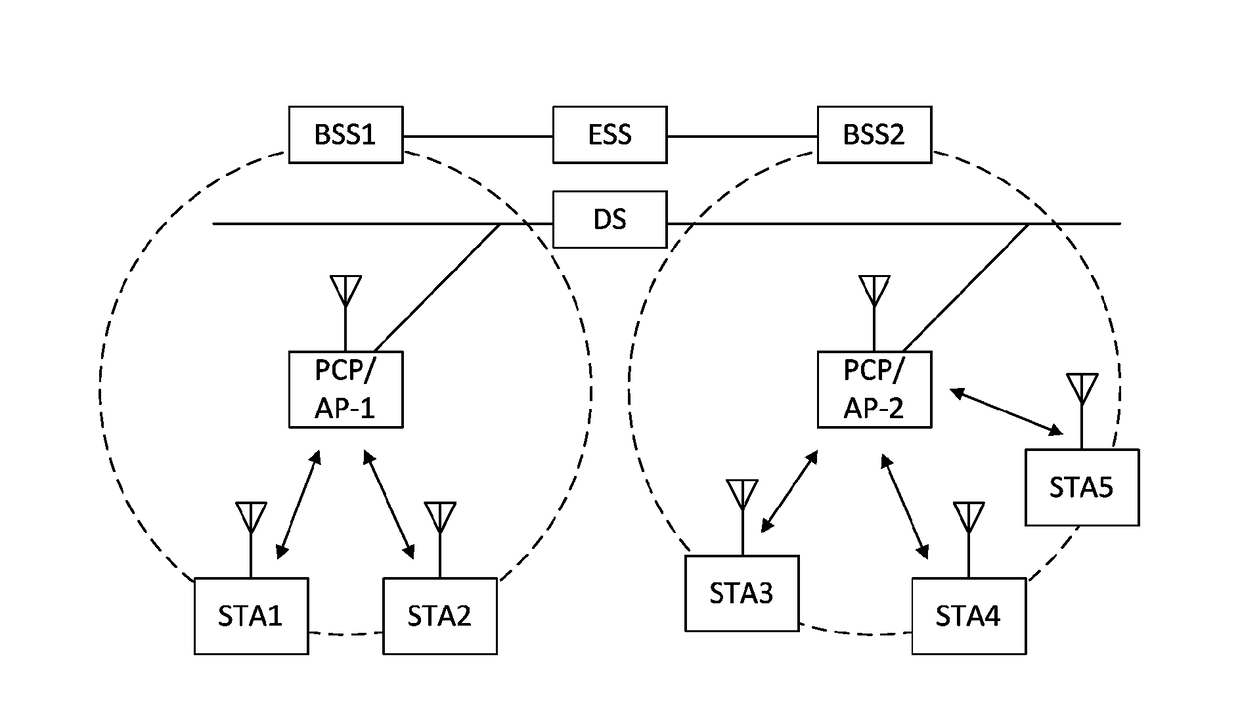
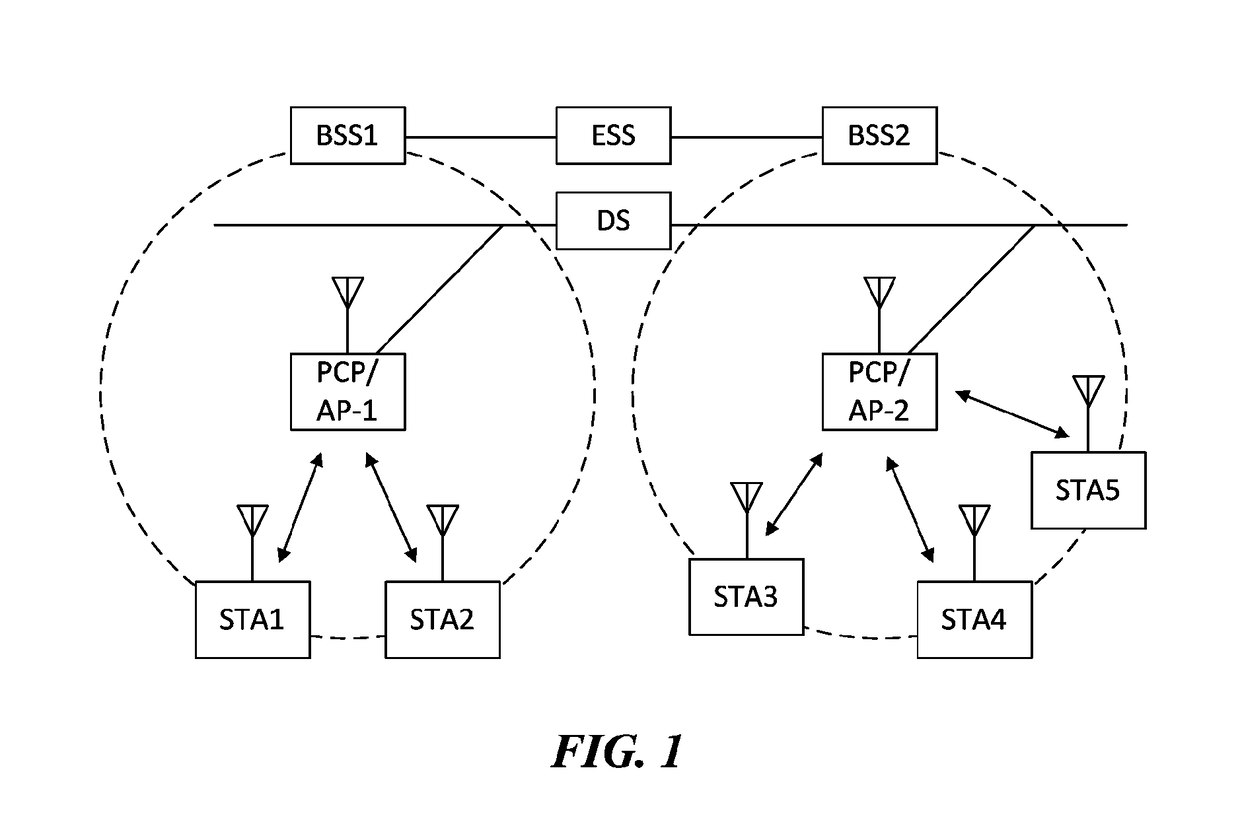
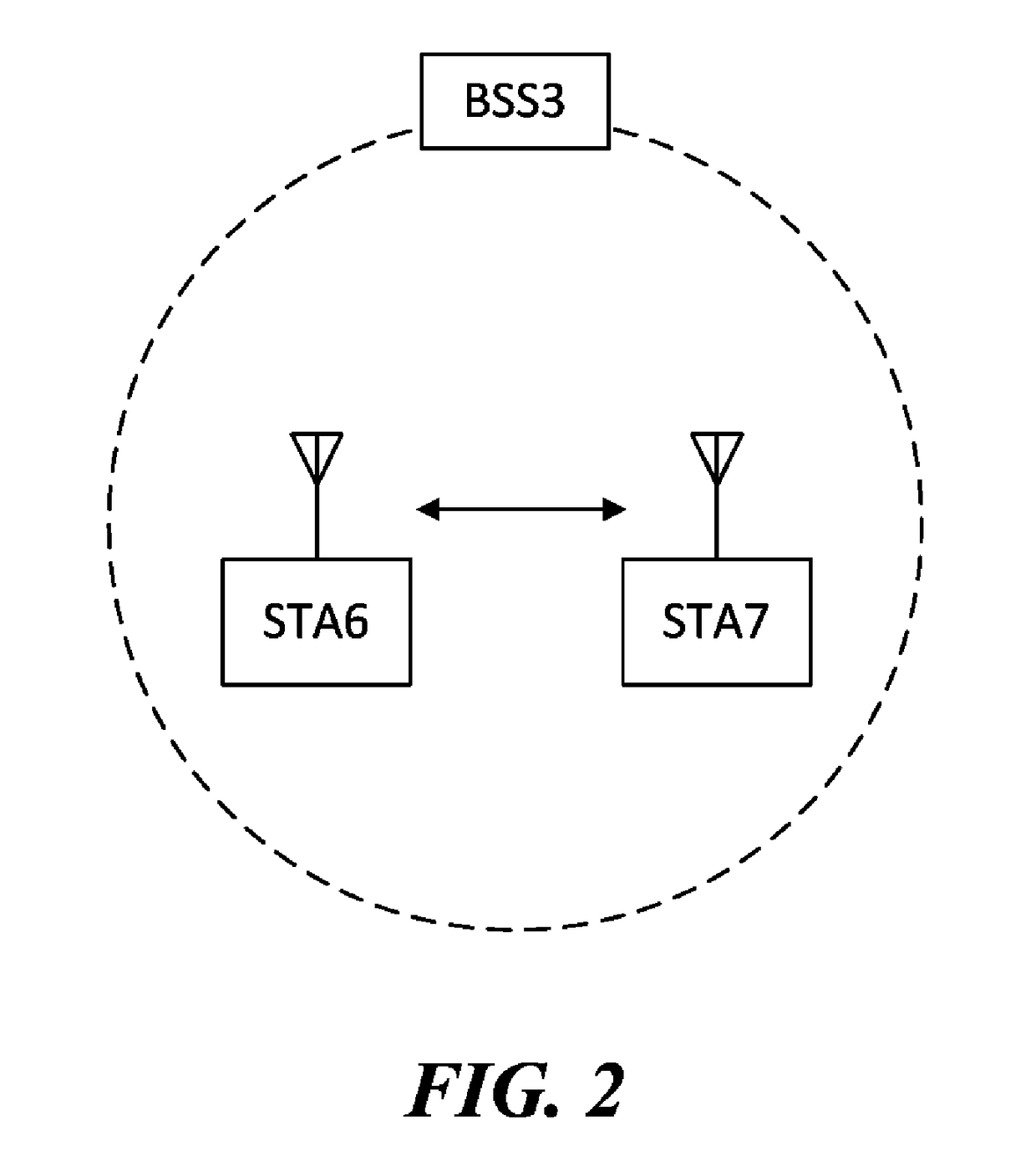
Apparatus for and method of synchronization and beaconing in a WLAN mesh network
ActiveUS20070014269A1Avoid collisionMaintain compatibilityAssess restrictionNetwork topologiesComputer scienceMesh networking
A novel and useful synchronization mechanism that functions to provide a uniform time base for mesh points in a WLAN mesh based network. The invention enables timing synchronization to a common reference clock base by advertising the common TSF within beacon transmissions. All MPs in a mesh share a common DTIM interval. The synchronization mechanism enables the mesh points to avoid collisions in the generation and transmission of beacons. The TBTT offsets of the current MP and its neighbors are advertised in beacons so that neighboring MPs that hear the beacons can select non-overlapping TBTT offsets. Each MP receives one or more beacons from its neighbors and compares the timing of its neighbors to that of itself and adopts the highest TSF (i.e. the fastest) in the mesh. Eventually, all MPs in the mesh will adjust their timing to that of the MP with the fastest clock. The reception of beacons by MPs from its neighbors is also advertised. This allows for MPs to verify that the beacons they send are actually heard and are not in collision with beacon transmissions of other MPs.
Owner:TEXAS INSTR INC
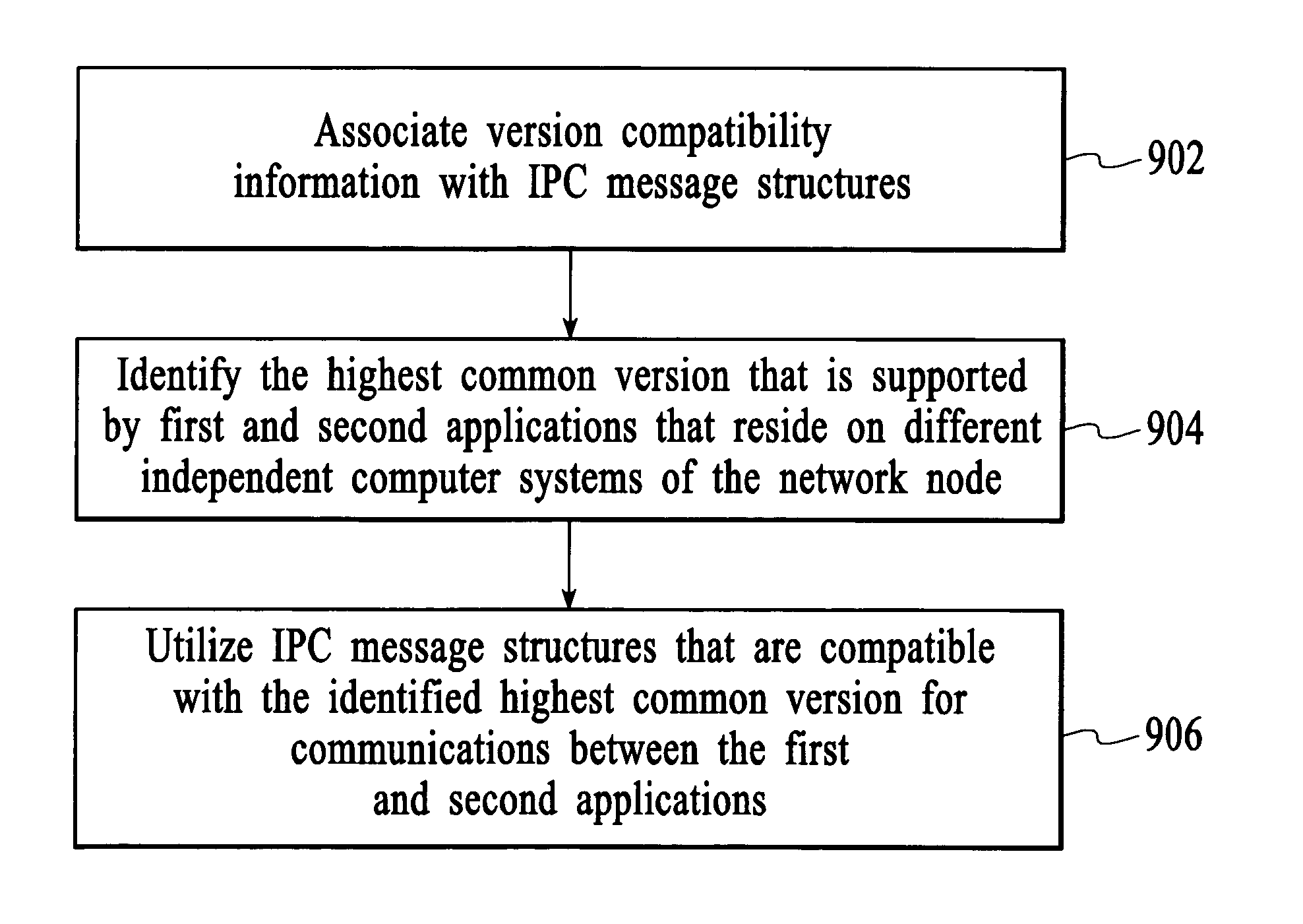
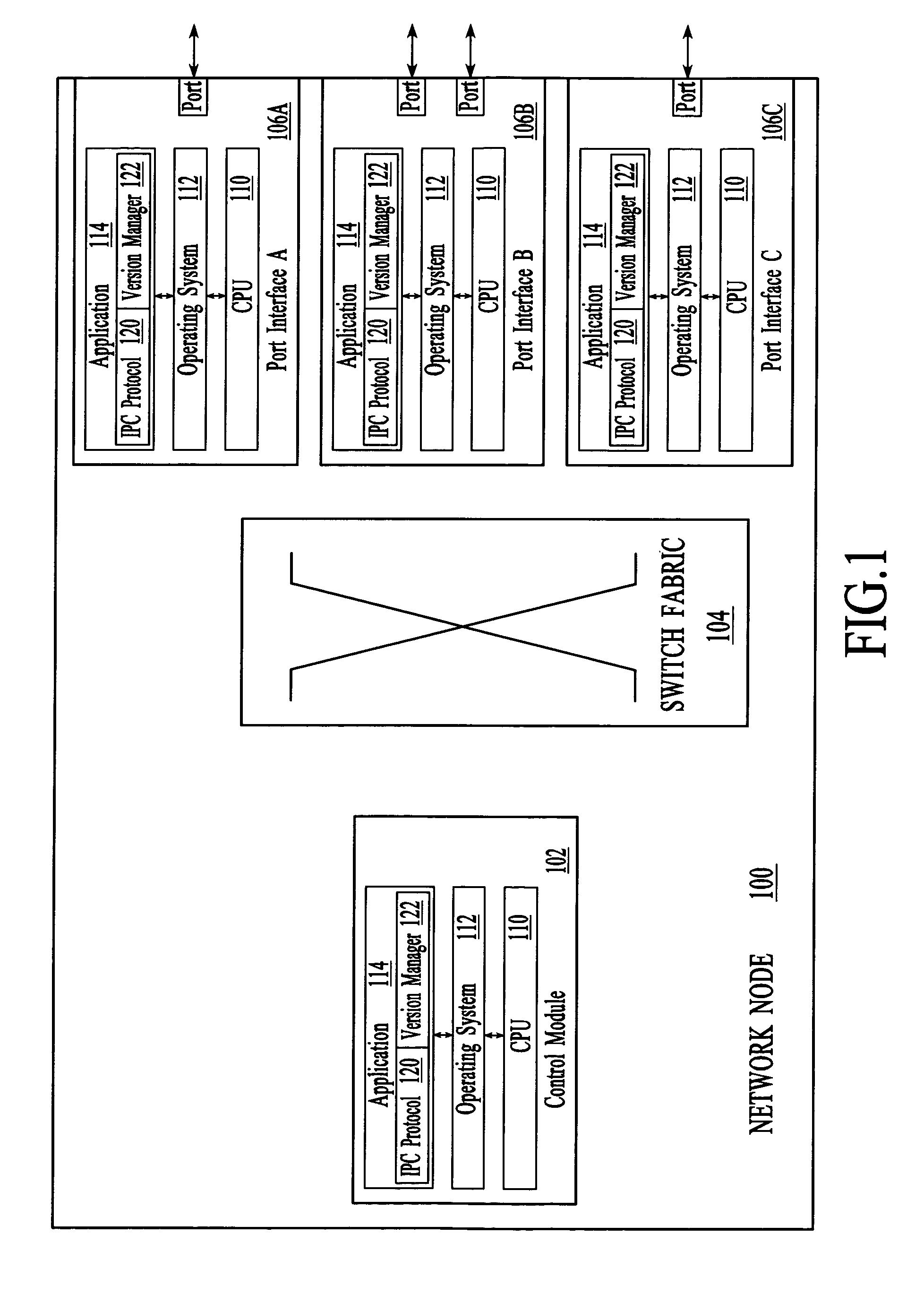
Implicit interprocess communications (IPC) versioning support
InactiveUS7653911B2Maintain compatibilityMultiprogramming arrangementsSpecific program execution arrangementsComputer compatibilityMessage structure
Owner:WSOU INVESTMENTS LLC
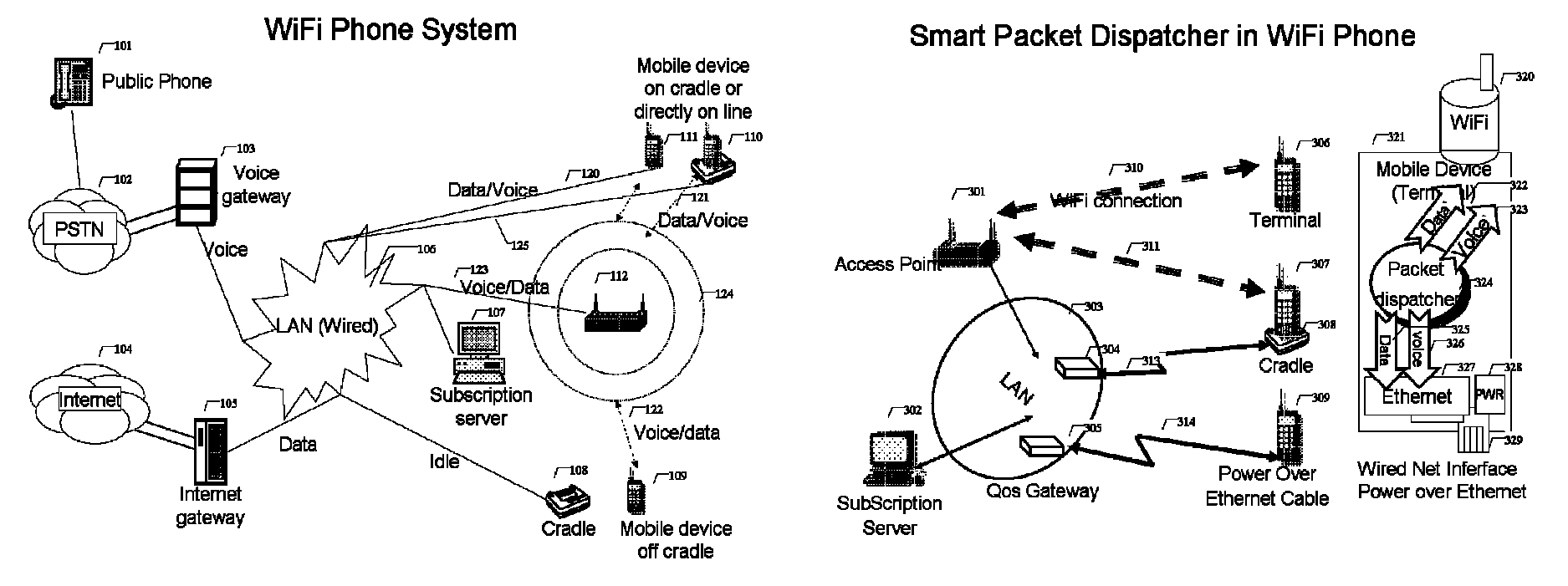
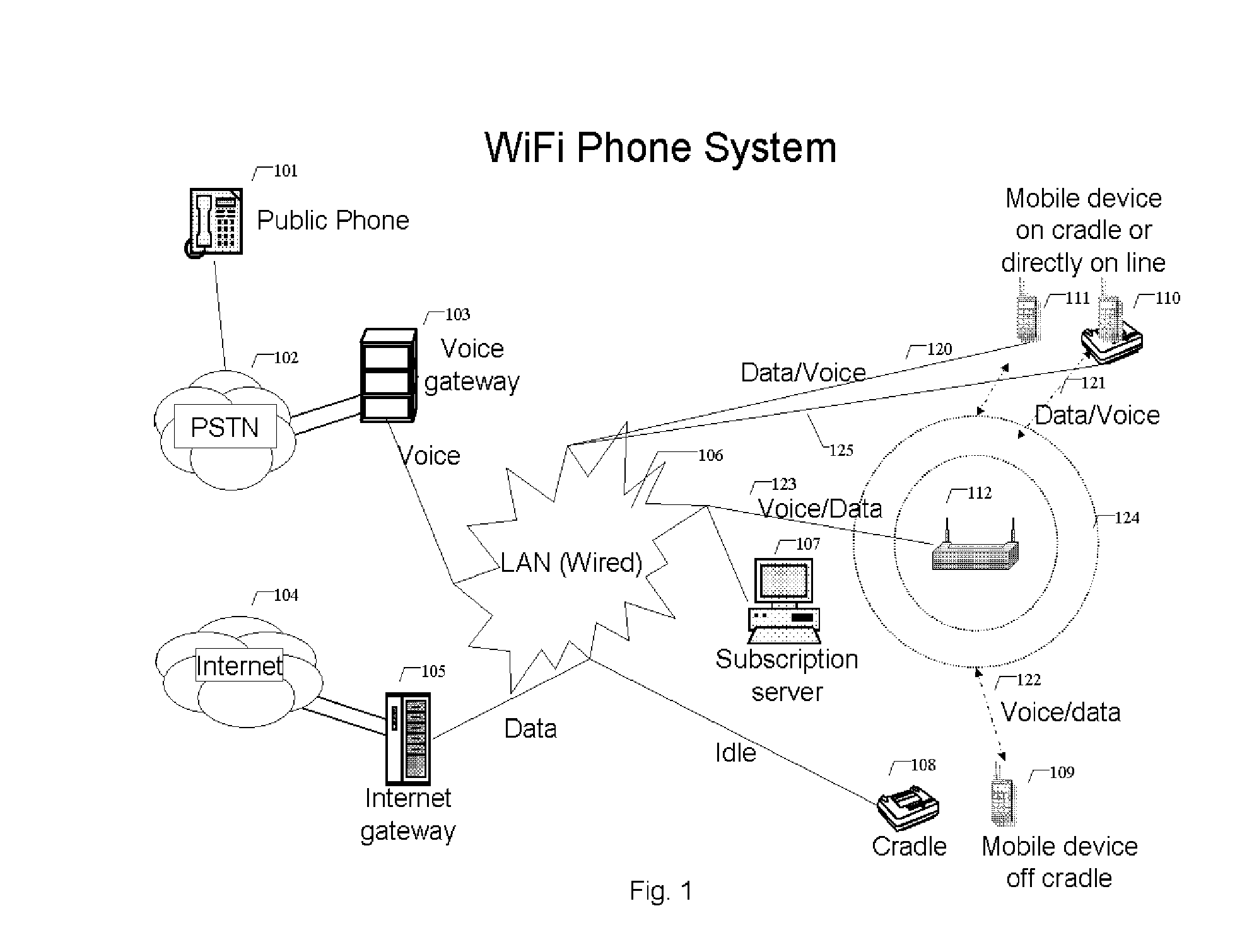
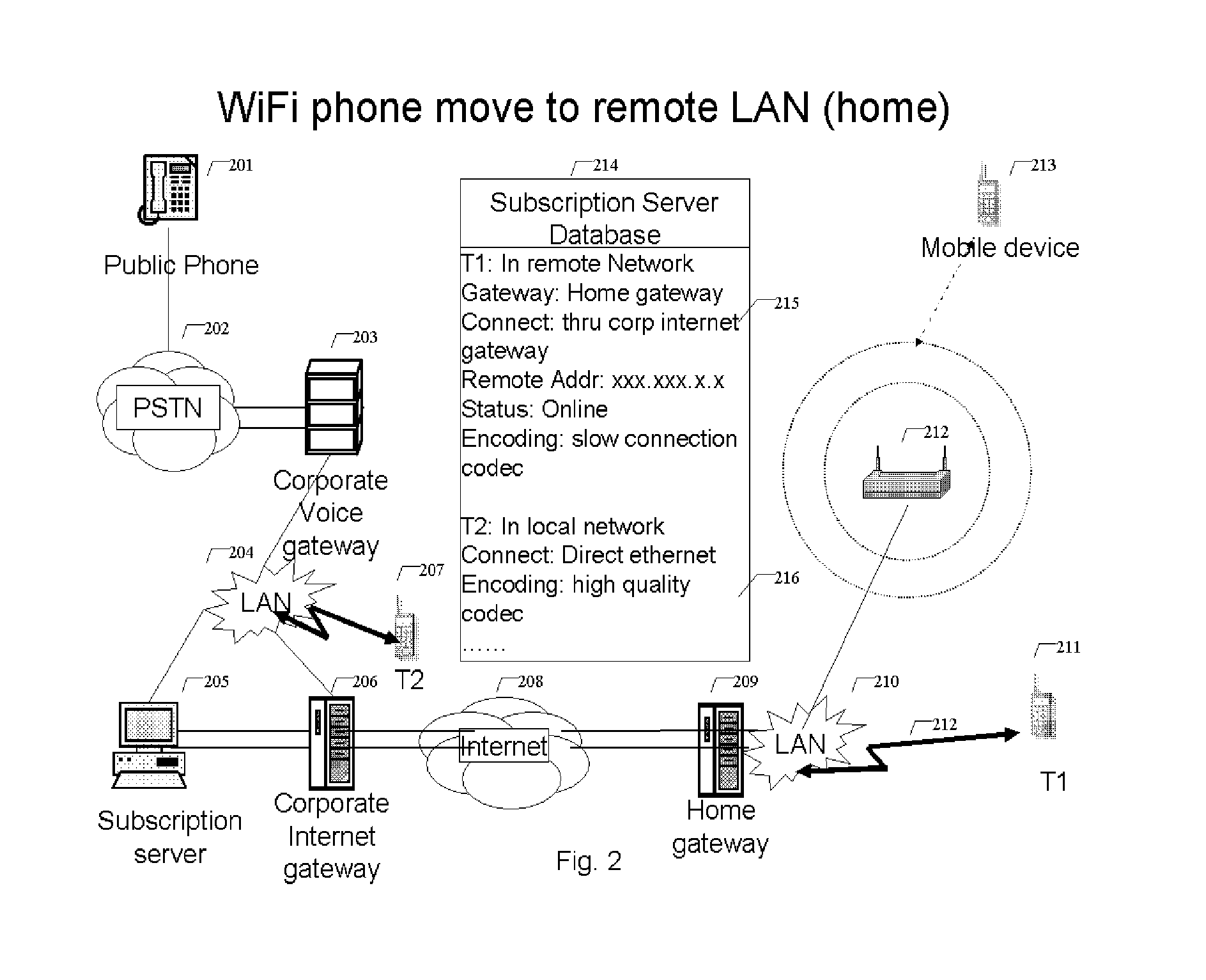
WiFi phone system
ActiveUS8503340B1Small rangeFast transferNetwork topologiesSubstation equipmentQuality of serviceData stream
A voice communication system over WiFi 802.11 network includes: WiFi phone devices, subscription servers and wireless access points. Through voice and data gateways the system can transfer both voice and data streams through WiFi; Phone devices with both wired and WiFi wireless interfaces can automatically switches between wired network and wireless network through WiFi access points. Subscription server maintains the current status of each device every time it changes location. Voice packets are prioritized over regular data packets and dispatched to different network interface by smart packet dispatcher. Quality of service for voice conversation over WiFi is achieved by various methods: piggy-tail method and ACK enhancements and other improvements to reduce delay and latency of real time voice packets.
Owner:XU YONGYONG
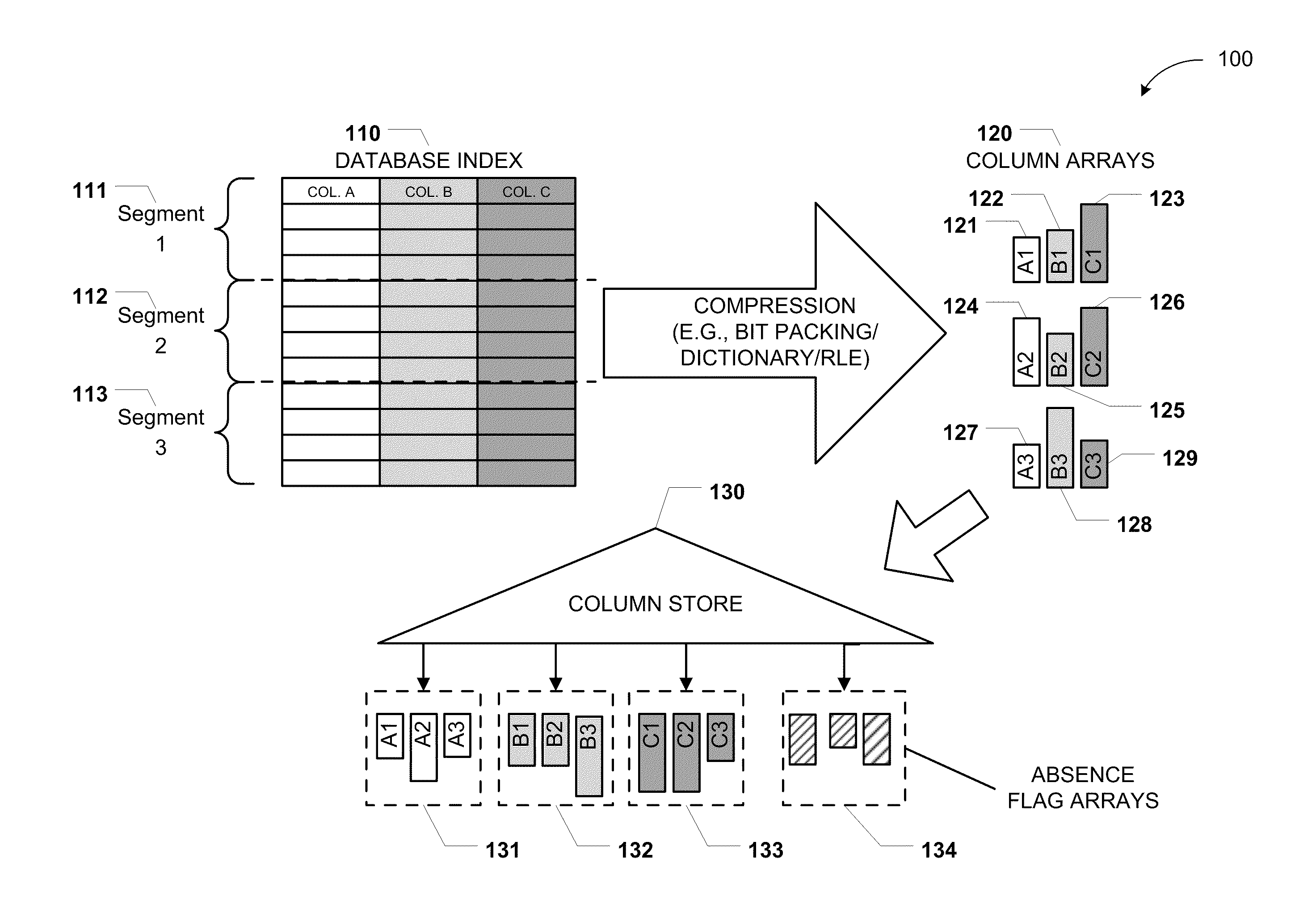
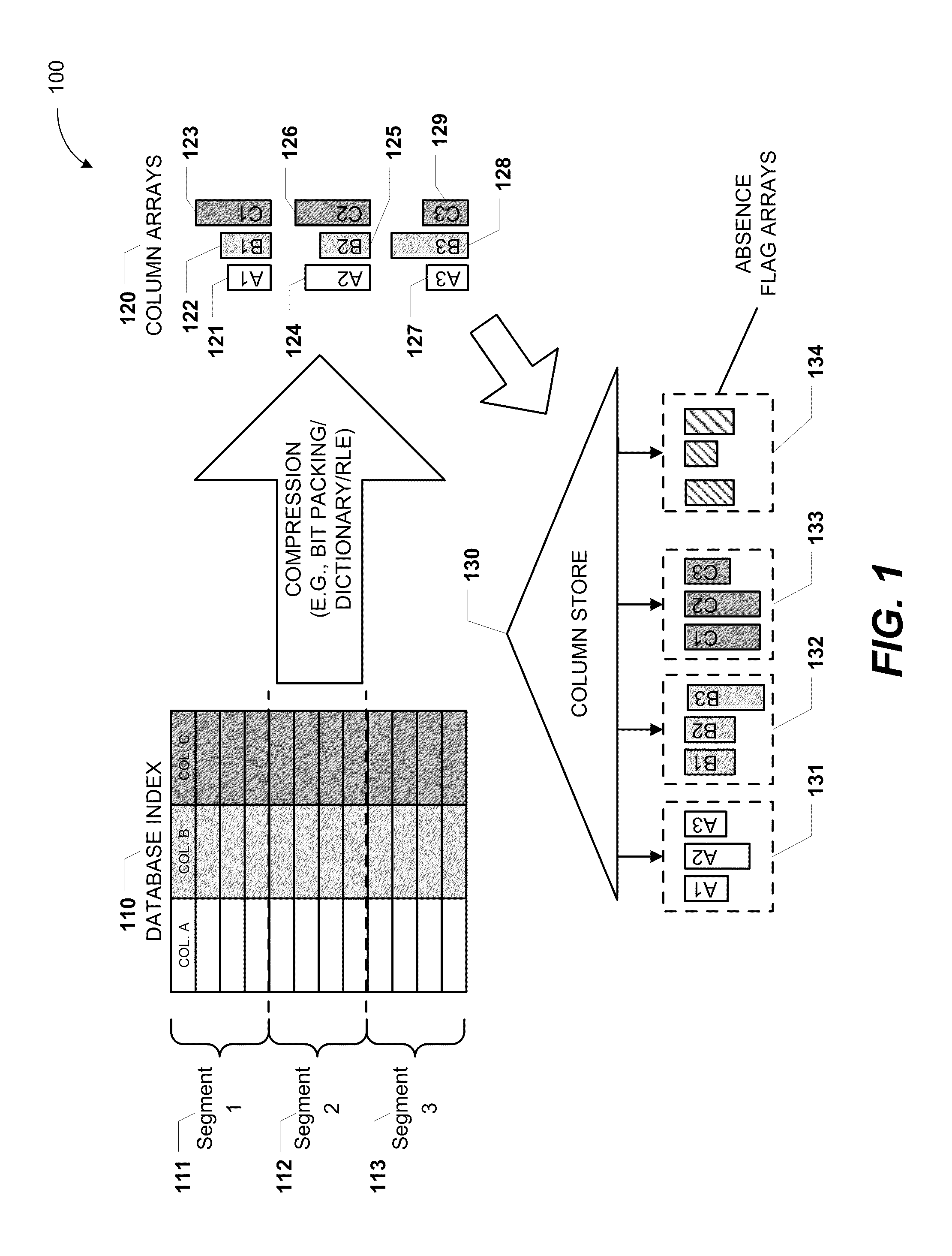
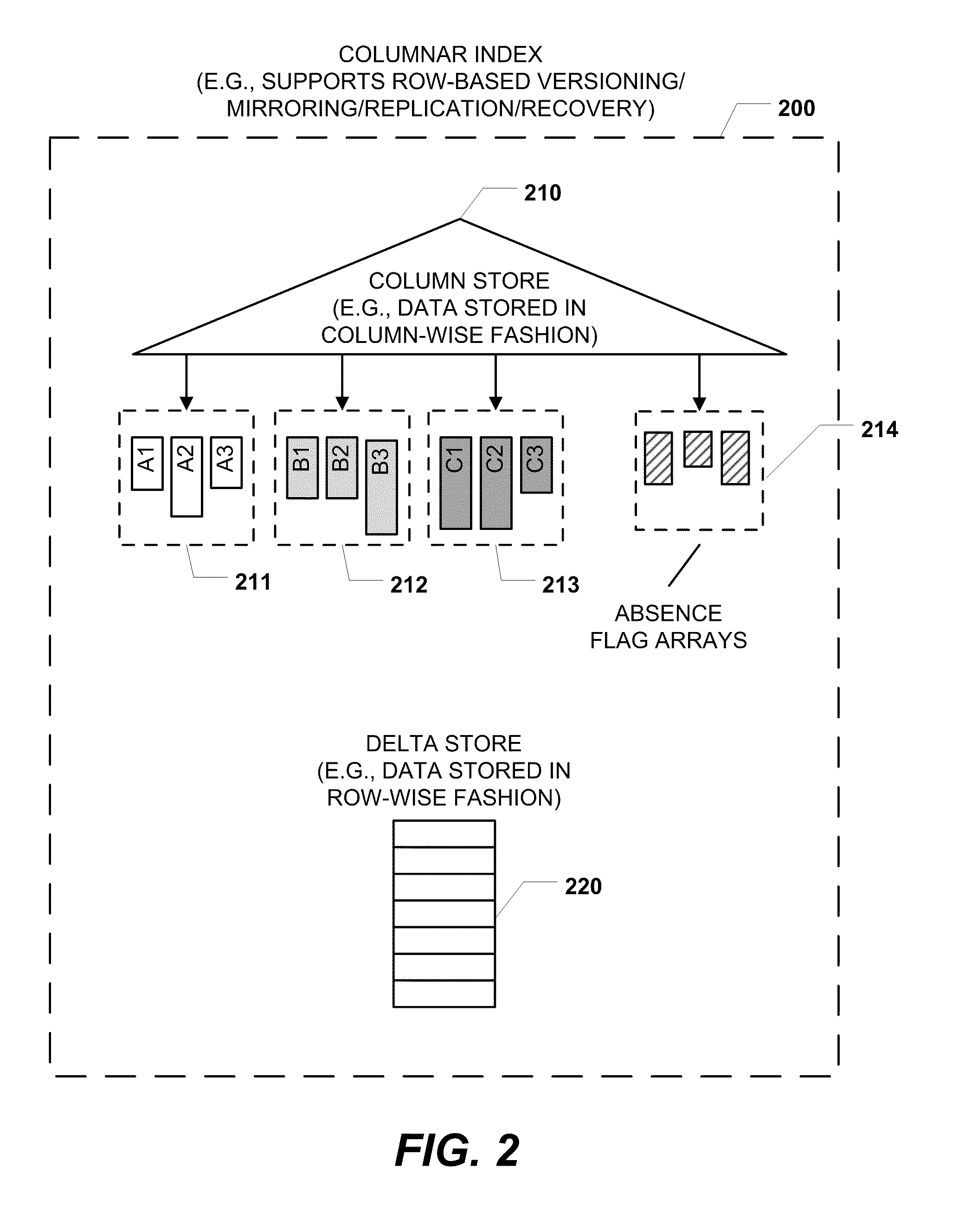
Columnar storage of a database index
ActiveUS20110219020A1Fast column-wise processing of queryMaintain compatibilityDigital data processing detailsHierarchical databasesArray data structureDatabase index
Methods, systems, and computer-readable media of columnar storage of a database index are disclosed. A particular columnar index includes a column store that stores rows of the columnar index in a column-wise fashion and a delta store that stores rows of the columnar index in a row-wise fashion. The column store also includes an absence flag array. The absence flag array includes entries that indicate whether certain rows have been logically deleted from the column store.
Owner:MICROSOFT TECH LICENSING LLC
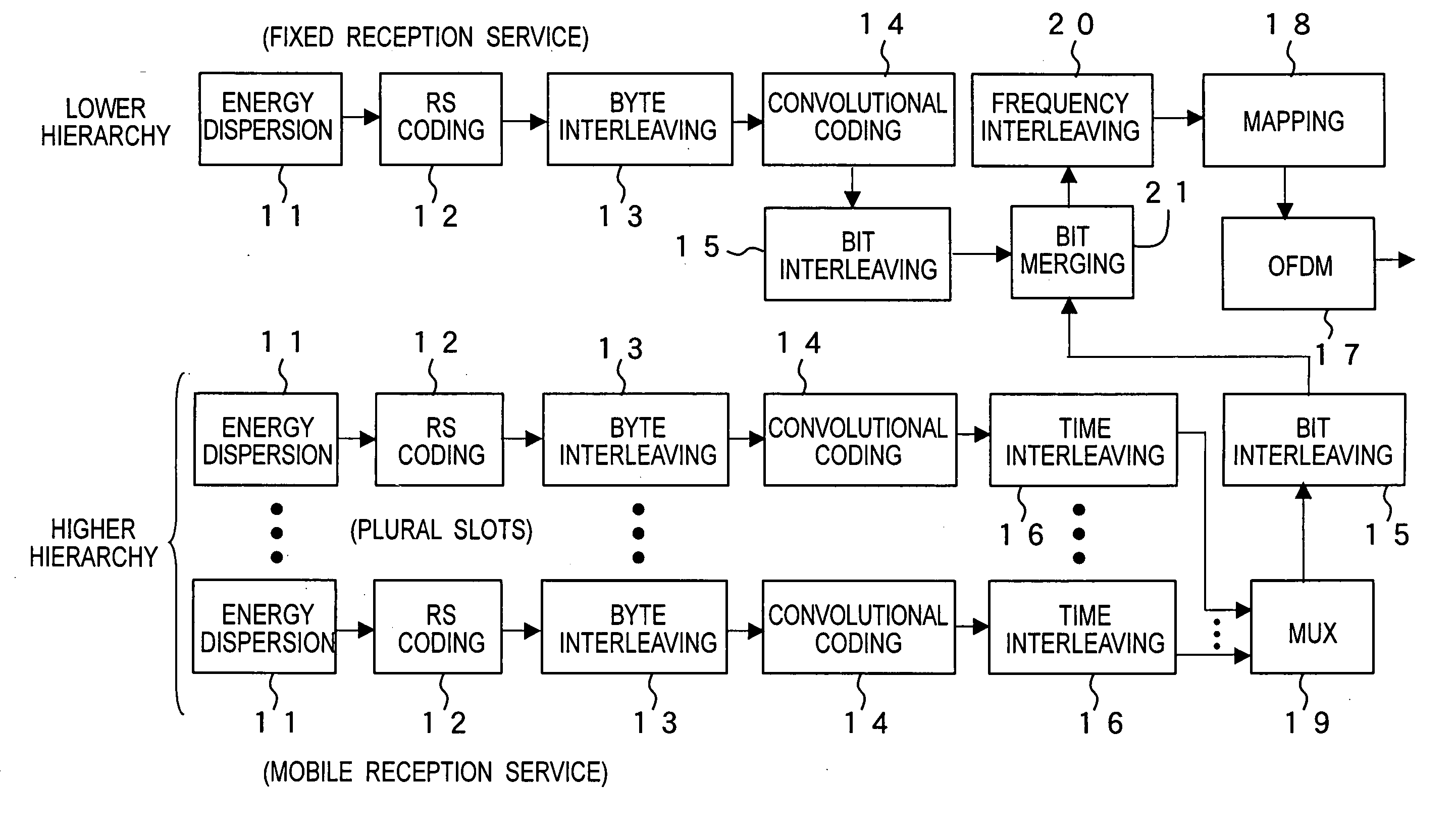
Ofdm signal transmission method, transmission apparatus, and reception apparatus
ActiveUS20070064588A1Maintain compatibilityReduce power consumptionTelevision system detailsColor television detailsDVB-TEnergy dispersion
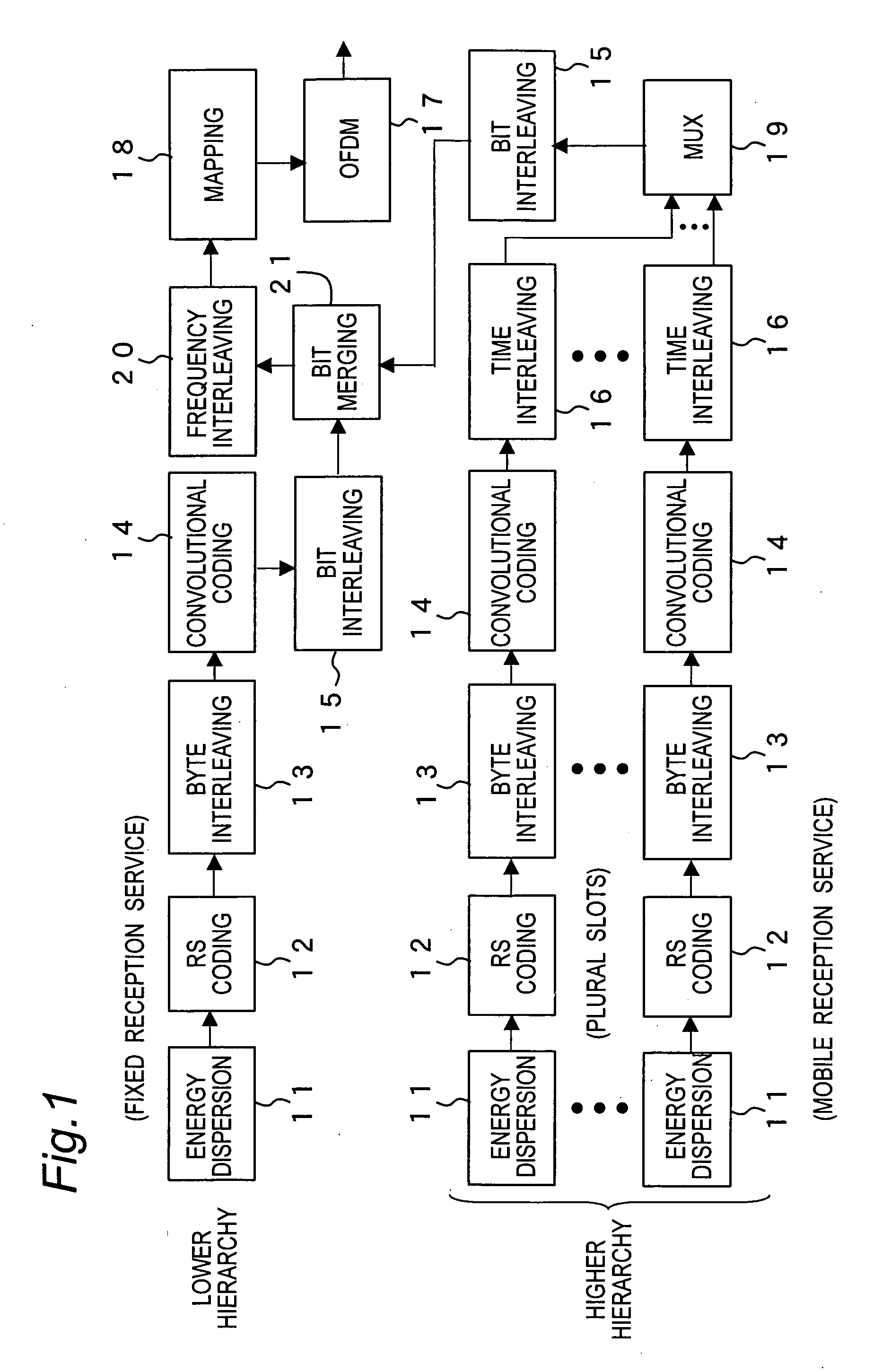
A transmission method of a digital broadcast which is compatible with the DVB-T terrestrial digital broadcast system in Europe and saves a battery in a mobile terminal is provided. Superframes in DVB-T are divided into units each including plural symbols so that an integer number of TS packets can be carried in each slot. At least one slot is used to transmit one service. Energy dispersion, Reed-Solomon coding, byte interleaving, convolutional coding, and time interleaving are applied to each service. When services for mobile terminal reception and services for fixed terminals are transmitted as the provided services, null packets may be transmitted before and after the slot carrying the mobile receiver service so that the fixed reception service and the mobile reception services are not mixed. If the fixed terminal handles TS packets of the mobile reception service as error packets, it could have no problem in reception.
Owner:PANASONIC CORP
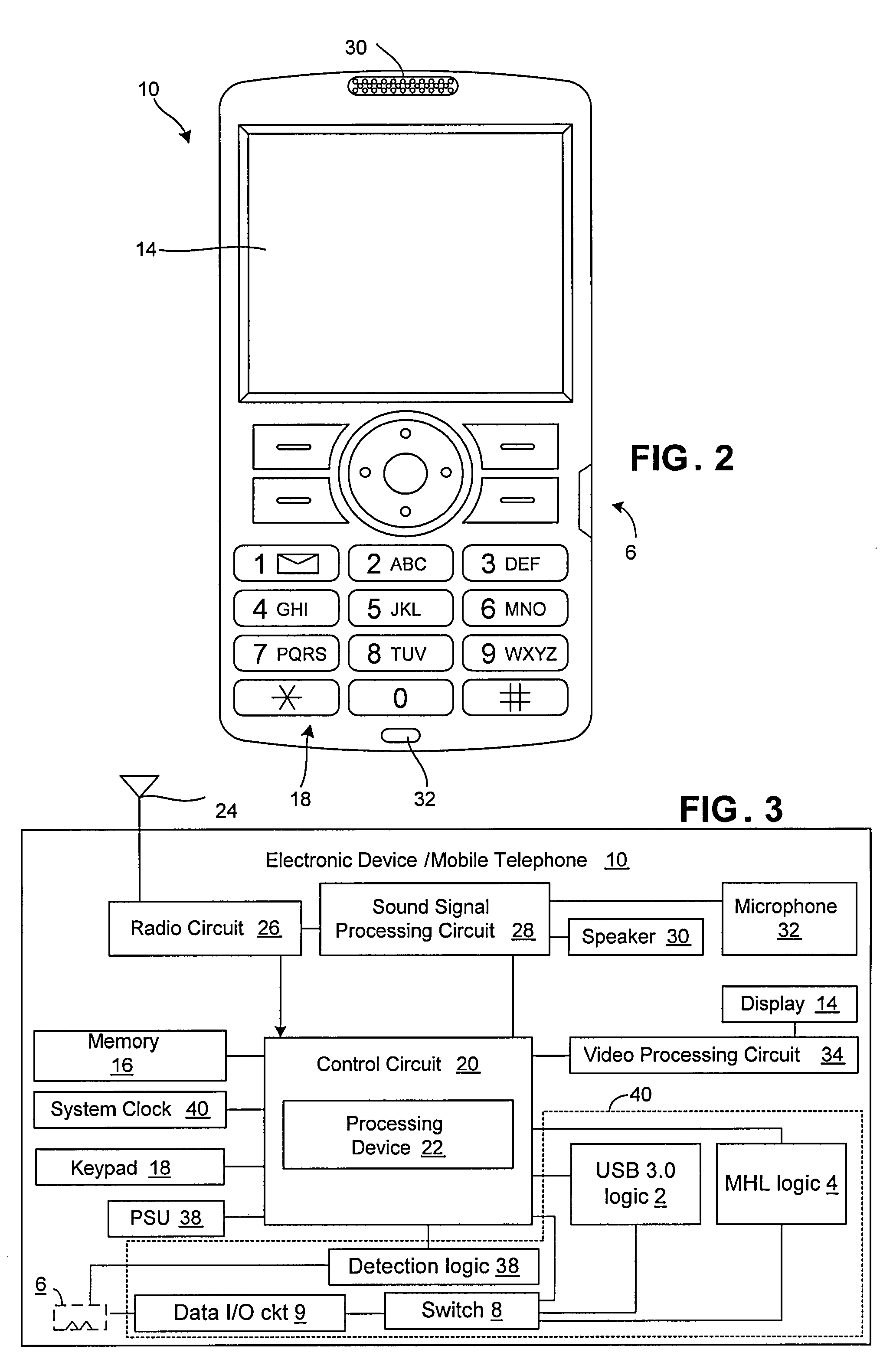
Multiplex mobile high-definition link (MHL) and USB 3.0
InactiveUS20090248924A1SizeAvailable spaceCathode-ray tube indicatorsNetwork connectionsData shippingUSB
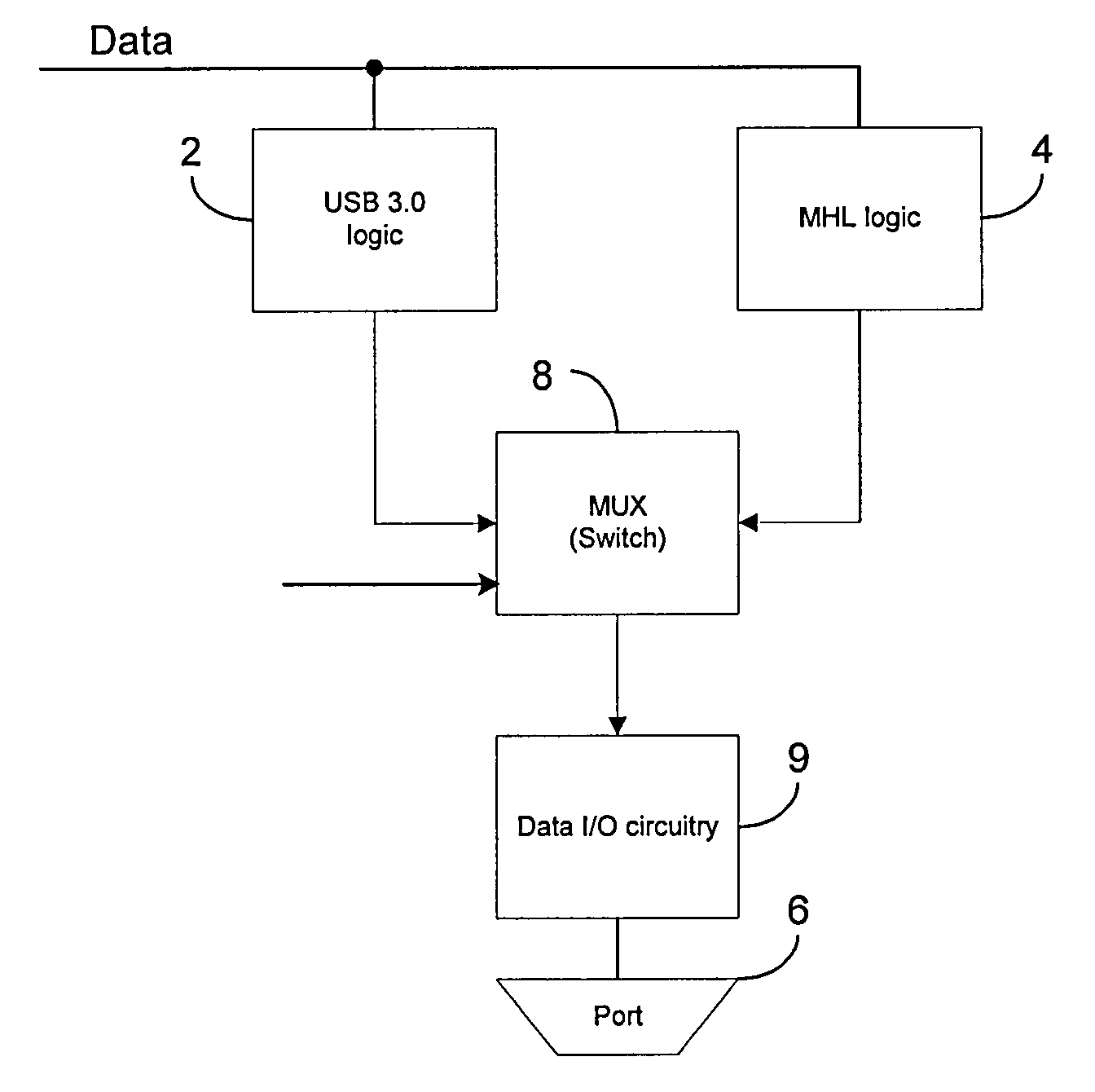
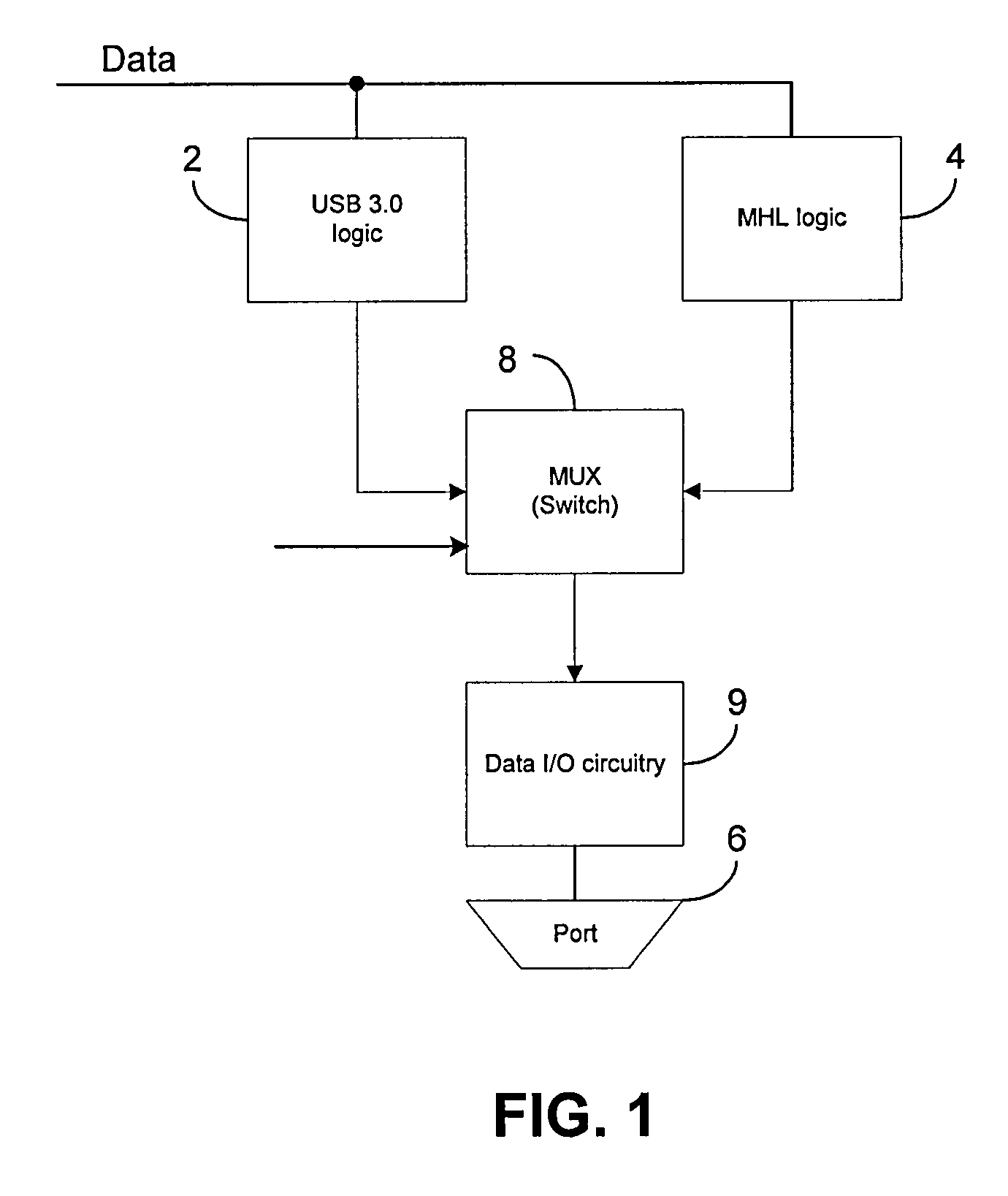
A portable electronic device includes a data input / output (I / O) circuit for communicating data to / from the electronic device, first logic operative to control the data I / O circuit in accordance with a first data transfer standard, wherein the first data transfer standard defines a first connector pin out, and second logic different from the first logic and operative to control the data I / O circuit in accordance with a second data transfer standard, wherein the second data transfer defines a second connector pin out different from the first connector pin out. The device also includes switching logic operative to selectively couple the first logic or the second logic to the data I / O circuit so as to enable operation of the data I / O circuit in accordance with the first data transfer standard or the second data transfer standard.
Owner:SONY ERICSSON MOBILE COMM AB
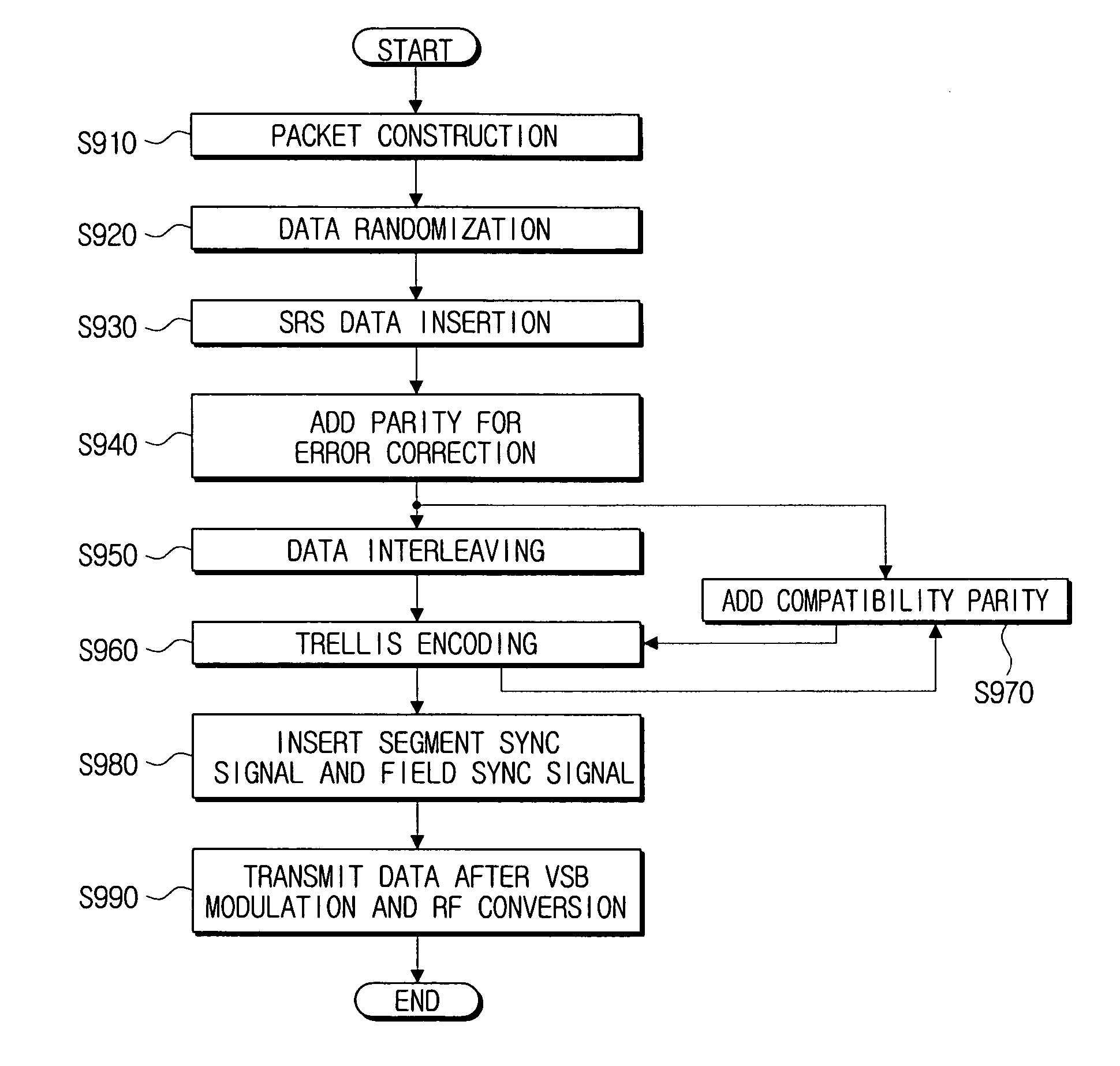
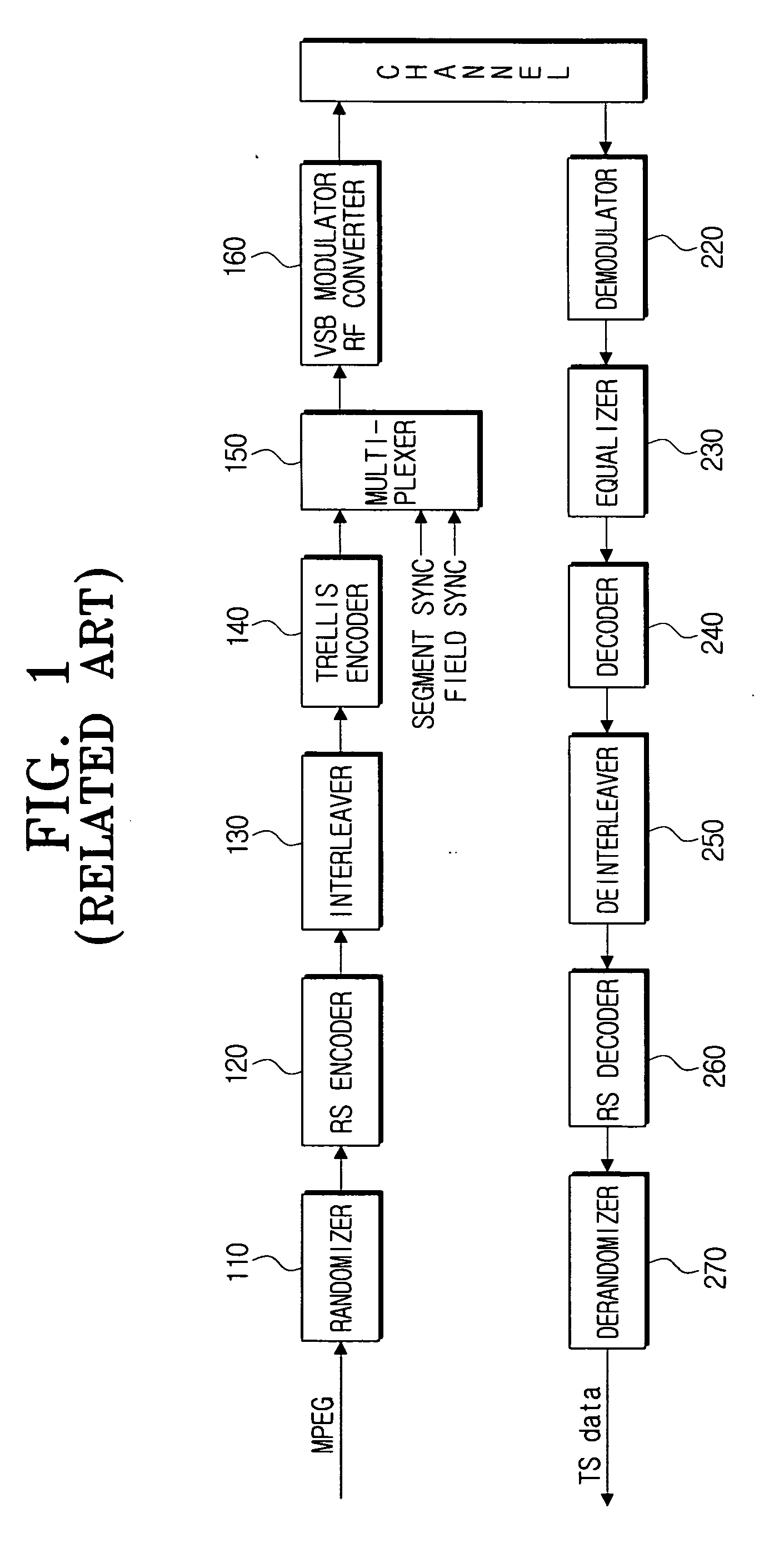
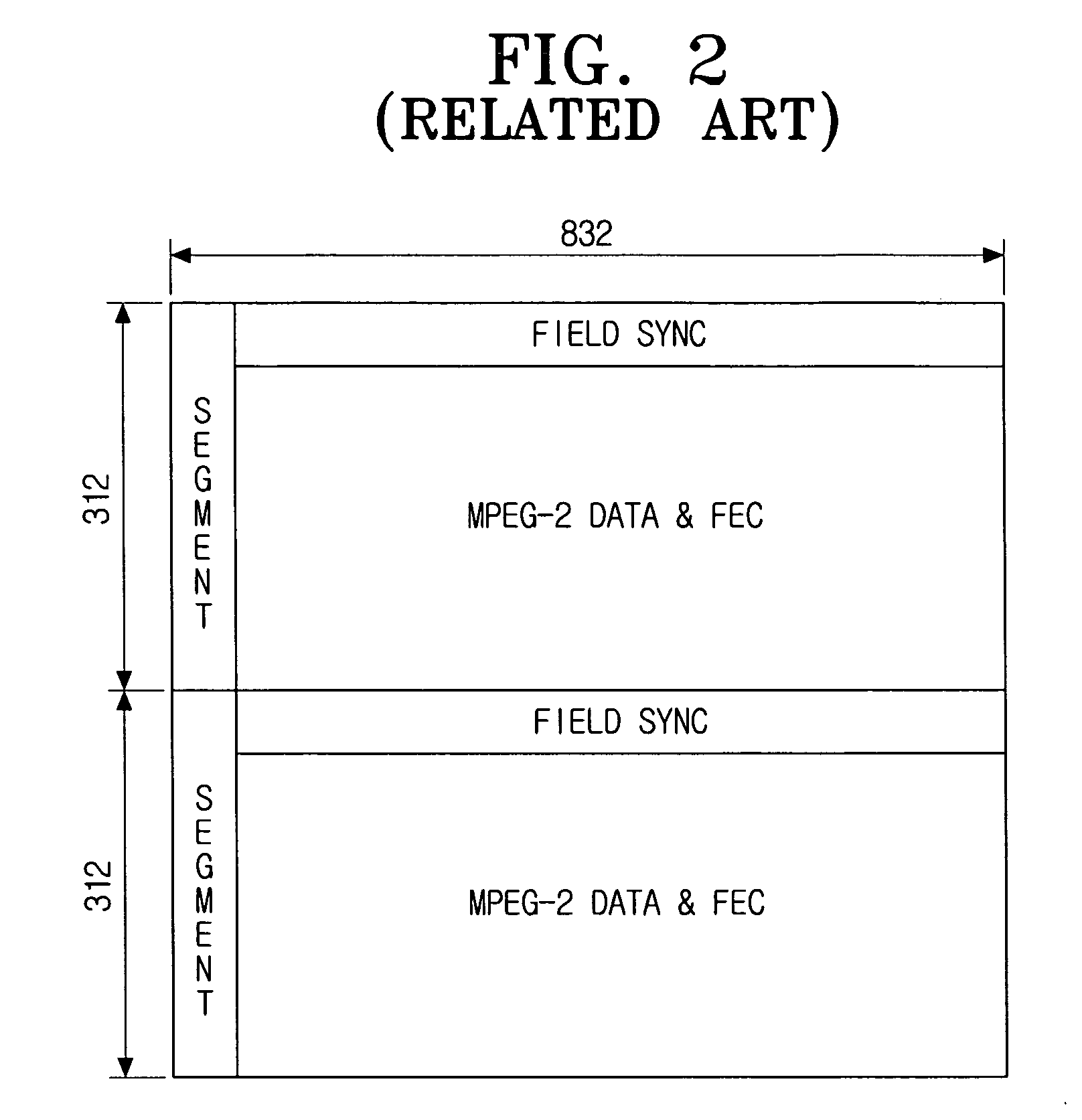
Method for formatting digital broadcast transport stream packet for improved receiving performance, digital broadcast transmitter, and signal processing method thereof
ActiveUS20060262863A1Maintain compatibilityPulse modulation television signal transmissionError correction/detection using concatenated codesSidebandBroadcast transmitter
A method of formatting a digital broadcast transport stream packet, a digital broadcast transmitter, and a signal processing method thereof, includes constructing a transport stream packet that includes a stuffing region for an insertion of a known supplementary reference signal (SRS) data therein, randomizing the packet that includes the stuffing region is randomized, and the SRS data is inserted into the stuffing region of the randomized packet. Adding a parity for an error correction to the packet into which the SRS data has been inserted, the packet to which the parity has been added is interleaved, and a trellis encoding of the interleaved packet is performed. Inserting a segment sync signal and a field sync signal into the trellis-encoded packet, and a vestigial side band (VSB) modulation and an RF conversion of the packet are performed to transmit the VSB-modulated and RF-converted packet.
Owner:SAMSUNG ELECTRONICS CO LTD
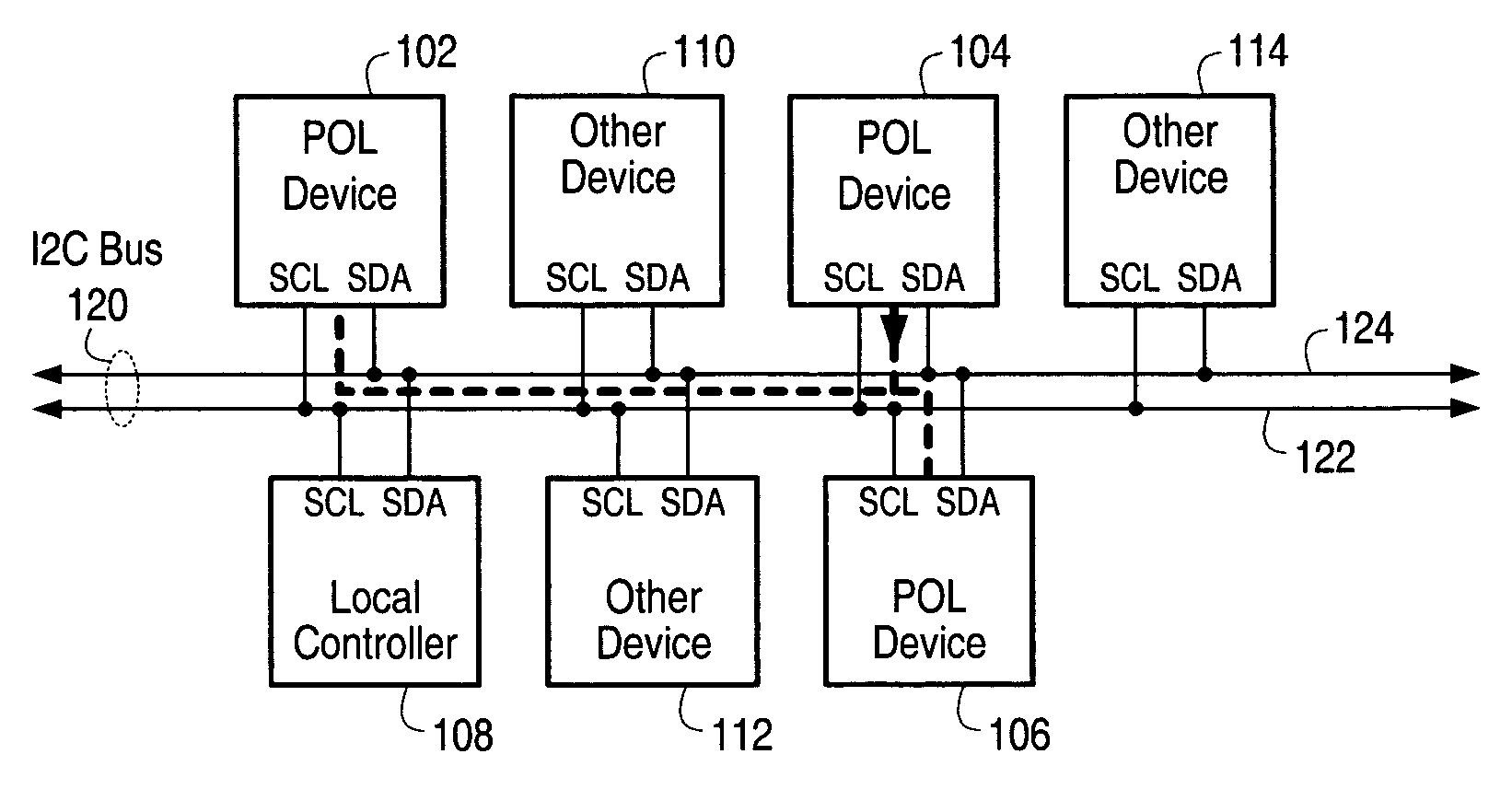
Method for using a multi-master multi-slave bus for power management
ActiveUS7653757B1Maintain compatibilityHardware monitoringPower supply for data processingProcessor registerAnalog signal
In one set of embodiments, a power management system comprises two or more devices, such as POL devices, configured to transmit and receive data over a shared bus, such as an I2C bus, according to the bus protocol of the shared bus. Each device may be configured with at least one respective address register, which may be programmed with an address uniquely identifying the device, and a mask register that may be configured to mask select bits of the respective address register, thereby enabling the device to identify device groups. In one embodiment, one of the devices identifying itself as a master device may distribute information to any of the other devices by transmitting the information, which may include commands and / or data, to itself, in effect targeting the address programmed into its own address register. The devices on the shared bus may be configured to monitor the bus for events, and respond to each event according to the requirements inherent within a transmitted command, thereby performing the necessary tasks to enable power management functions without the need for interconnecting analog signal lines.
Owner:INTERSIL INC
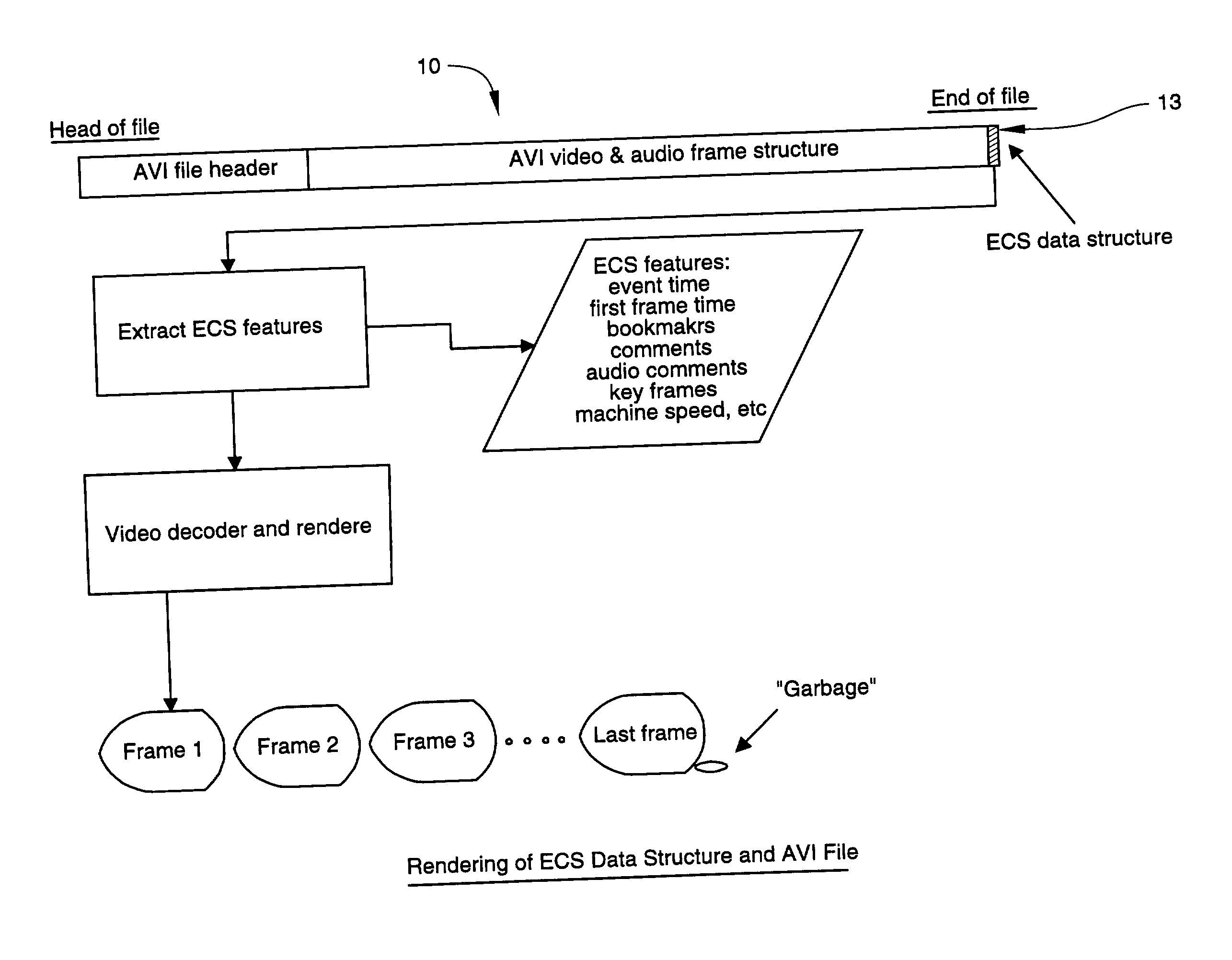
Video event capturing system and method
InactiveUS20040052501A1Improving data transportabilityGood transportabilityTelevision system detailsColor television signals processingDigital videoReverse order
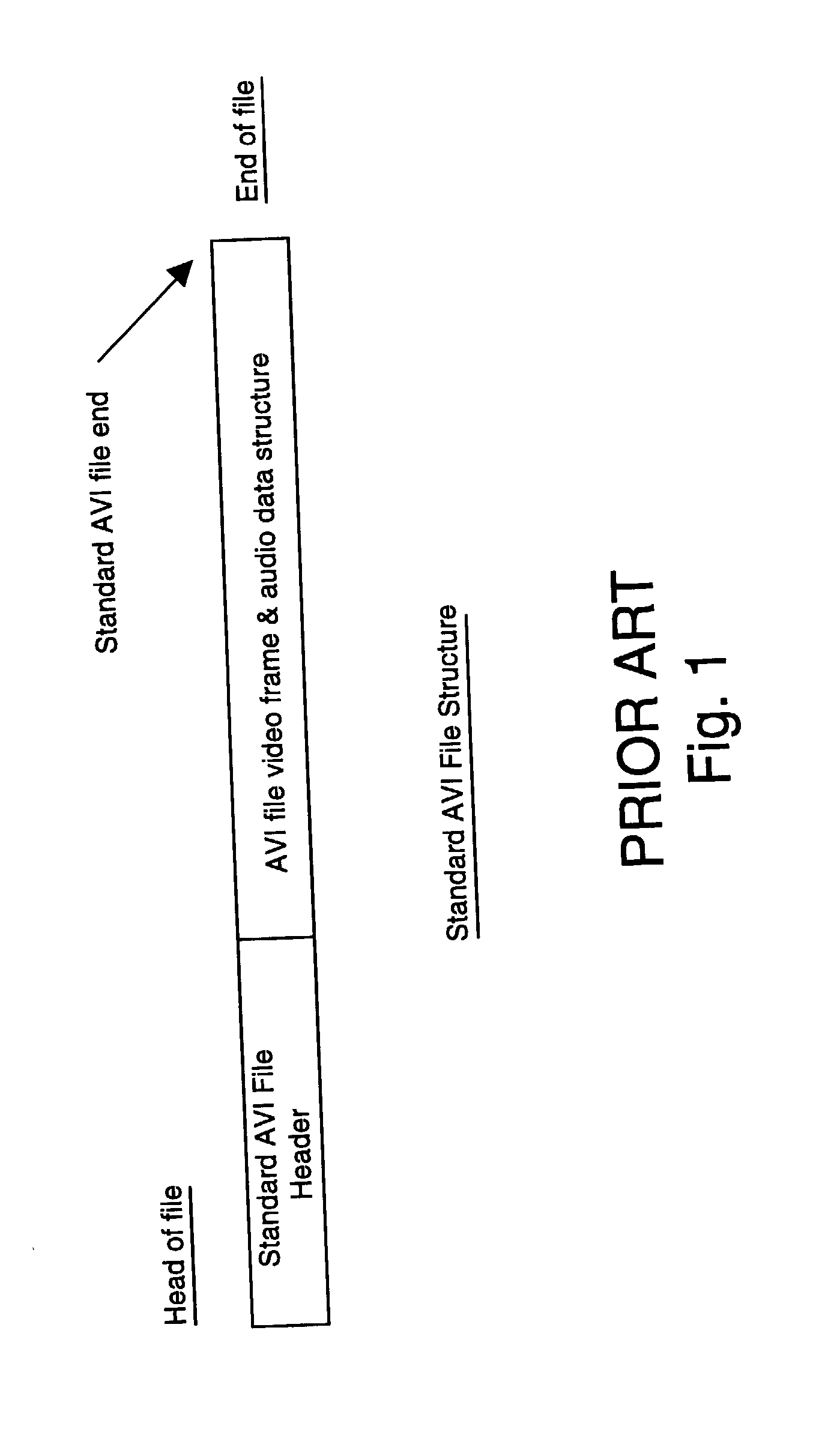
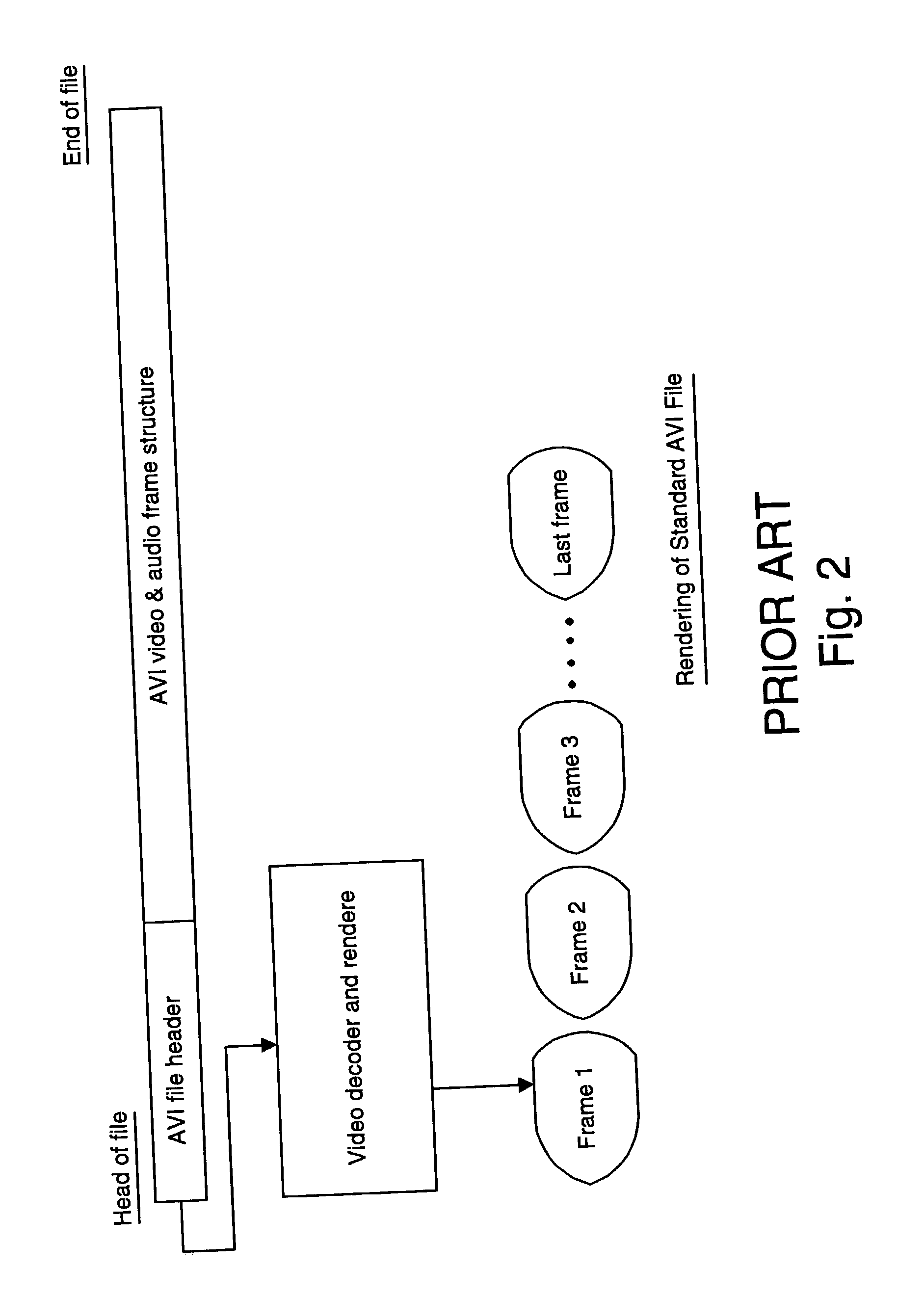
A process monitoring and event capturing system, including at least one video detector for monitoring a process and outputting a video signal and at least one event signal representing an event condition of the process being monitored. A recorder records the video signal and the event signal in a digital video file of a digital storage device having a predetermined data structure. The video file is structured to include at least one event feature data structure at the end of the video file for storing the event signal representative of the captured event condition. The digital storage device includes a temporary data file for storing video segments for examination by the user. The file format structure stores event data from the end of the data file forward in reverse order, permitting additions of data without affecting the previous file structure.
Owner:TAM EDDY C
Communication method, communication system, transmission method, transmission apparatus, receiving method and receiving apparatus
InactiveUS20070296859A1Maintain compatibilityTransmission easilyTelevision system detailsPicture reproducers using cathode ray tubesCommunications systemComputer science
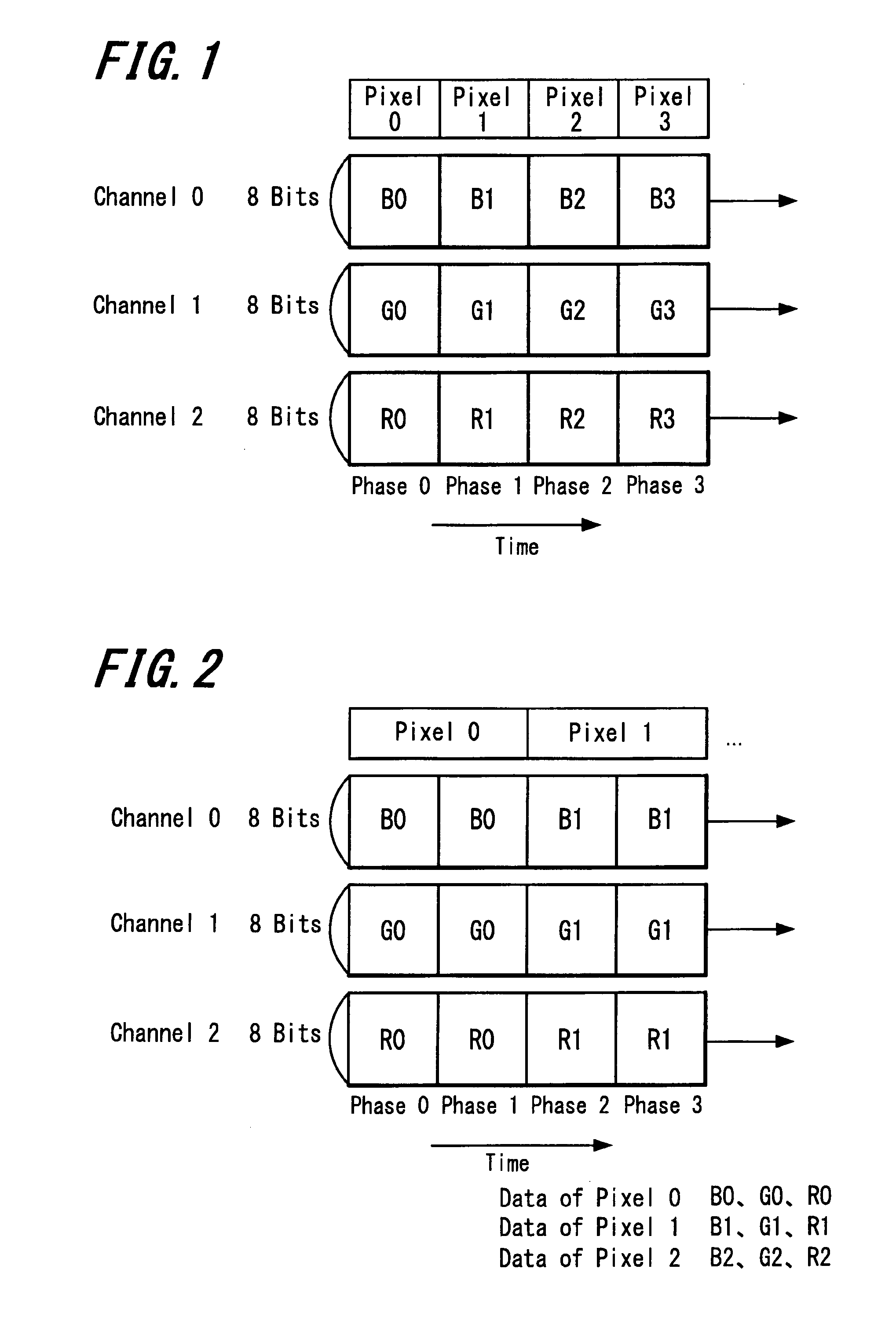
A communication method of transmitting video data which may include a predetermined number of bits as a unit from a source device to a sink device in sync with pixel clock and using individual transmission lines for respective color data or the like, may include preparing video data for three-dimensional display including the video data for a left eye and the video data for a right eye; forming the data for the left eye and for the right eye including the predetermined number of bits per pixel respectively; adding one of the data for the left eye and data for the right eye to the other thereof per pixel; forming the data of one pixel including twice the predetermined number of bits and transmitting the data at a timing in sync with the pixel clock; and transmitting the data for three-dimensional display from the source device to the sink device.
Owner:SONY CORP
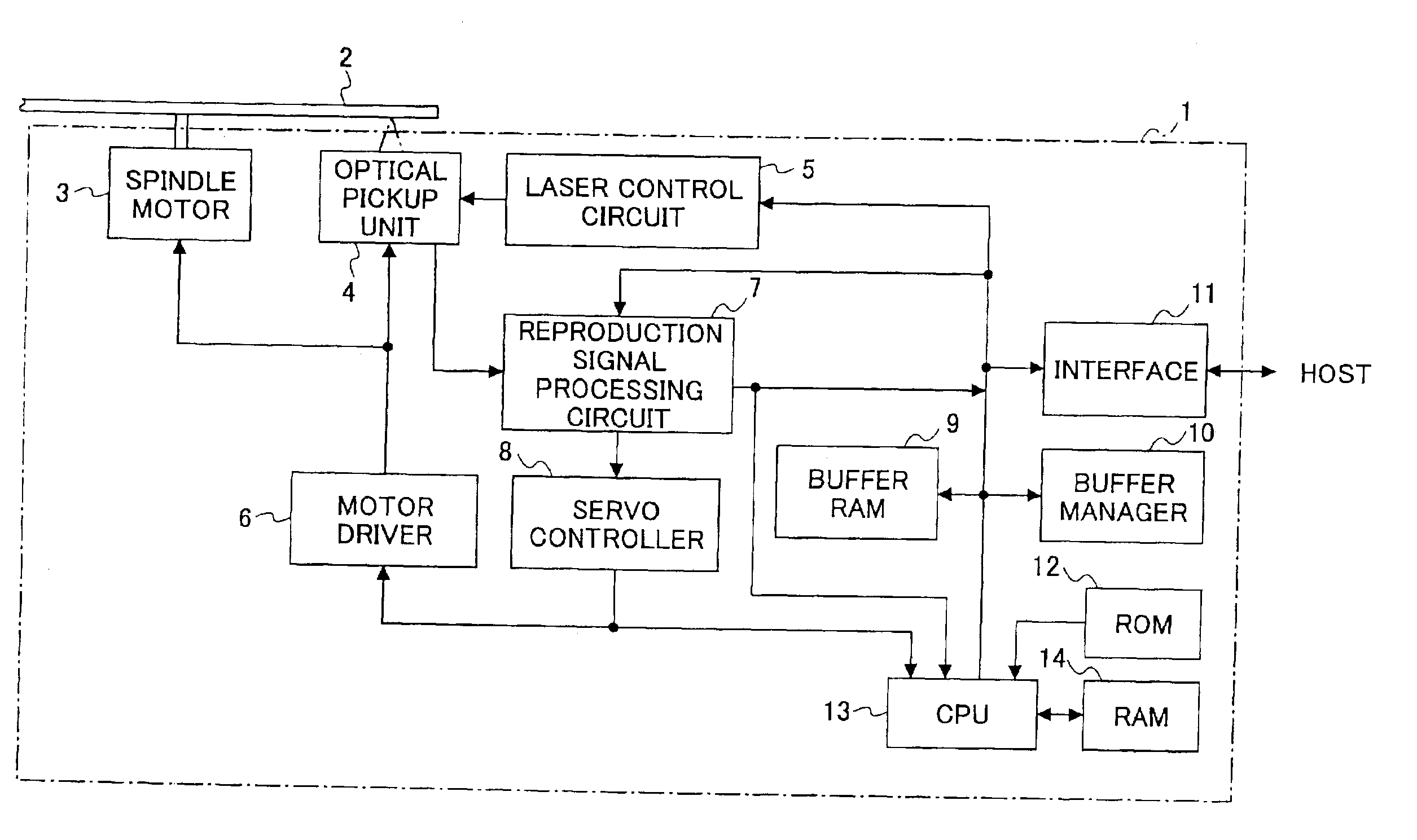
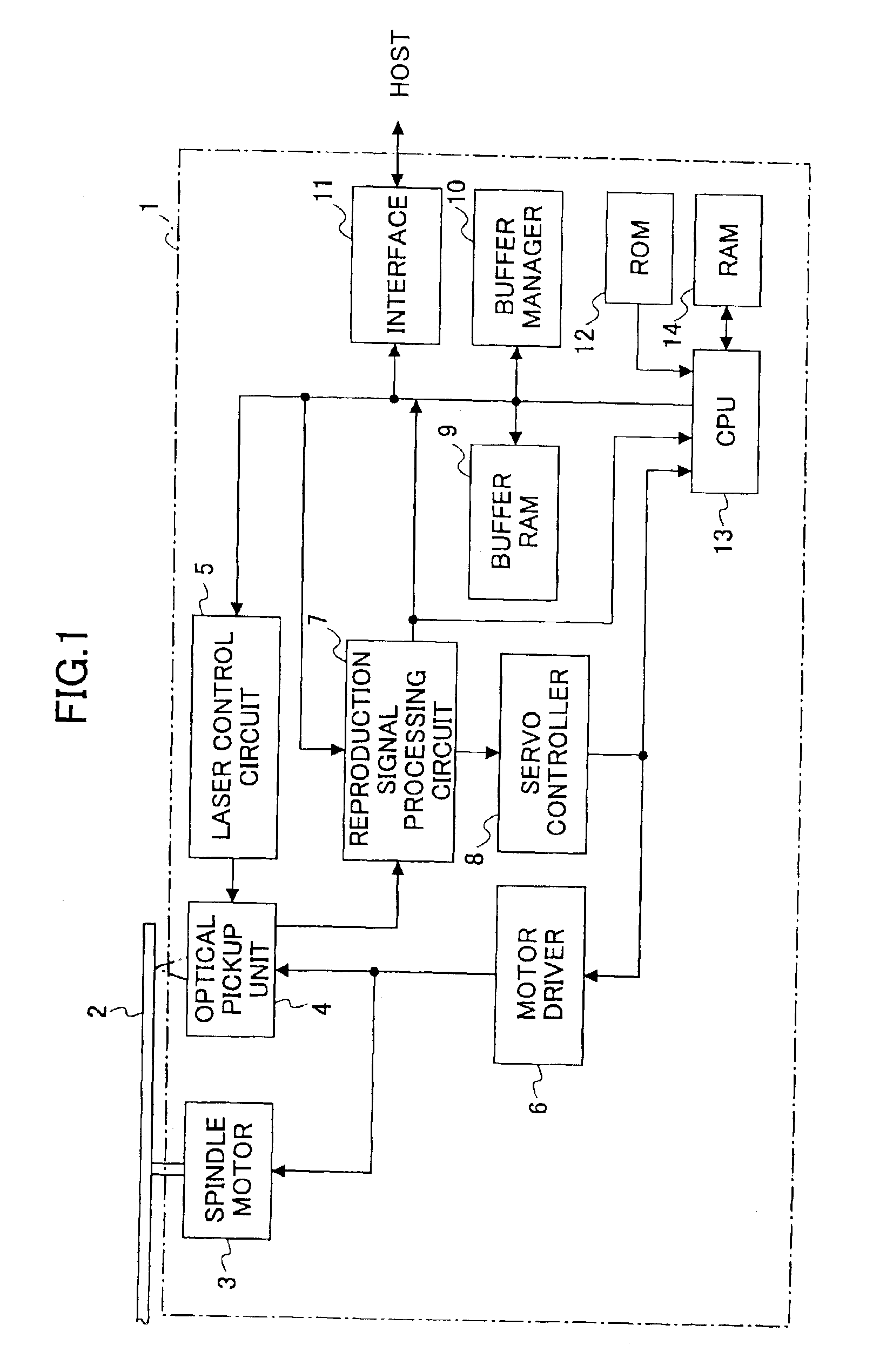
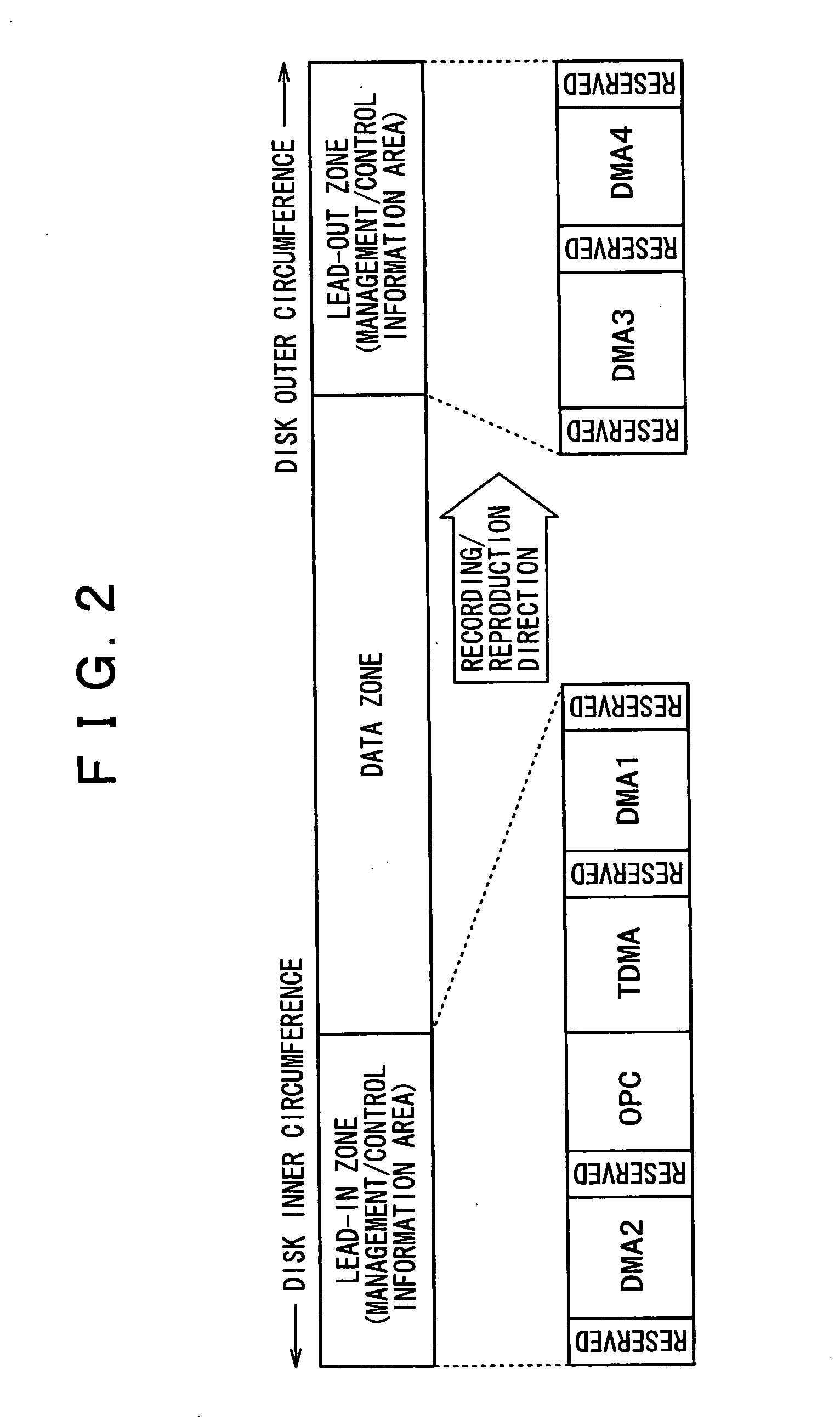
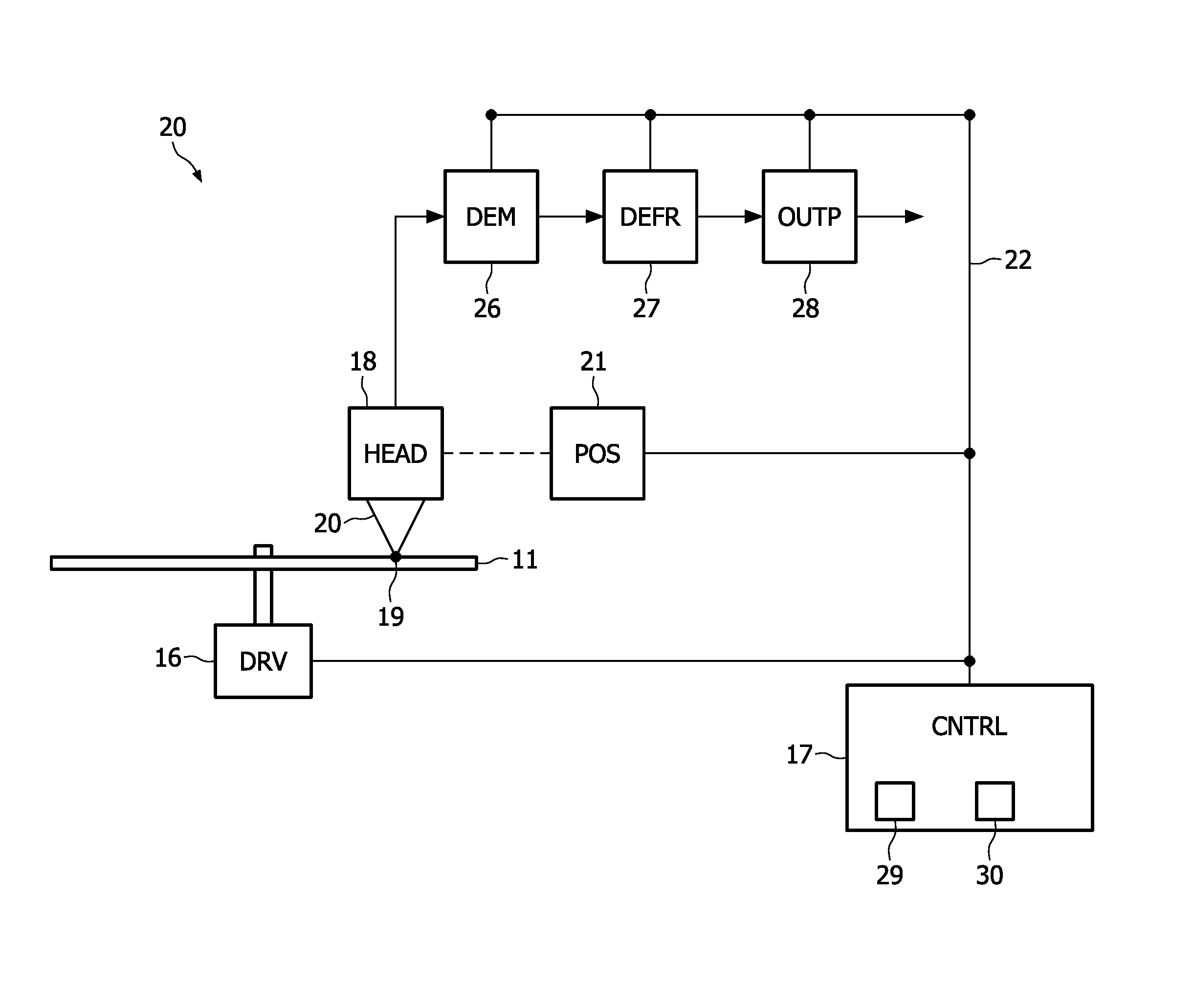
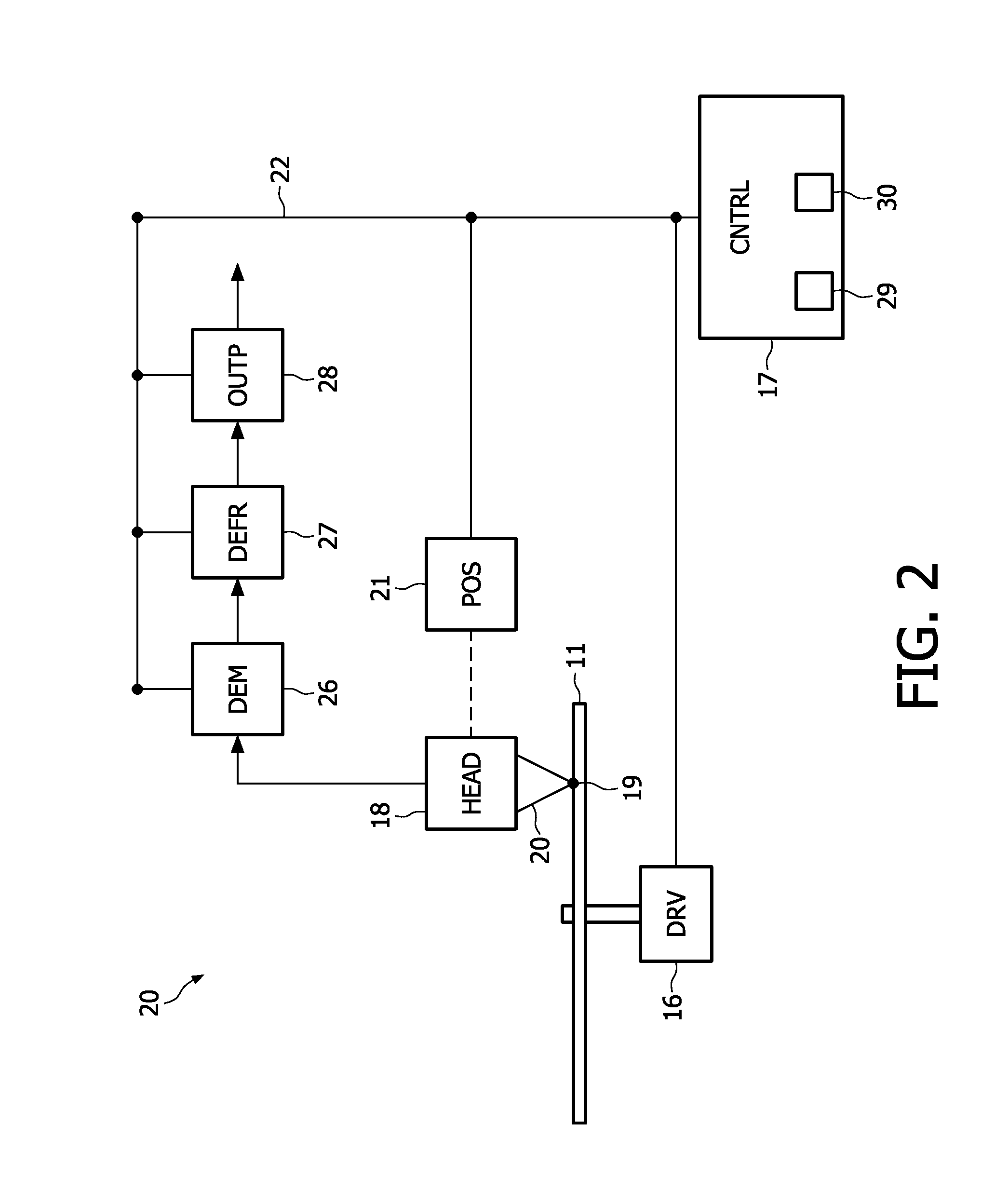
Information reproducing apparatus, data management information obtaining method, data management information obtaining program, and storage medium
InactiveUS6918003B2Without degrading performanceEasy to getTelevision system detailsRecord information storageData managementData recording
An information reproducing apparatus reproducing information of an information recording medium is disclosed. In the information recording medium, a record area is divided into a plurality of data areas. Also, data are recorded for each of the divided data areas. In addition, management information relating to the data recording is recorded in a predetermined management information area. Further, the management information is updated and recorded in a new management information area every time a predetermined data recording is completed. A receiving part receives, from an external device, an obtaining request for the management information. The obtaining request includes designation of a specific management information area. A management information obtaining part obtains the management information relating to the data recording from the specific management information area designated in the received obtaining request. A reporting part reports, to the external device, the management information relating to the data recording obtained from the designated specific management information area.
Owner:RICOH KK
Apparatus for and method of synchronization and beaconing in a WLAN mesh network
ActiveUS7564826B2Avoid collisionMaintain compatibilityAssess restrictionNetwork topologiesMesh pointTime synchronization
Owner:TEXAS INSTR INC
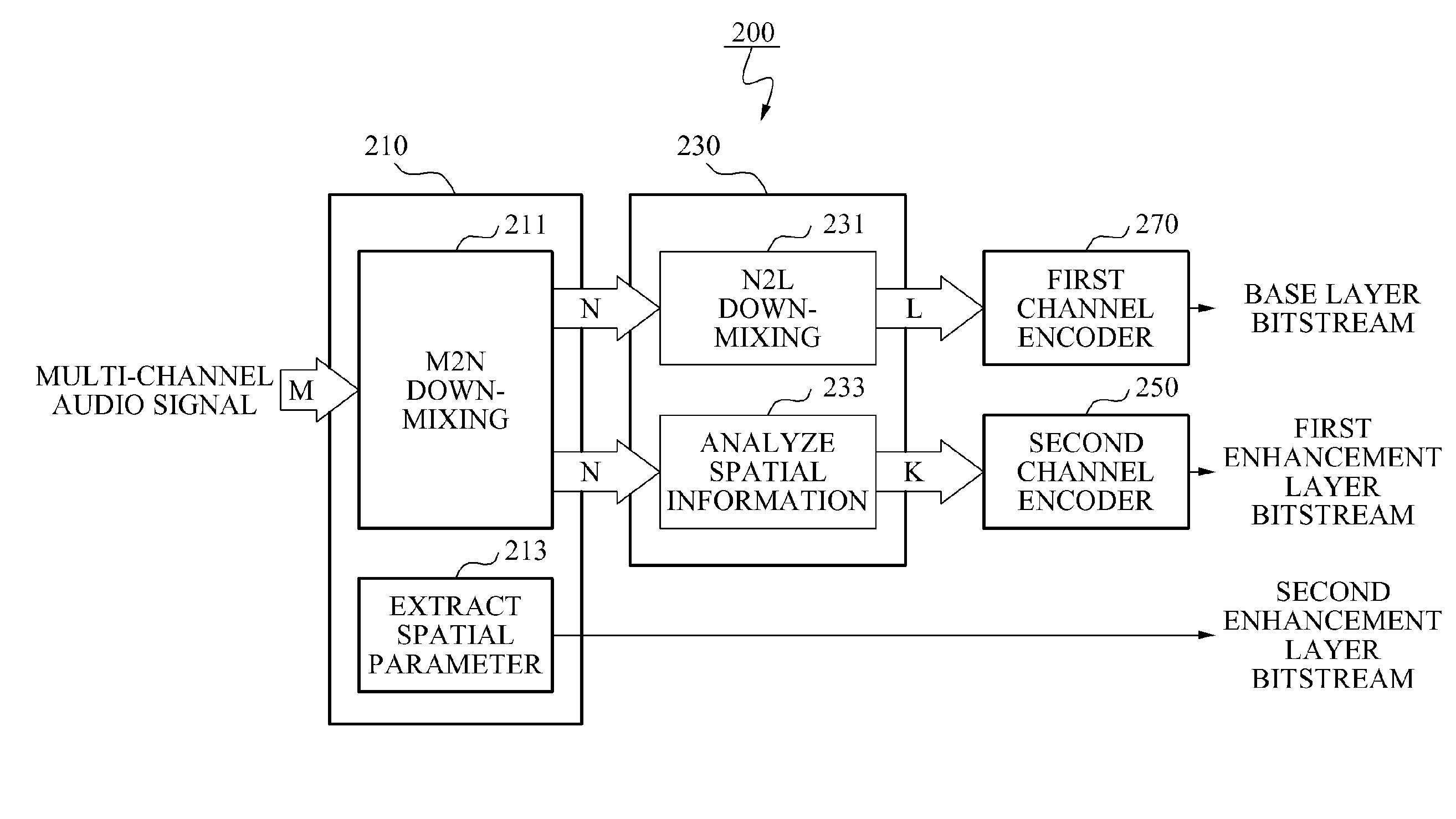
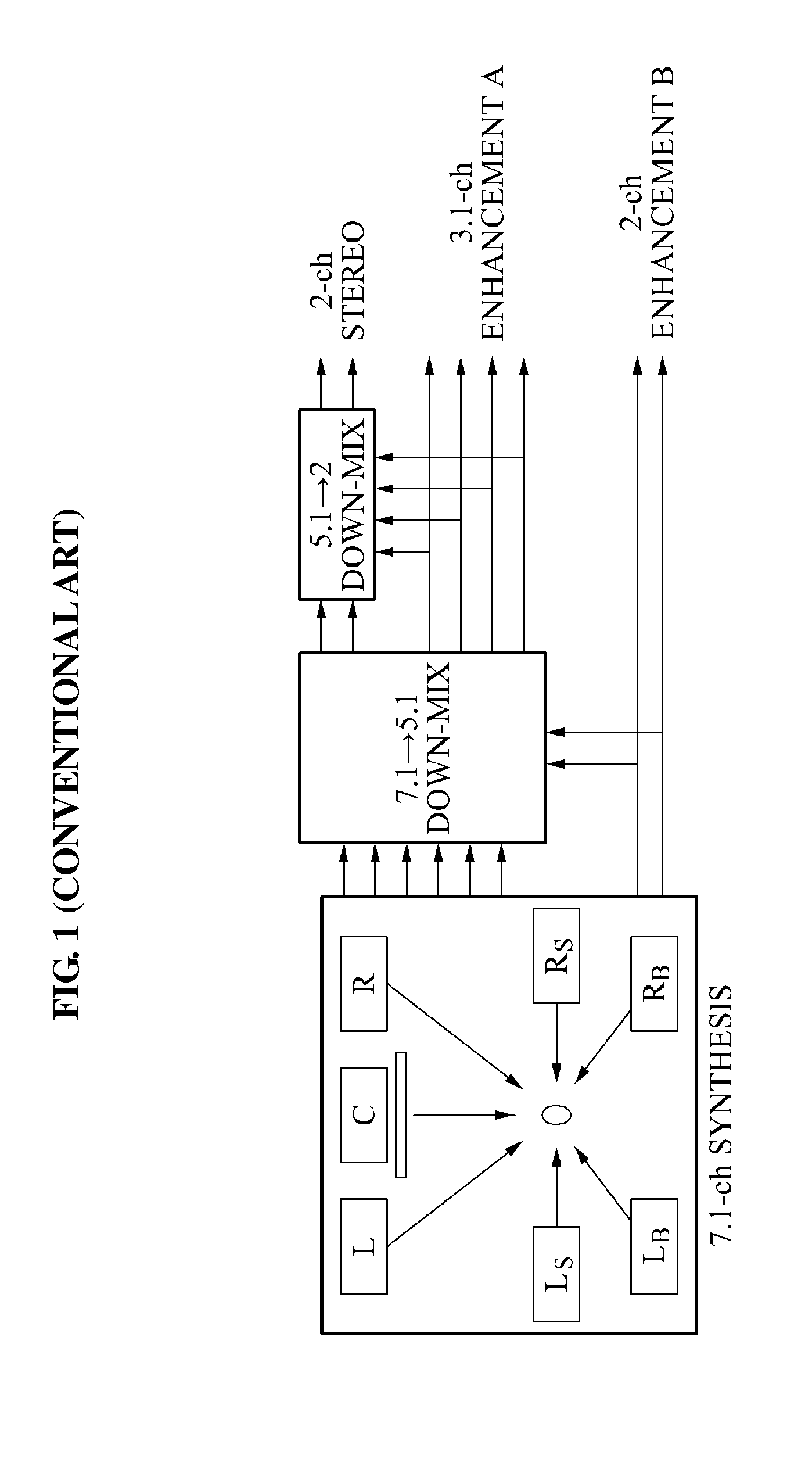
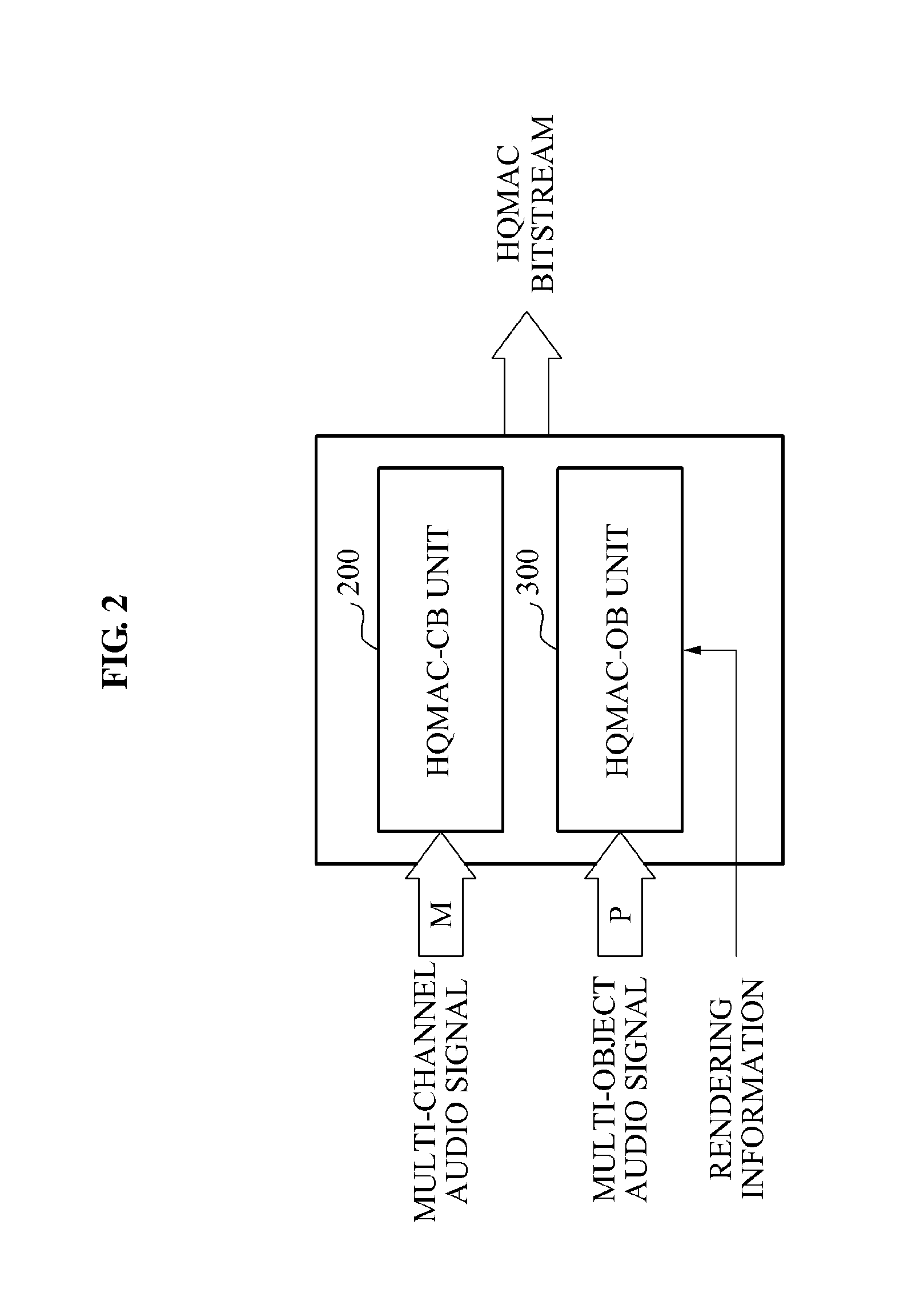
Encoding and decoding apparatuses for high quality multi-channel audio codec
InactiveUS20100324915A1Maintain compatibilityReduce bandwidthSpeech analysisTransmissionAudio signalAudio frequency
Owner:ELECTRONICS & TELECOMM RES INST
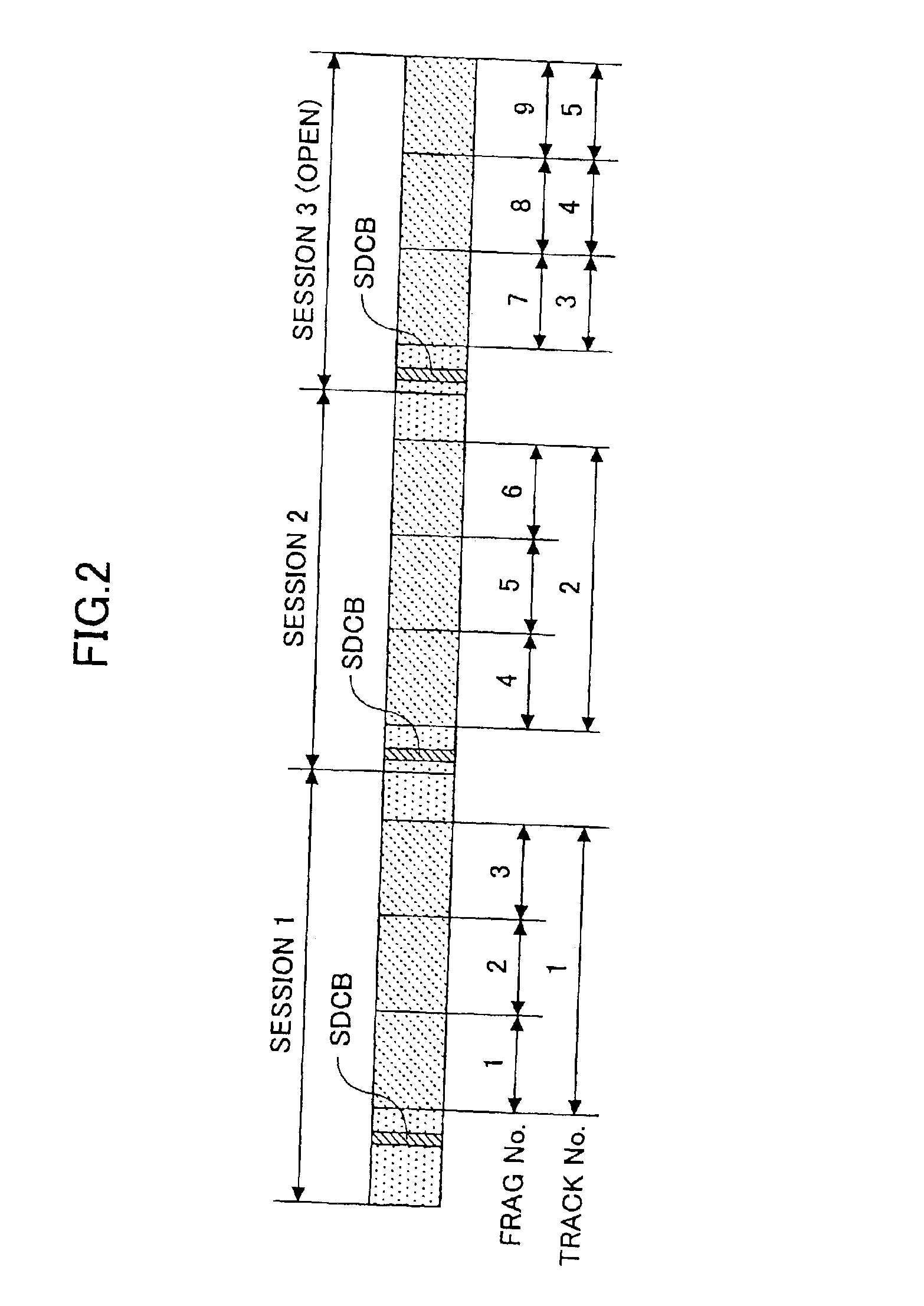
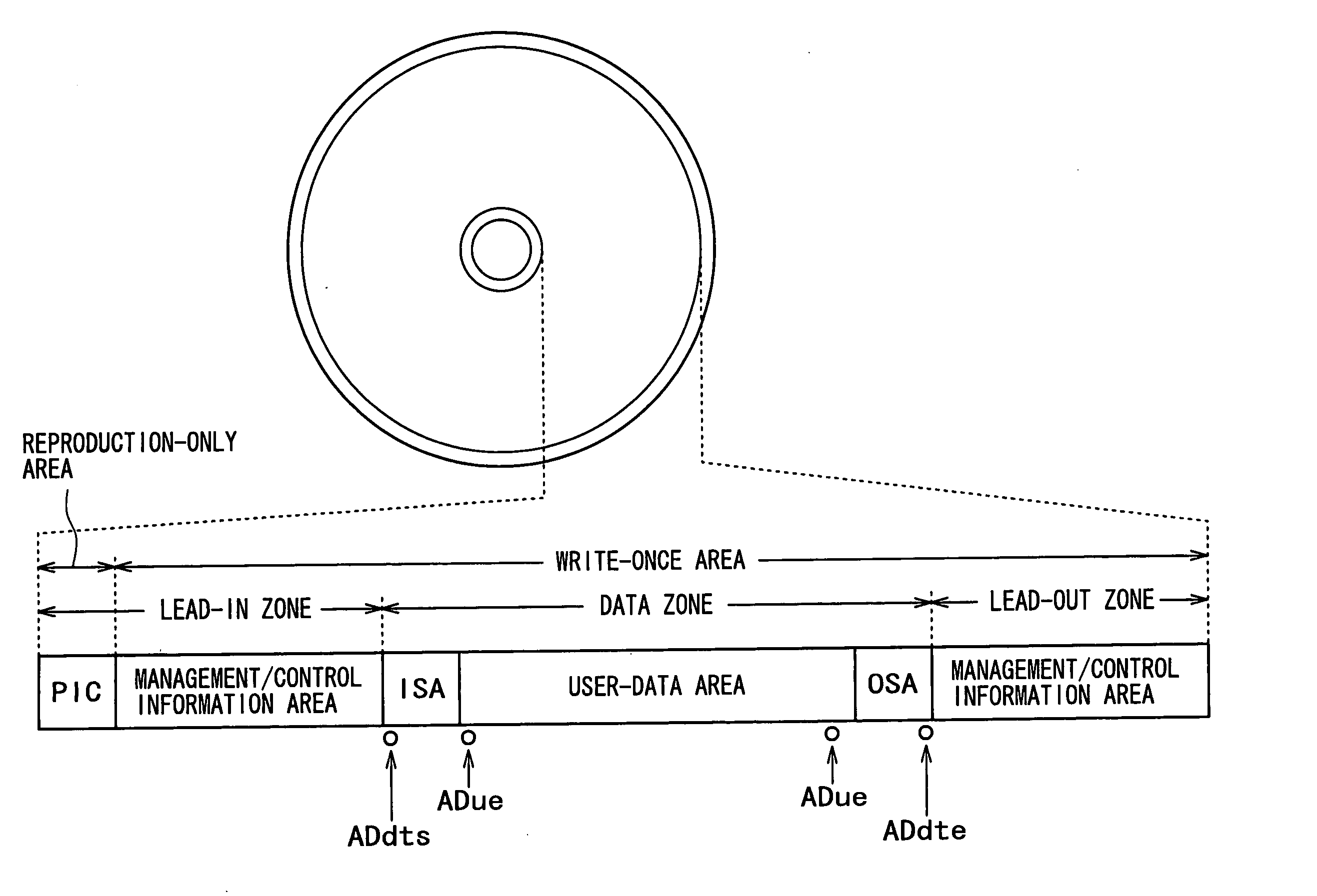
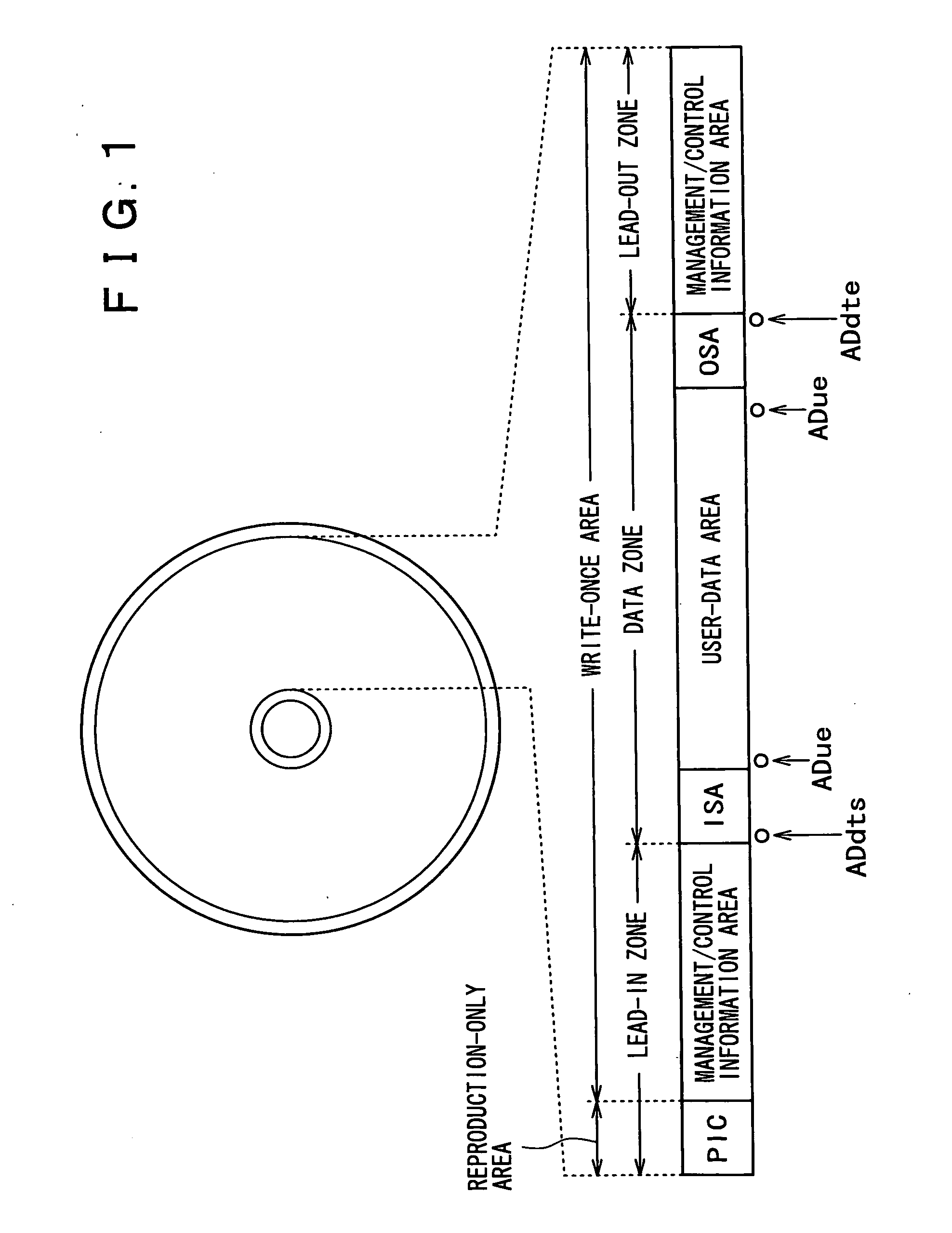
Recording medium, recording device, reproduction device, recording method and reproduction method
InactiveUS20050207262A1Easy to masterImprove efficiencyRecord information storageOptical erasing systemsUsabilityDatabase
In order to enhance the usability of a write-once recording medium, the recording medium is provided with an ordinary recording / reproduction area, an alternate area, a first alternate-address management information area and a second alternate-address management information area. In addition, written / unwritten state indication information is recorded in a predetermined area. The second alternate-address management information area is an area allowing alternate-address management information recorded therein to be renewed by adding alternate-address management information to the area. Alternate-address management information recorded in the second alternate-address management information area includes information recorded in a first information format and information recorded in a second information format. The first information format shows an alternate source address and an alternate destination address for each data unit. On the other hand, the second information format shows an alternate source address and an alternate destination address for a group of a plurality of physically continuous data units. The second information format is used as a format for effectively carrying out an alternate-address process on such a group of data units. All alternate-address management information is recorded in the first alternate-address management information area in the first information format.
Owner:SONY CORP
3D mode selection mechanism for video playback
ActiveUS20100303442A1Easy to switchMaintain compatibilityTelevision system detailsAudio/video recordingComputer graphics (images)Mode selection
The invention relates to a signal comprising video information and associated playback information, the video information and associated playback information being organized according to a playback format, the video information comprising a primary video stream for two-dimensional (2D) display, and an additional information stream for enabling three-dimensional (3D) display, wherein that the associated playback information comprises display information indicating the types of display possible. The invention also relates to a method and device for playback of such a signal, the method comprising receiving the video information and the associated playback information, processing the display information to determine that both two-dimensional (2D) display possible and three-dimensional (3D) display are possible for the received video information; determining a playback setting of a playback device indicating whether the video information should be displayed two-dimensional (2D) or three dimensional (3D); and processing for display either the primary video stream or the primary video stream and the additional information stream, in accordance with the playback setting of the playback device.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
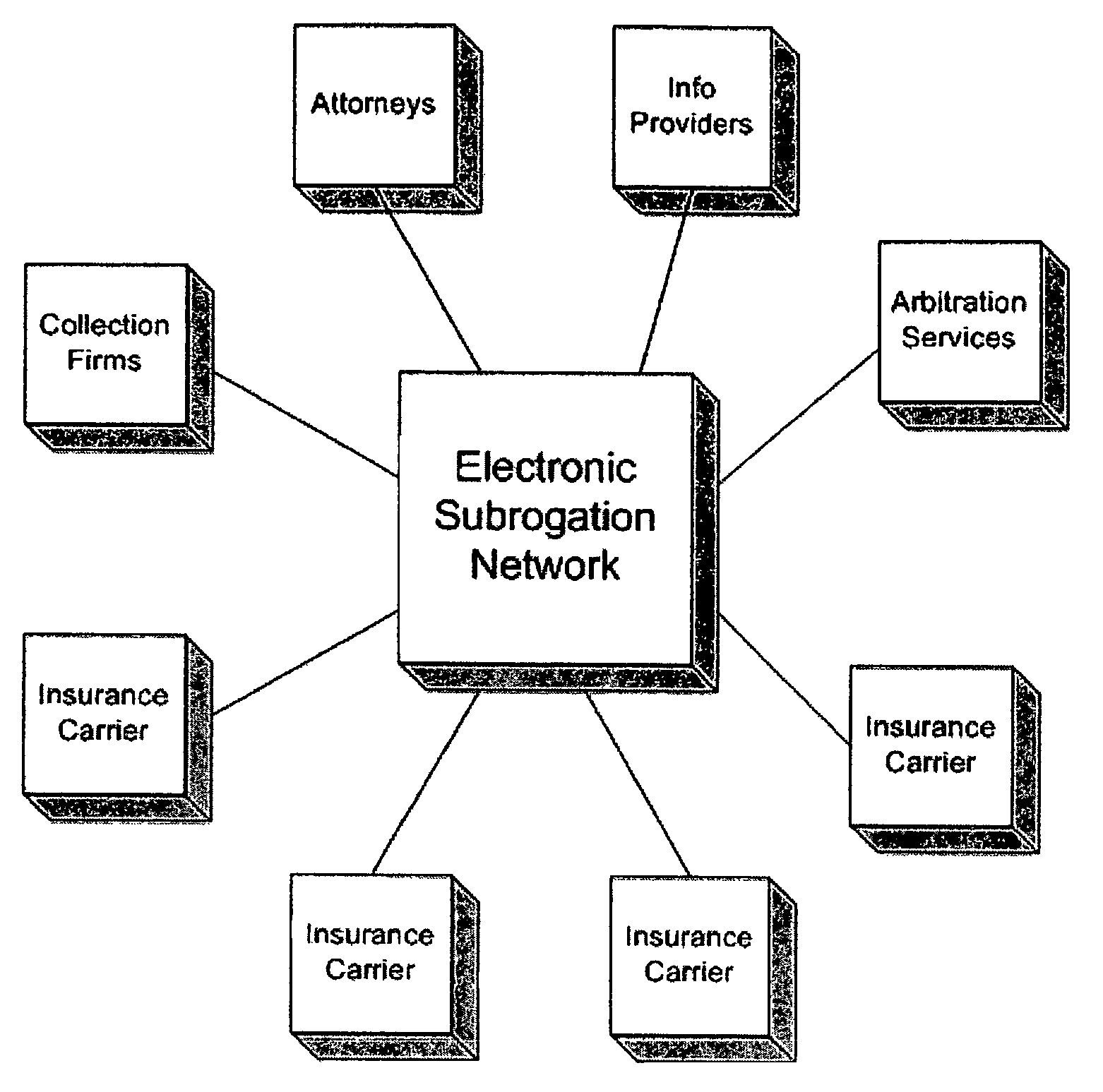
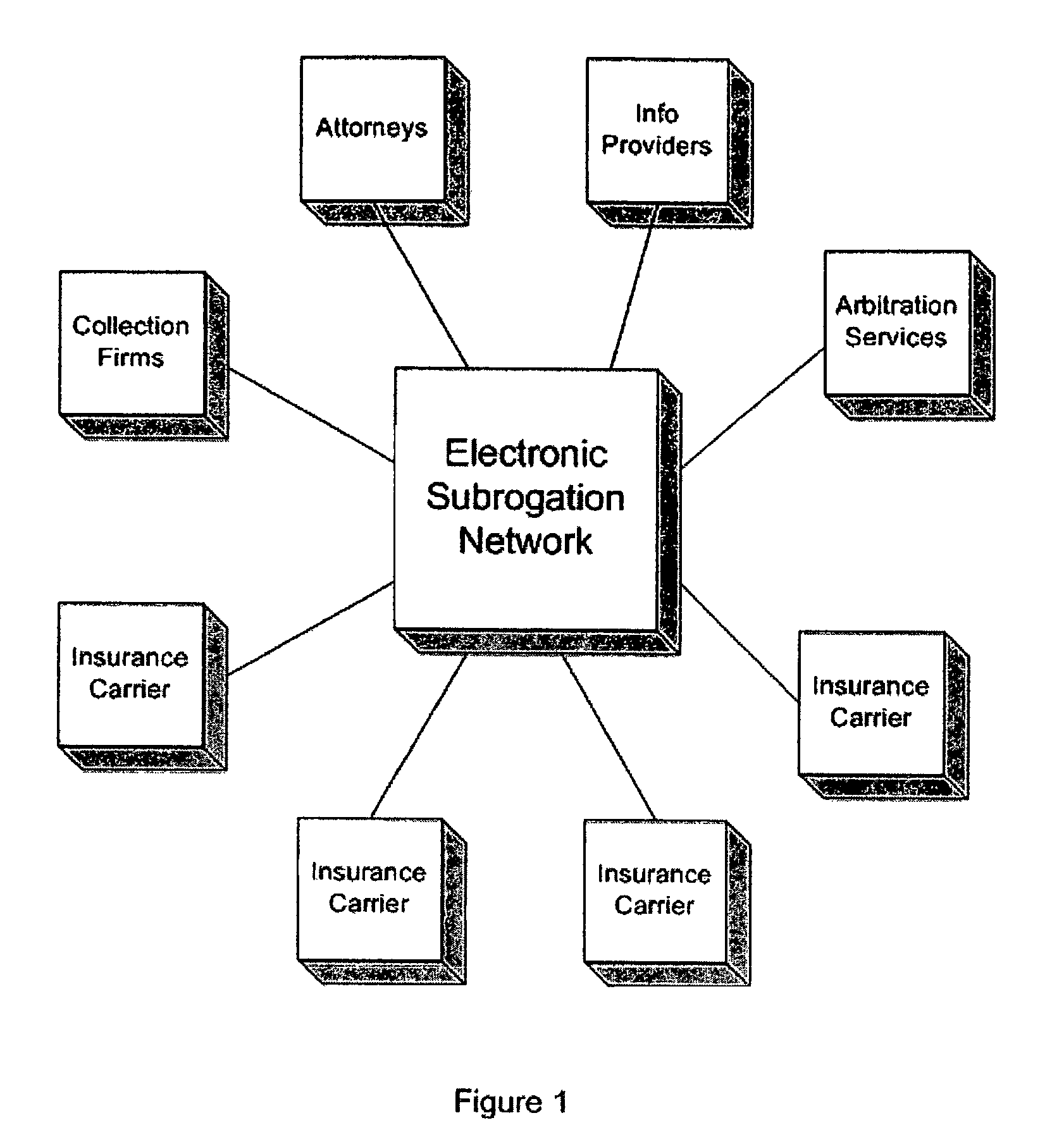
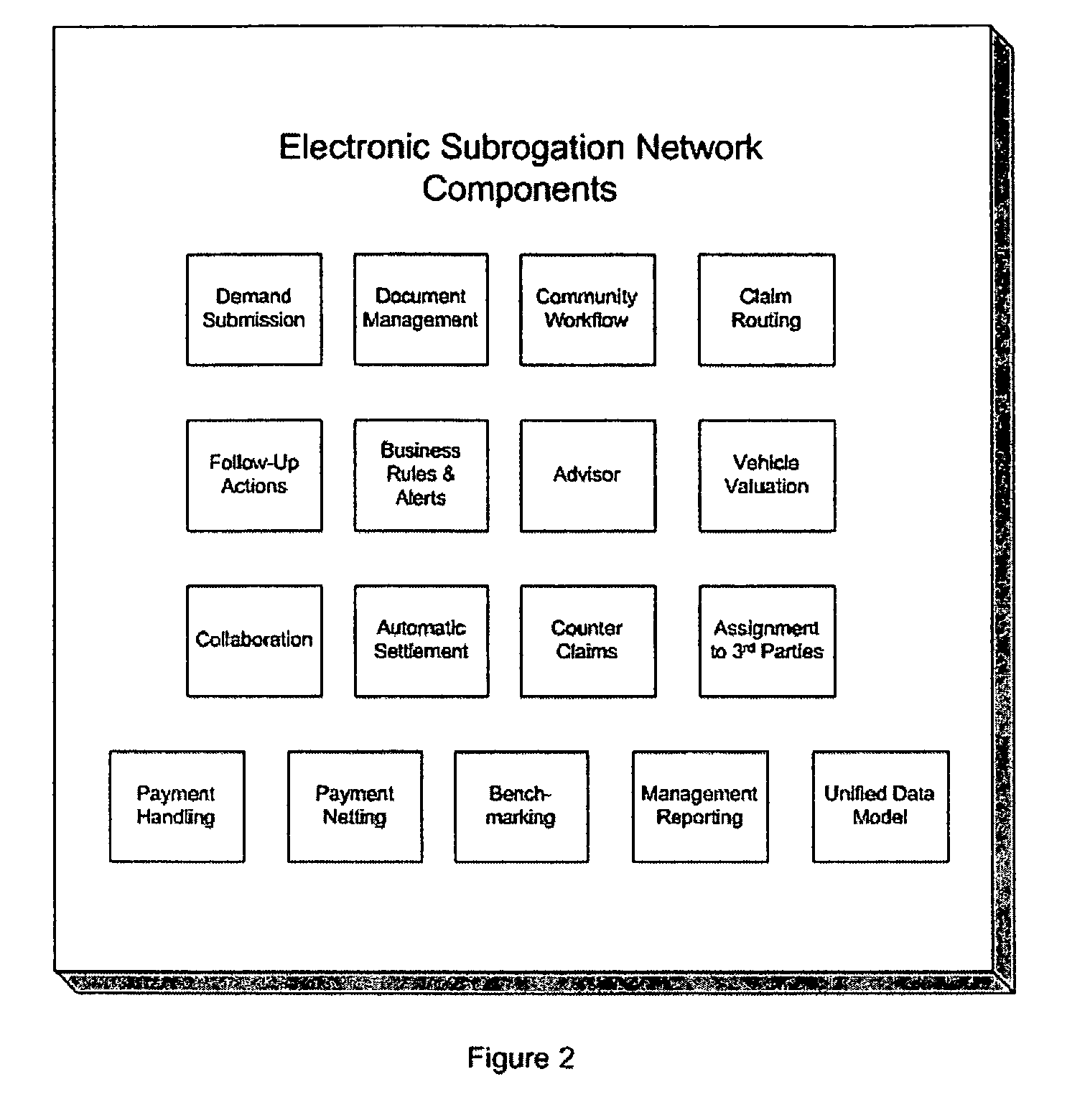
System and process for electronic subrogation, inter-organization workflow management, inter-organization transaction processing and optimized web-based user interaction
ActiveUS7962385B2Reduced processing power requirementsReduce message sizeFinanceOffice automationInter organizationalTransaction processing system
An intelligent electronic subrogation network (“ESN”) automates intra-organization workflow, inter-organization workflow and collaboration for insurance subrogation. This ESN is facilitated by a novel system architecture and process that includes an inter-organizational workflow management system, an inter-organizational transaction processing system, and a unique mechanism for optimizing and enriching web-based user interaction within any such system.
Owner:ARBITRATION FORUMS
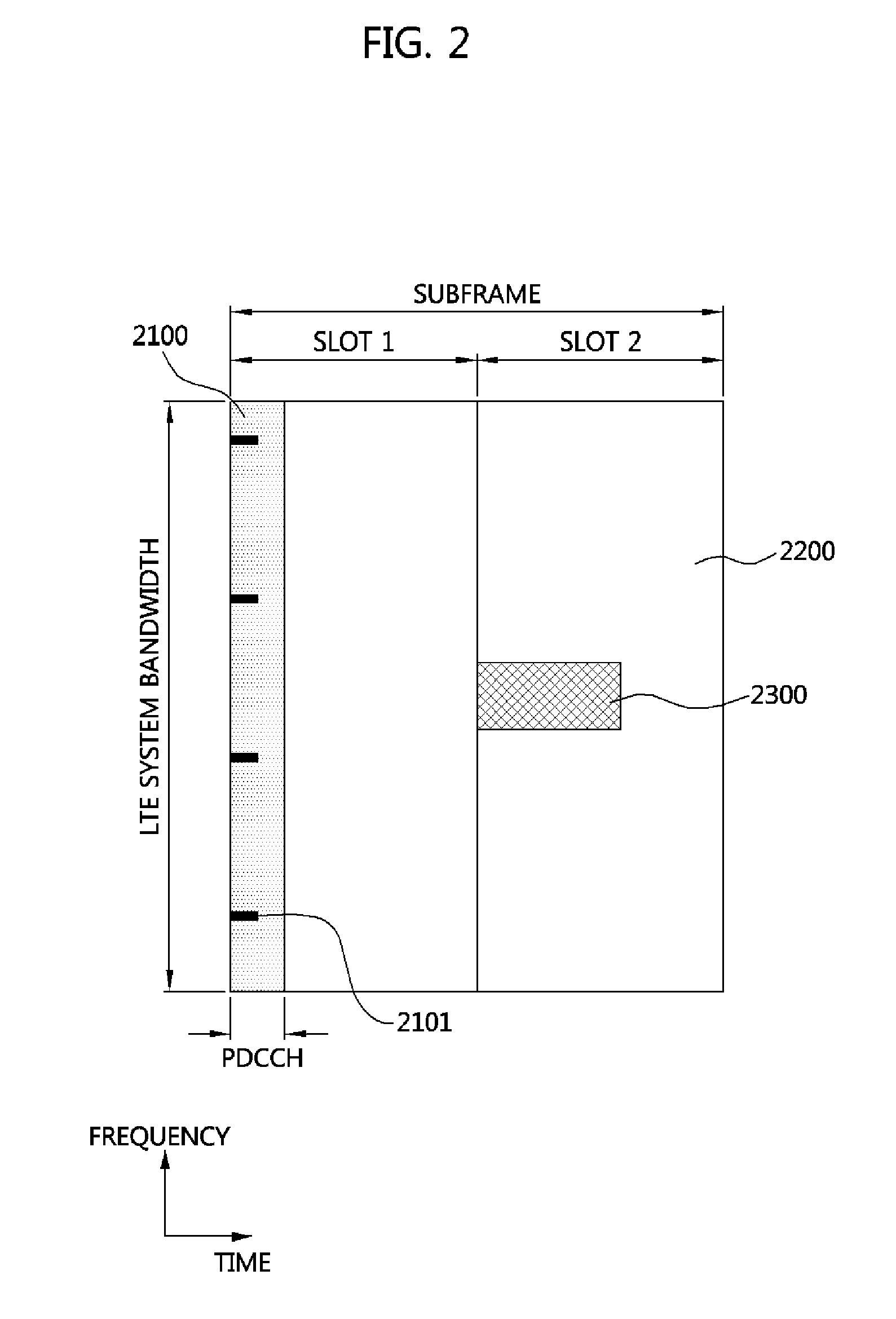
Method and apparatus for performing random access in a multi-carrier system
ActiveUS20110235609A1Maintain backward compatibilityShorten the timeTransmission path divisionSignal allocationCarrier signalPreamble
A method of a user equipment performing random access in a multi-carrier system comprises receiving mapping information through downlink component carriers, sending a random access preamble through uplink component carriers, and receiving a random access response through a specific downlink component carriers determined based on the mapping information.
Owner:LG ELECTRONICS INC
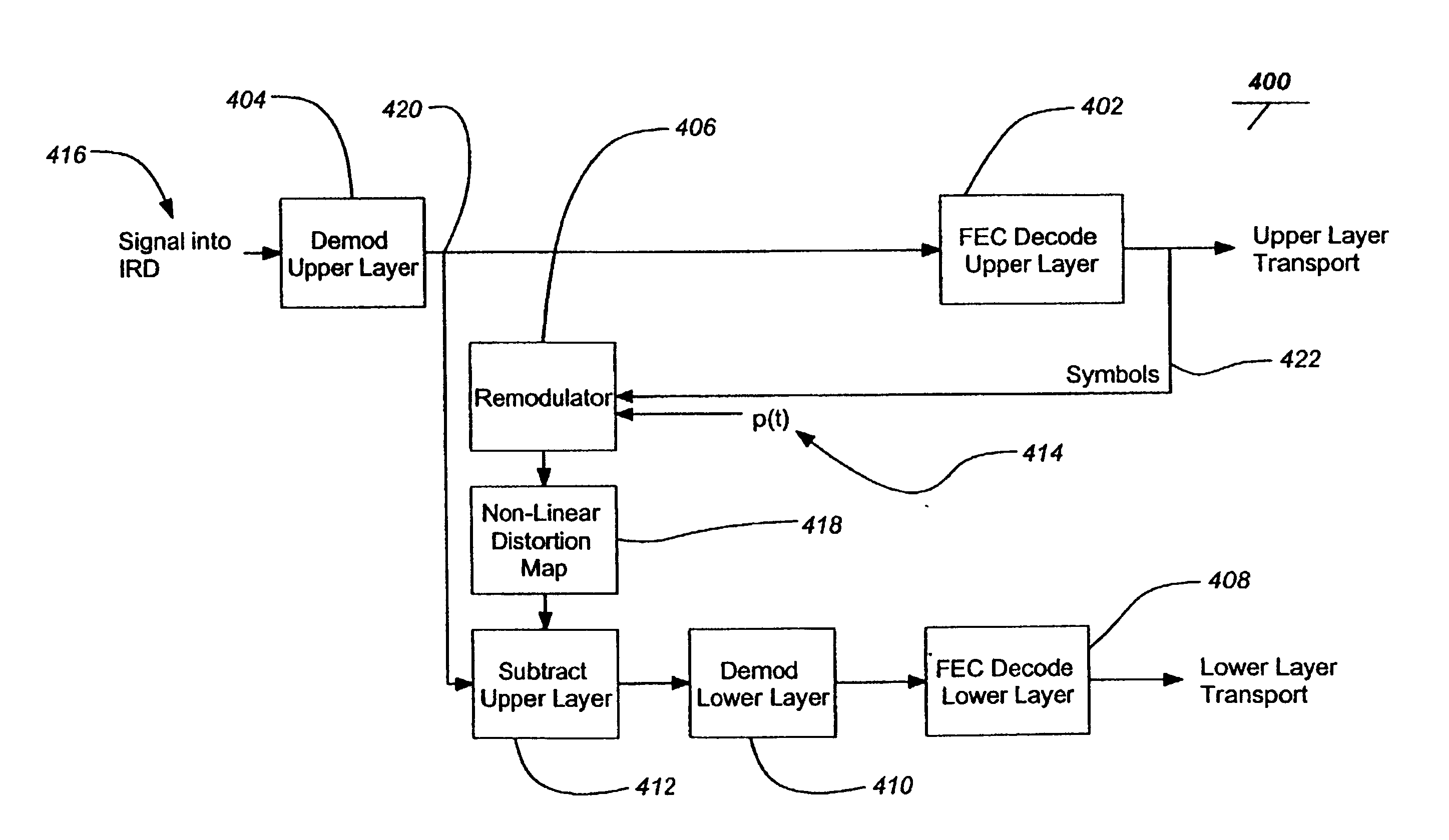
Layered modulation for digital signals
InactiveUS7209524B2Increase capacityGood serviceError detection/prevention using signal quality detectorResource management arrangementsCarrier signalDigital signal
Signals, systems and methods for transmitting and receiving layered modulation for digital signals are presented. A layered signal for transmitting data, comprises a first signal layer including a first carrier and first signal symbols for a first digital signal transmission and a second signal layer including a second carrier and second signal symbols for a second signal, transmission disposed on the first signal layer, wherein the layered signal has the first carrier demodulated and first layer decoded to produce the first signal symbols for a first layer transport, the first signal symbols are remodulated and subtracted from the layered signal to produce the second signal layer, and the second signal layer has the second carrier demodulated and decoded to produce the second signal symbols for a second layer transport.
Owner:THE DIRECTV GROUP
Method and an apparatus for stream conversion, a method and an apparatus for data recording, and data recording medium
InactiveUS20040208135A1Good compatibilityImprove convertibilitySpecial service provision for substationTelevision system detailsMultiplexingNetwork packet
For encoding externally input AV signal to MPEG-TS that enables quickly conversion from MPEG-TS to MPEG-PS, data unit (Multiplexing Unit) is defined which includes a plurality of packet and has data size corresponding to data amount of one pack in MPEG-PS, and MPEG-TS is encoded for each defined data unit. Furthermore, time stamp information (ATS) added to a packet of MPEG-TS which is converted to MPEG-PS and time stamp information (SCR) added to a packet of the converted MPEG-PS are correlated with a predetermined formula.
Owner:PANASONIC CORP +2
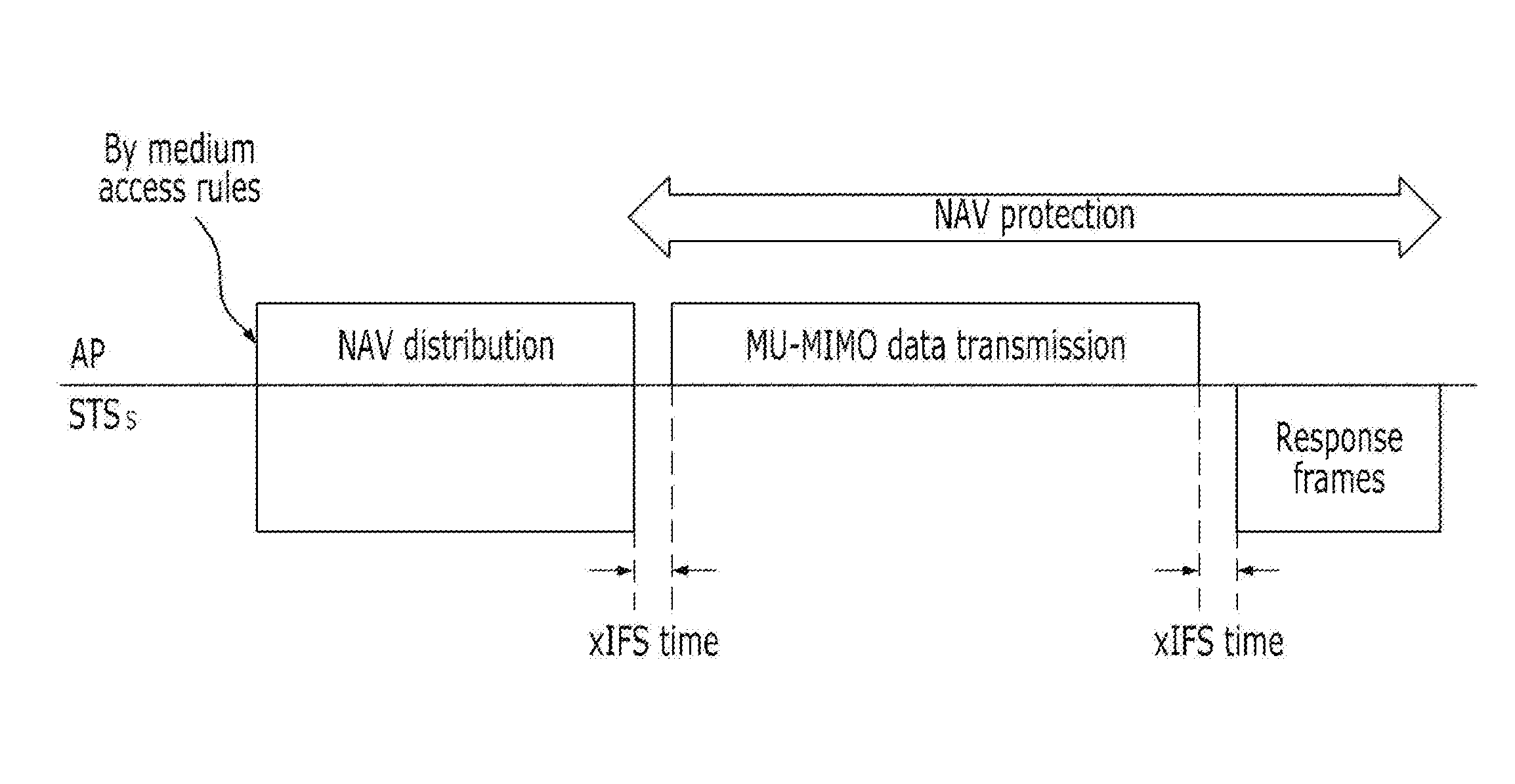
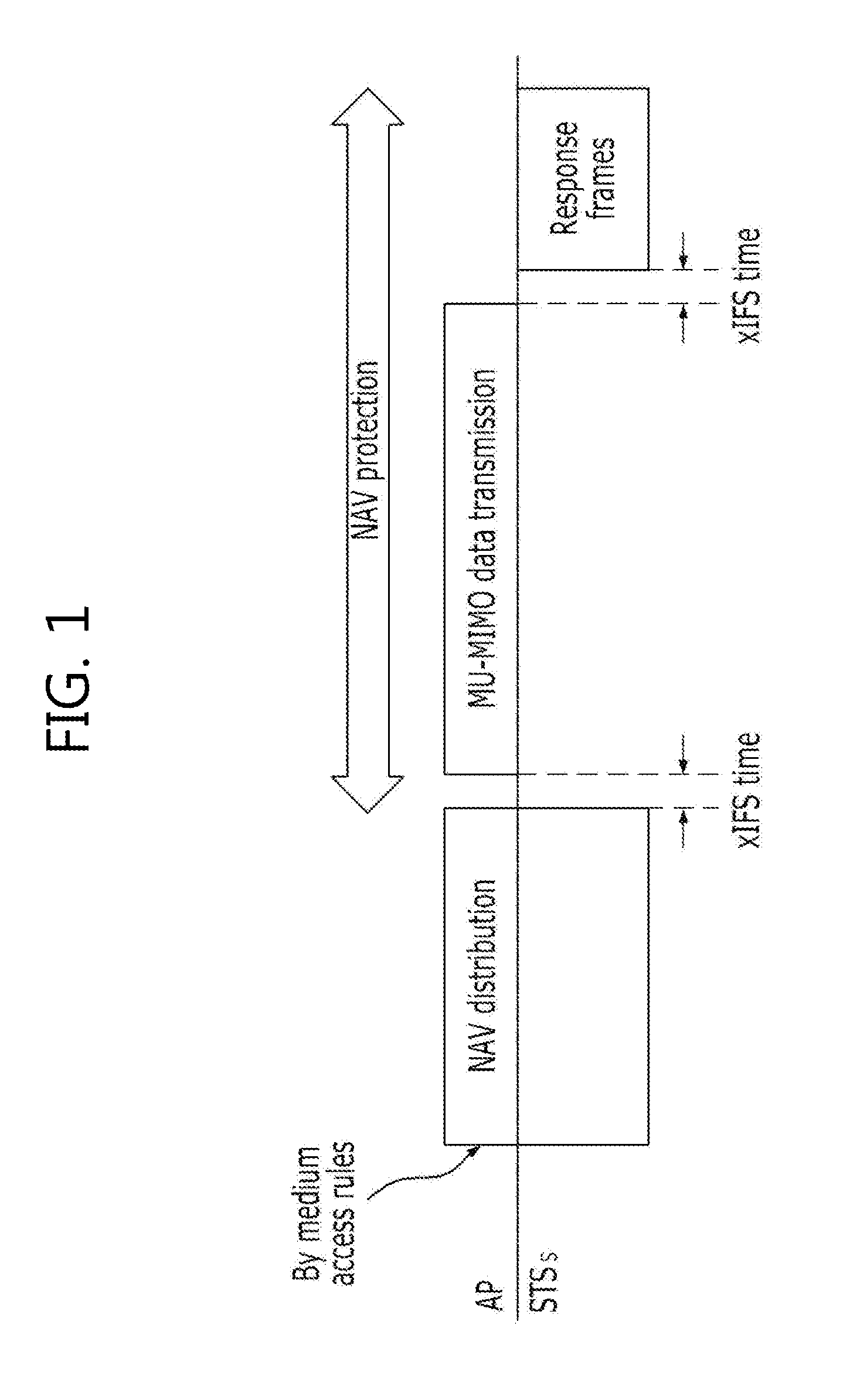
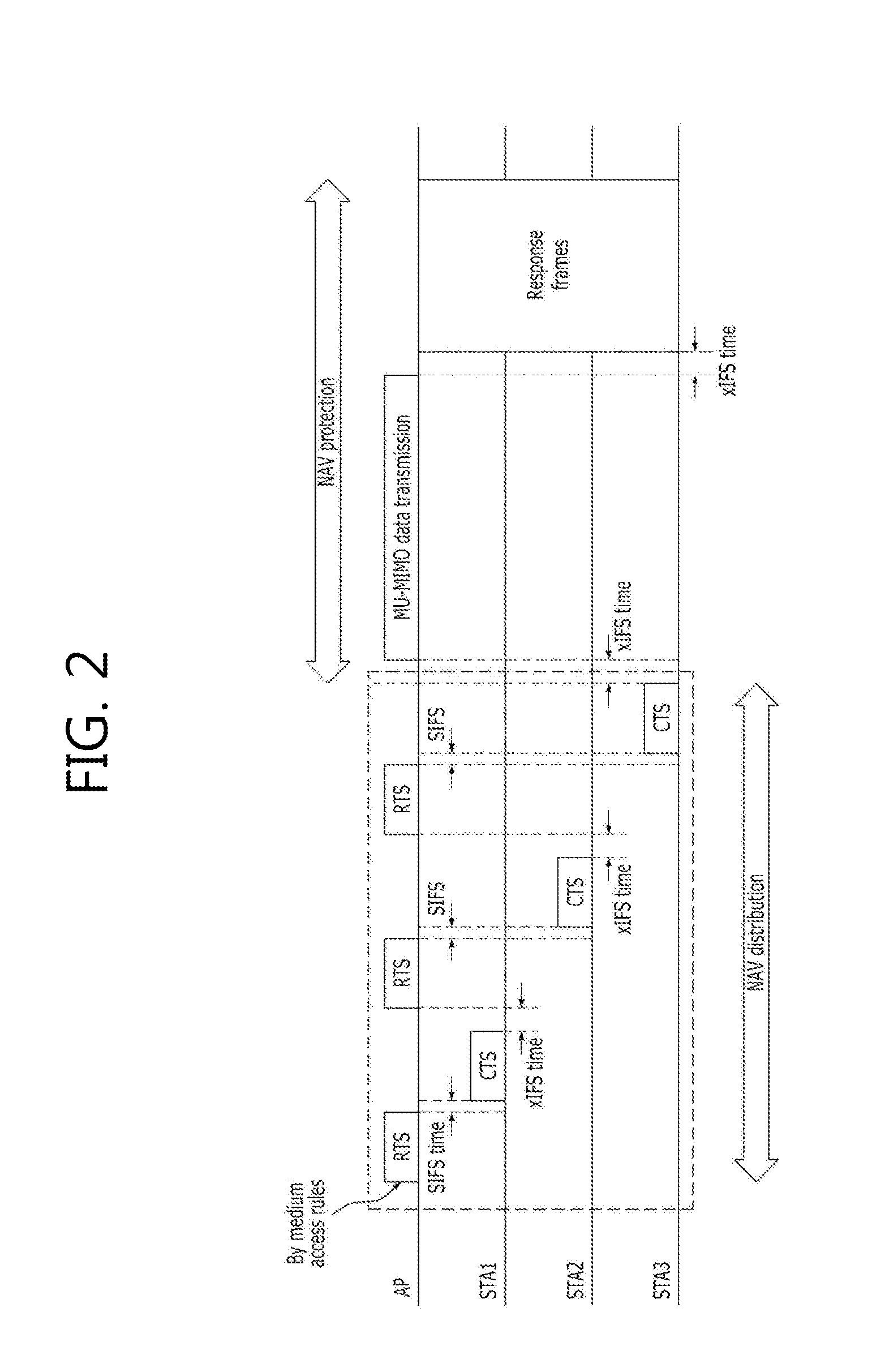
Method for protecting data in a mu-mimo based wireless communication system
ActiveUS20120236840A1Maintain compatibilityImprove throughputNetwork topologiesRadio transmissionCommunications systemMultiple input
The present invention relates to a method for protecting MU-MIMO (Multi User-Multiple Input Multiple Output) data through a multi-RTS / CTS frame exchange in a MU-MIMO based wireless communication system. The method of the present invention comprises: a process where an indicator for VHT data protection is added to an RTS frame, using the structure of an RTS / CTS frame for an existing legacy terminal during the multi RTS / CTS frame exchange; a process where an access point designates and then transmits the duration period of the RTS frame while transmitting the RTS frame; and a process where wireless terminals up to the (n-1)th terminal designate the duration period of the CTS frame as ‘0’ and send the designated duration period, while only the n-th wireless terminal designates NAV for data protection.
Owner:ELECTRONICS & TELECOMM RES INST
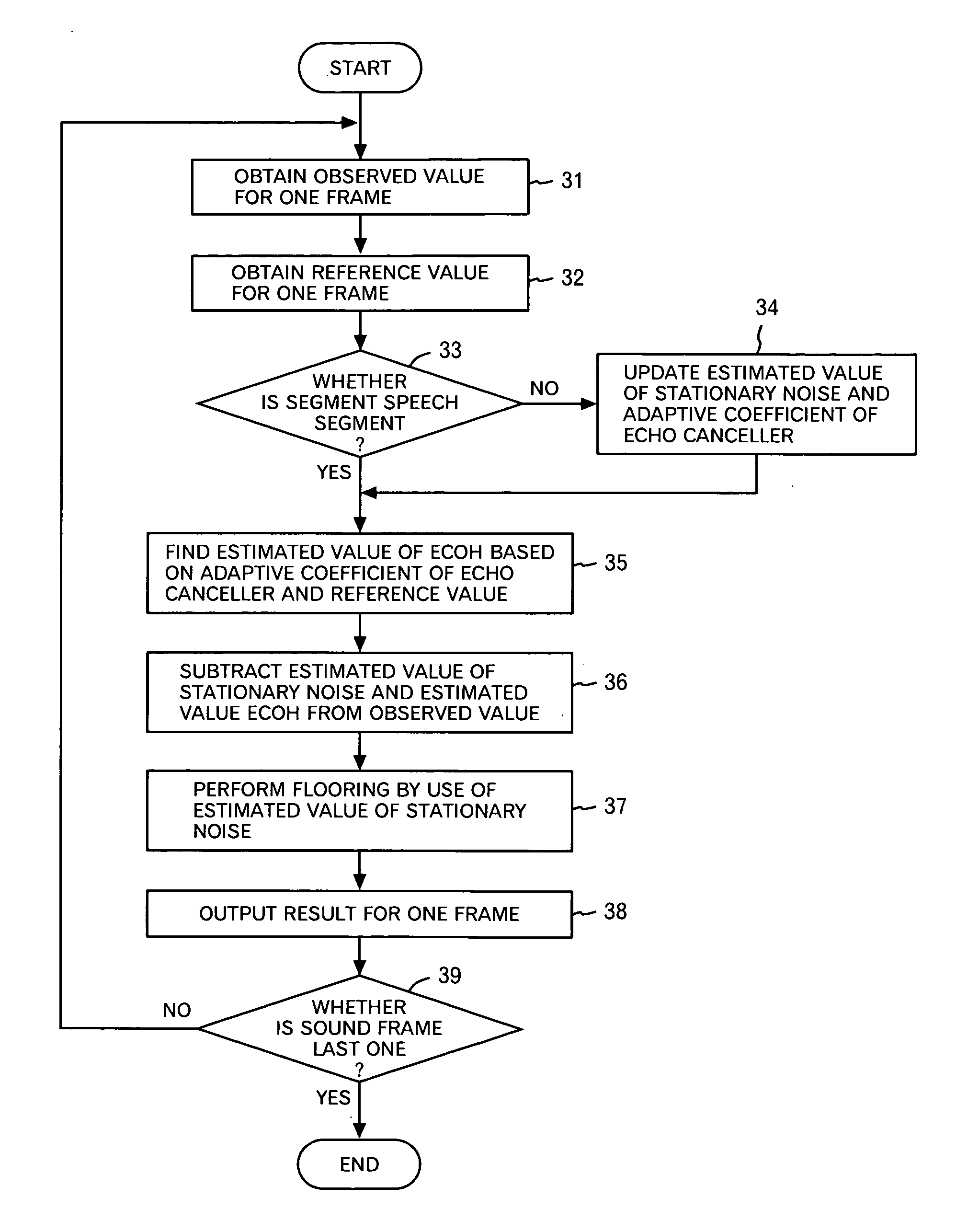
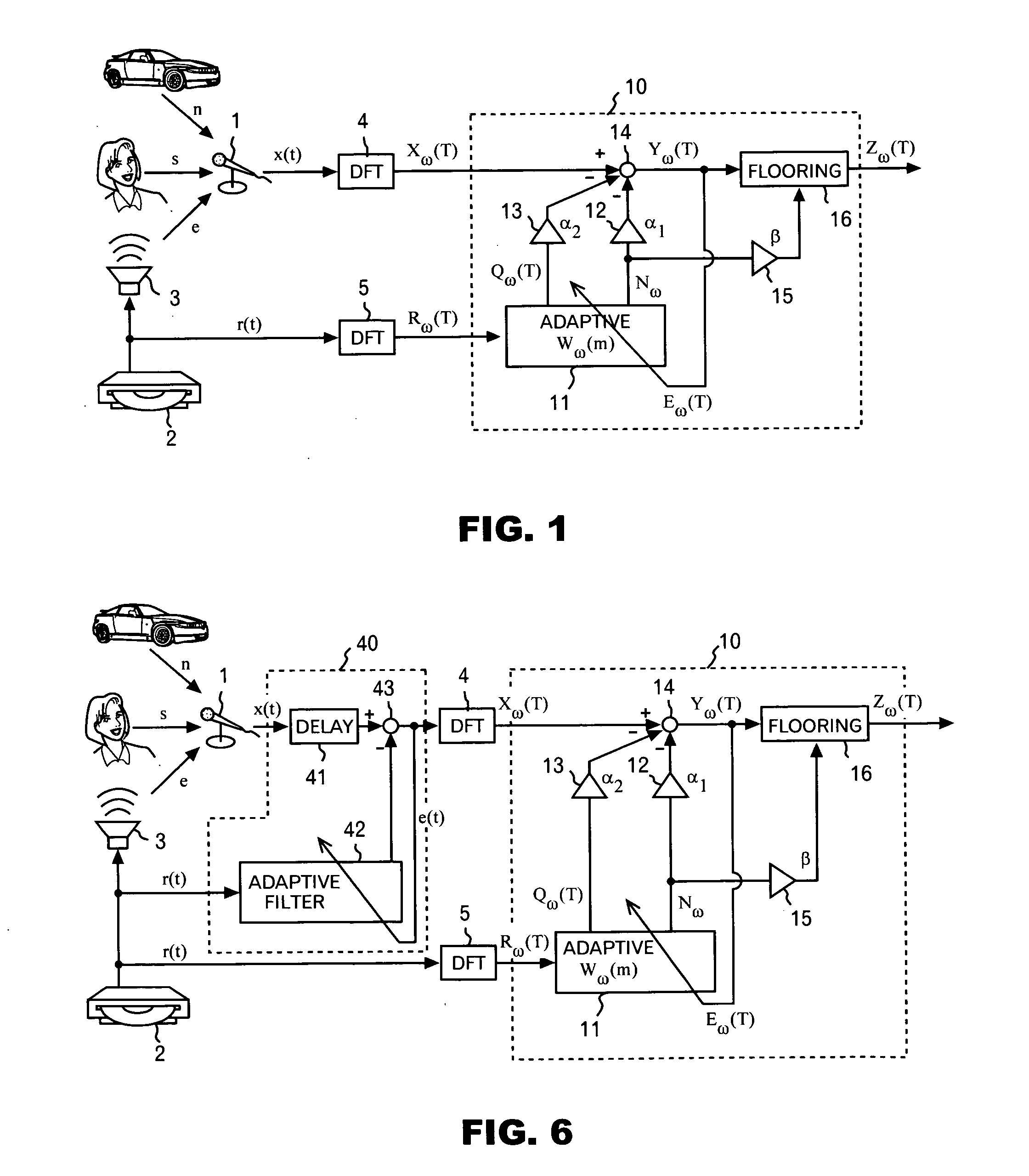
Noise reduction device, program and method
InactiveUS20060136203A1Improve Noise RobustnessMaintain compatibilitySpeech analysisStationary noiseAdaptive learning
A noise reduction device is configured by use of: means for calculating a predetermined constant, and a predetermined reference signal Rω(T) in the frequency domain, respectively by use of adaptive coefficients Wω(m), and for thereby obtaining estimated values Nω and Qω(T) respectively of stationary noise components, and non-stationary noise components corresponding to the reference signal, which are included in a predetermined observed signal Xω(T) in the frequency domain; means and for applying a noise reduction process to the observed signal on the basis of each of the estimated values, and for updating each of the adaptive coefficients on the basis of a result of the process; and an adaptive learning means and for repeating the obtaining of the estimated values and the updating of the adaptive coefficients, and for thereby learning each of the adaptive coefficients.
Owner:IBM CORP
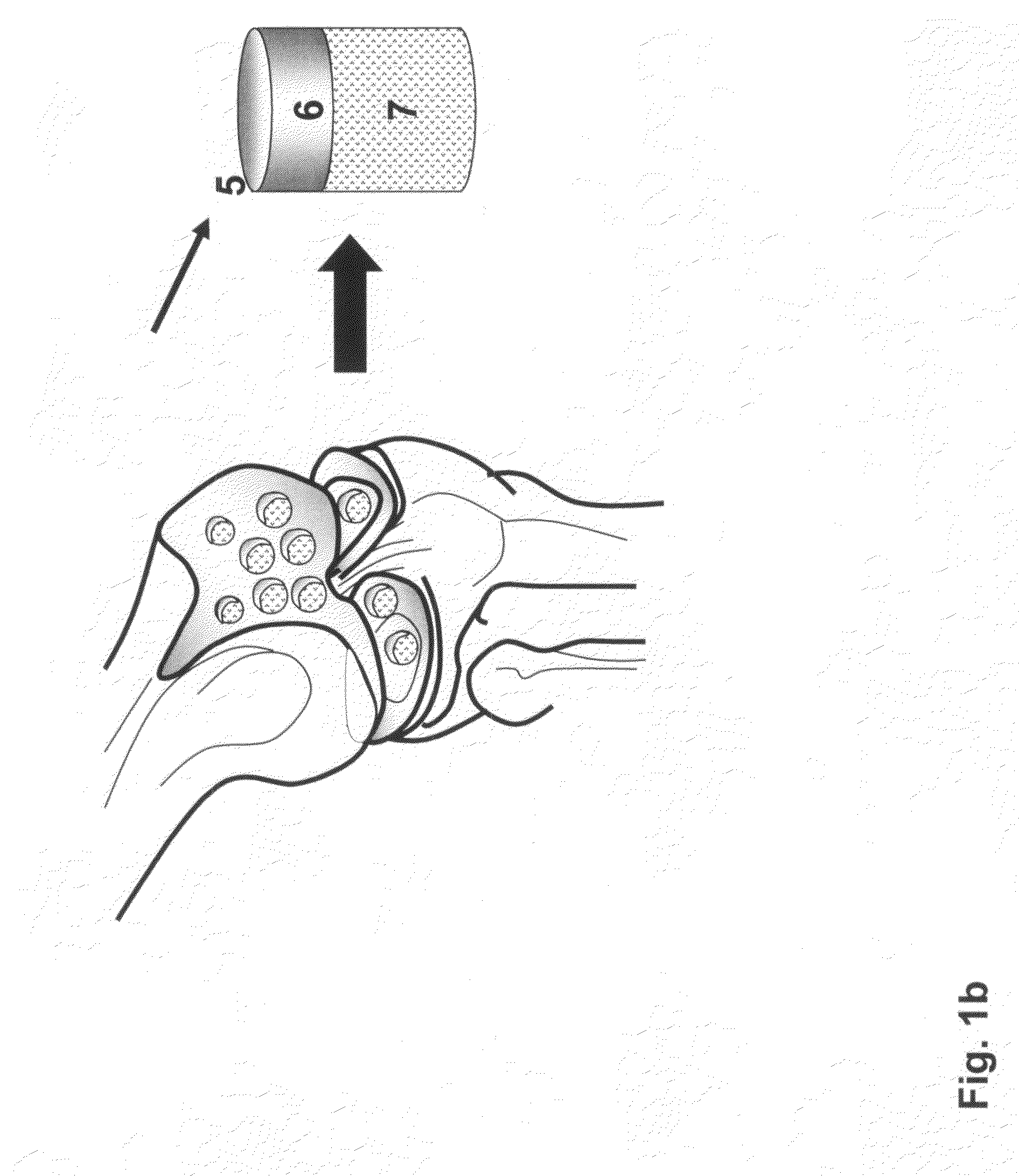
Implantation of cartilage
InactiveUS20090024224A1Promote recellularizationRestrict subsequent apoptosisBone implantSurgeryRepair cartilageViable cell
The invention is directed towards a process for implanting a cartilage graft into a cartilage defect and sealing the implanted cartilage graft with recipient tissue by creating a first bore down to the bone portion of the cartilage defect, creating a second shaped bore that is concentric to and on top of the first bore to match the shape and size of the cartilage graft, treating the first bore and the second shaped bore at the defect site with a bonding agent, treating the circumferential area of the cartilage graft with a bonding agent, inserting the cartilage graft into the defect site and wherein the superficial surface of the cartilage graft is at the same height as the surrounding cartilage surface. The first and second bonding agents may be activated by applying a stimulation agent to induce sealing, integration, and restoration of the hydrodynamic environments of the recipient tissue. The invention is also directed towards a process for repairing a cartilage defect and implanting a cartilage graft into a human or animal by crafting a cartilage matrix into individual grafts, cleaning and disinfecting the cartilage graft, applying a pretreatment solution to the cartilage graft, removing cellular debris using an extracting solution to produce a devitalized cartilage graft, implanting the cartilage graft into the cartilage defect with or without an insertion device, and sealing the implanted cartilage graft with recipient tissue. The devitalized cartilage graft is optionally recellularized in vitro, in vivo, or in situ with viable cells to render the tissue vital before or after the implantation. The devitalized cartilage graft is also optionally stored between the removing cellular debris and the recellularizing steps. The invention is further directed toward a repaired cartilage defect.
Owner:LIFENET HEALTH
Bearer control mode (nw-only or user-only) handling in intersystem handover
ActiveUS20110065435A1More flexibility in controlMaintain backward compatibilityConnection managementWireless network protocolsRadio access networkHandover
A method, a gateway node, a policy control node and a infrastructure network for handling a handover of a User Equipment communicating wirelessly with the infrastructure network. A first gateway node receives handover information indicative of a handover of the user equipment between two radio access networks the first gateway node determining a bearer control mode on the basis of the handover information the first gateway node transmitting control information determined on the basis of the bearer control mode to a policy control node the first gateway node controlling the bearer binding on the basis of the bearer control mode.
Owner:TELEFON AB LM ERICSSON (PUBL)
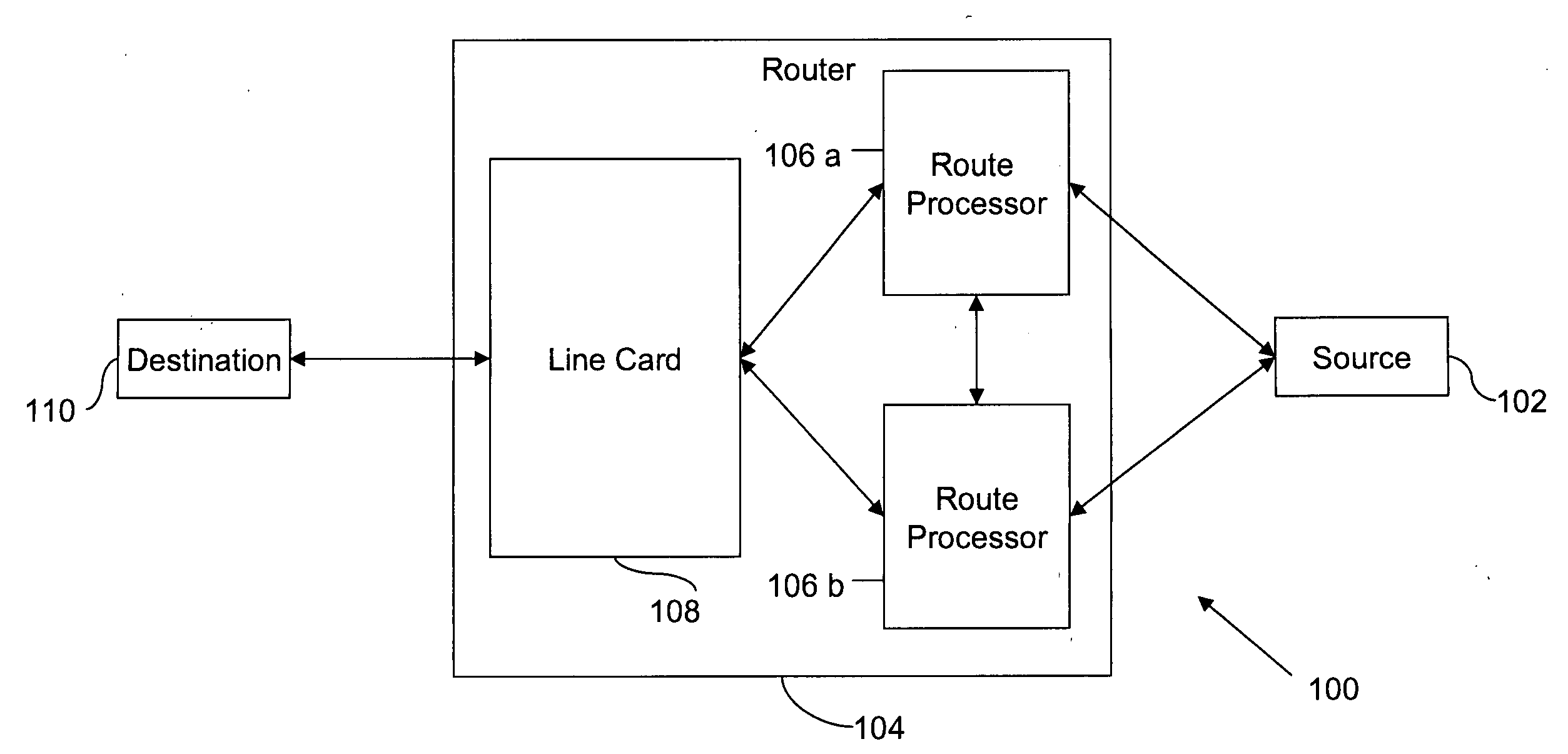
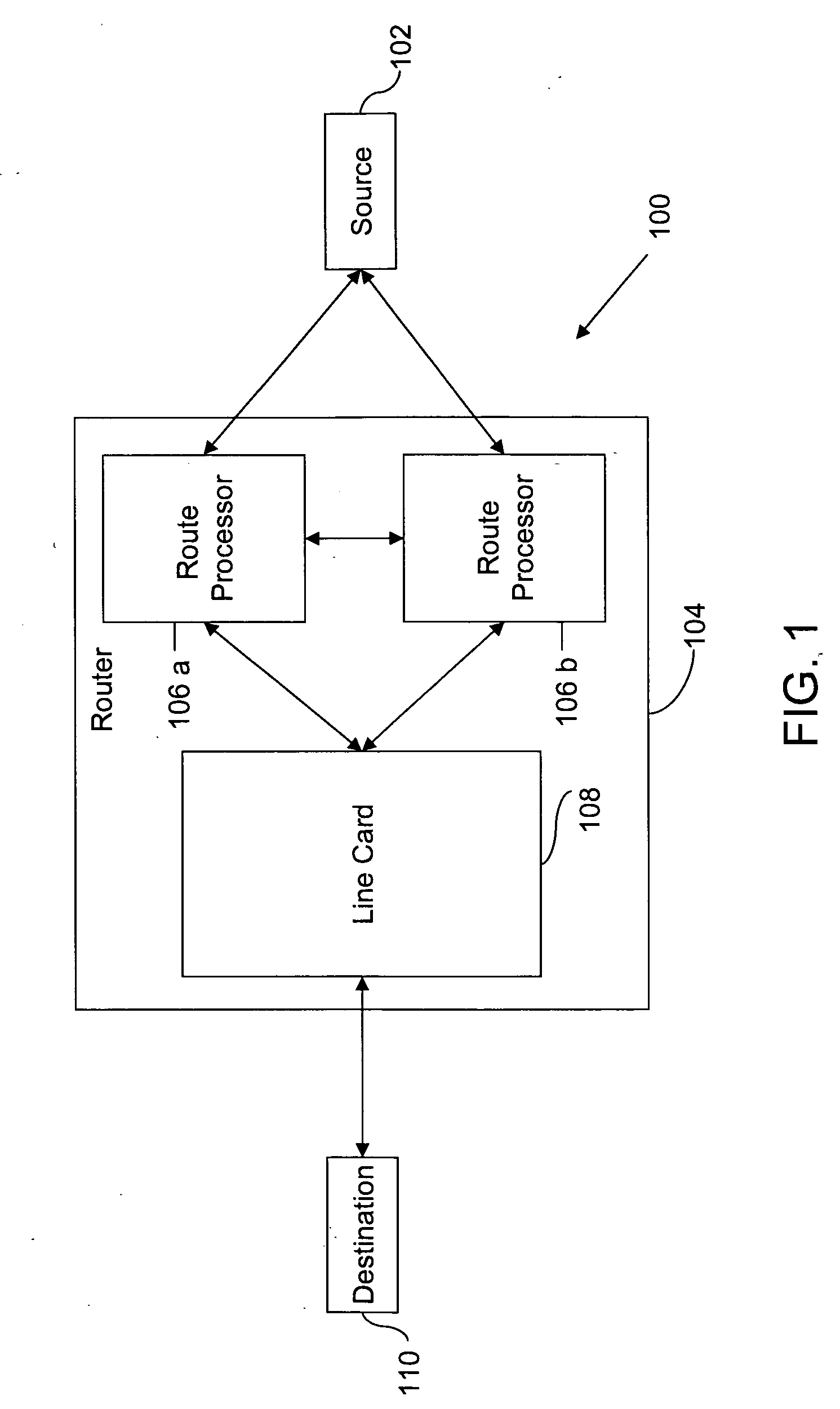
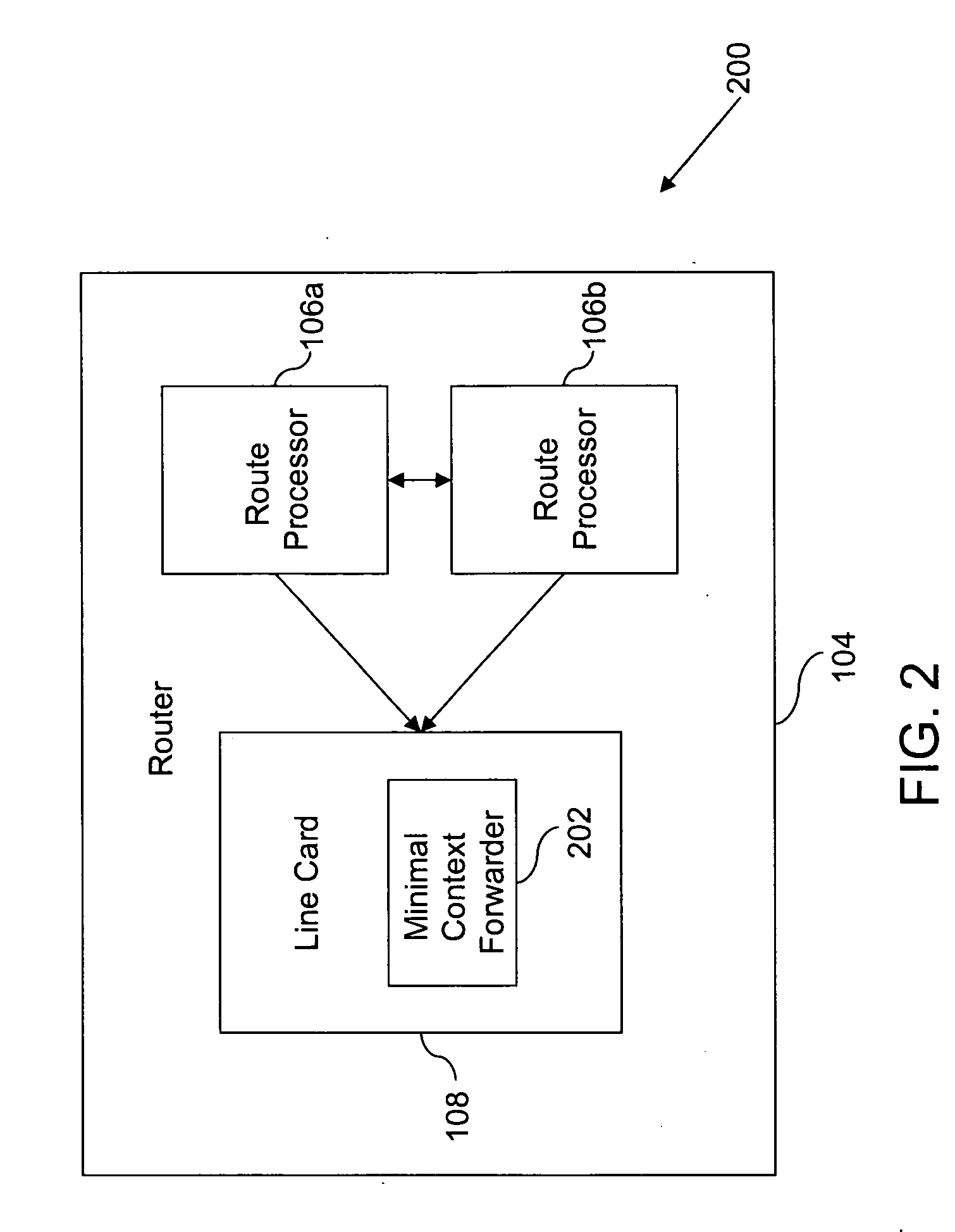
Method and system for minimizing disruption during in-service software upgrade
ActiveUS20070162565A1Avoid disruption and disruptionMinimize timeMultiple digital computer combinationsTransmissionLine cardReal-time computing
A method and a system for in-service software upgrade in a Hot Standby Redundant Distributed (HSRD) system are provided. A standby route processor in HSRD system is updated with upgraded software. The standby route processor is synchronized with an active route processor present in the HSRD system. The control of routing process is switched over to the standby route processor (with the upgraded software) from the active route processor. During the switchover, a minimal context forwarder on the line card begins execution and continues forwarding packets across a network, while a line card in the HSRD system is being reloaded and configured.
Owner:CISCO TECH INC
Device and process for determining the position of an engine
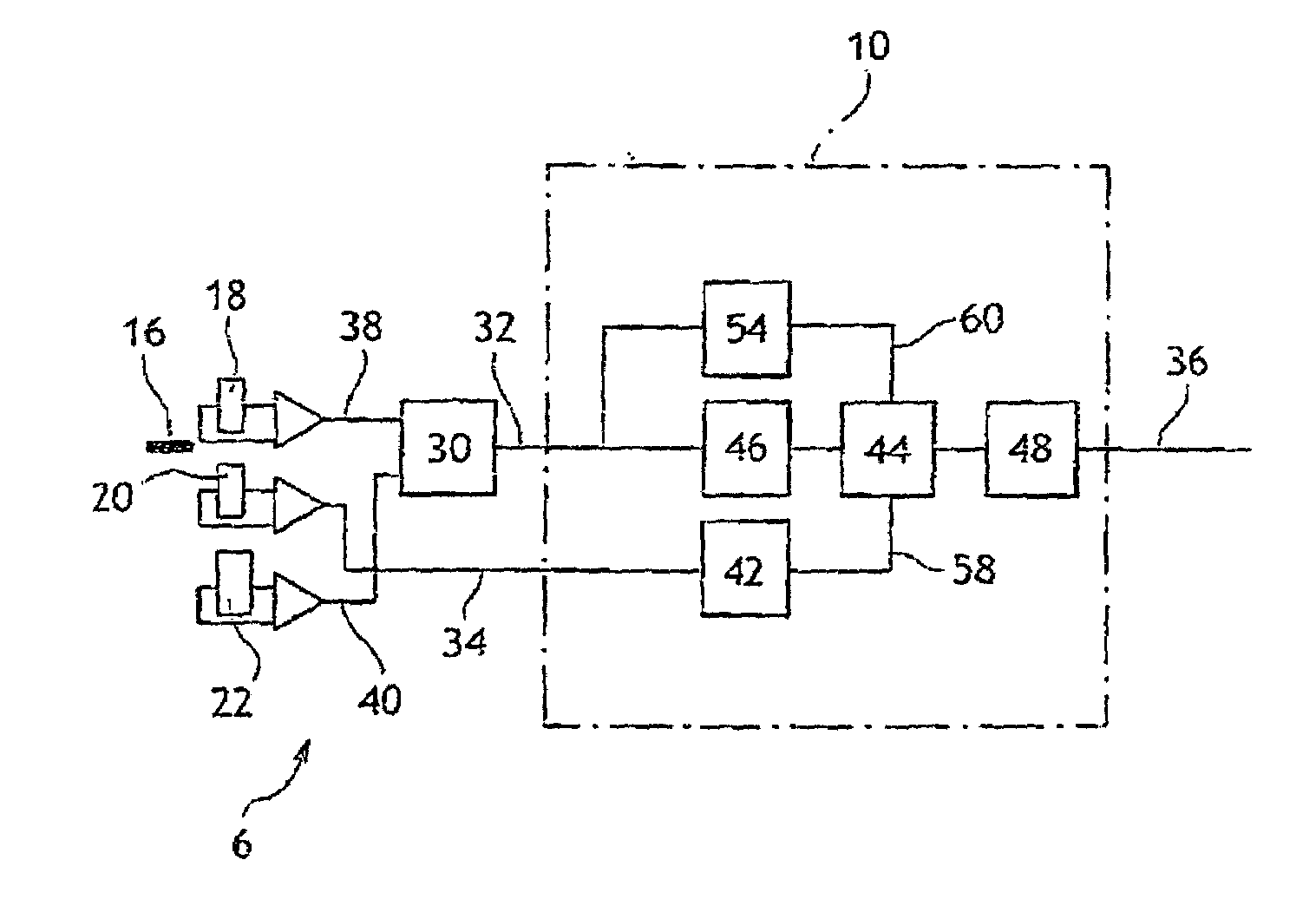
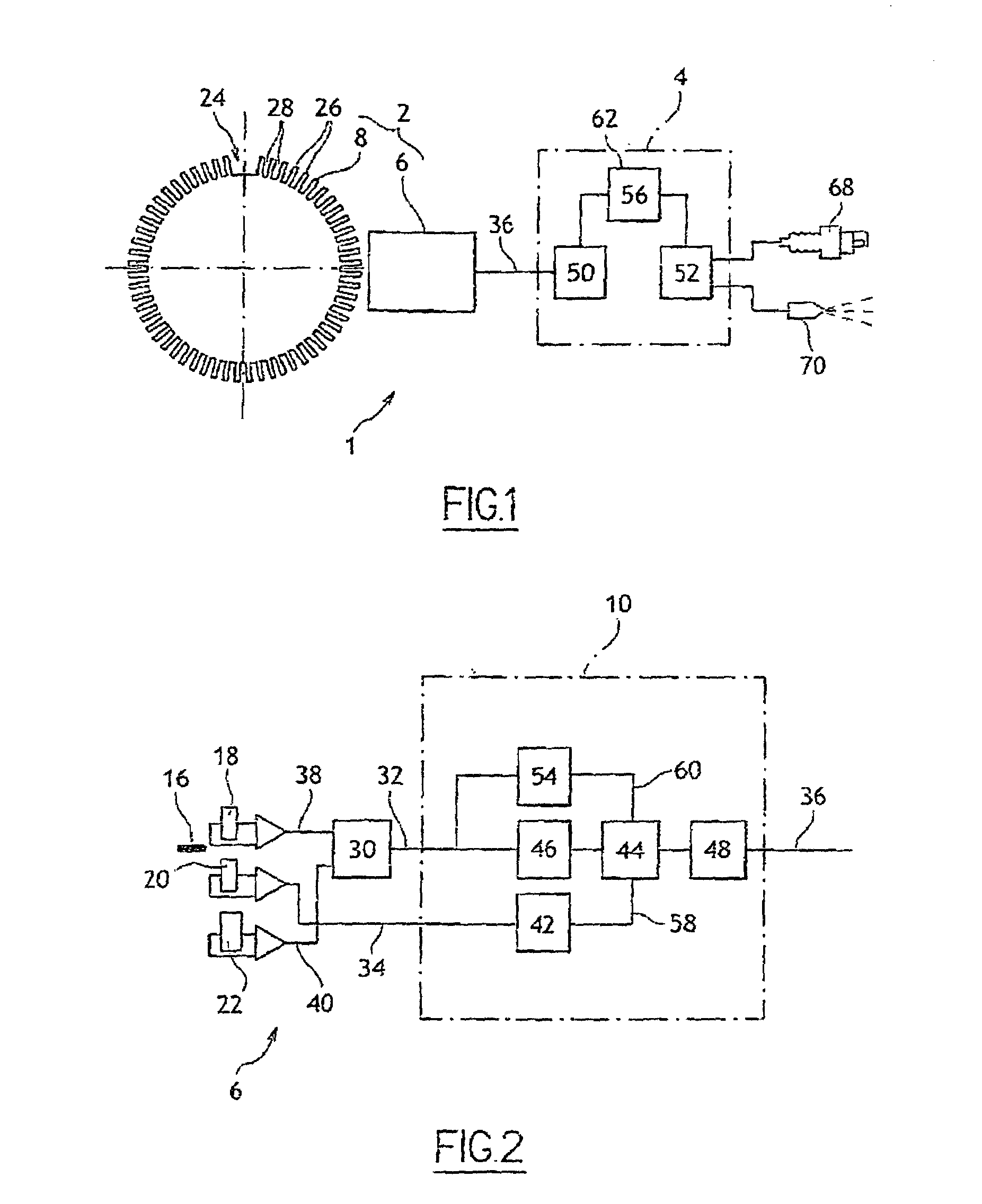
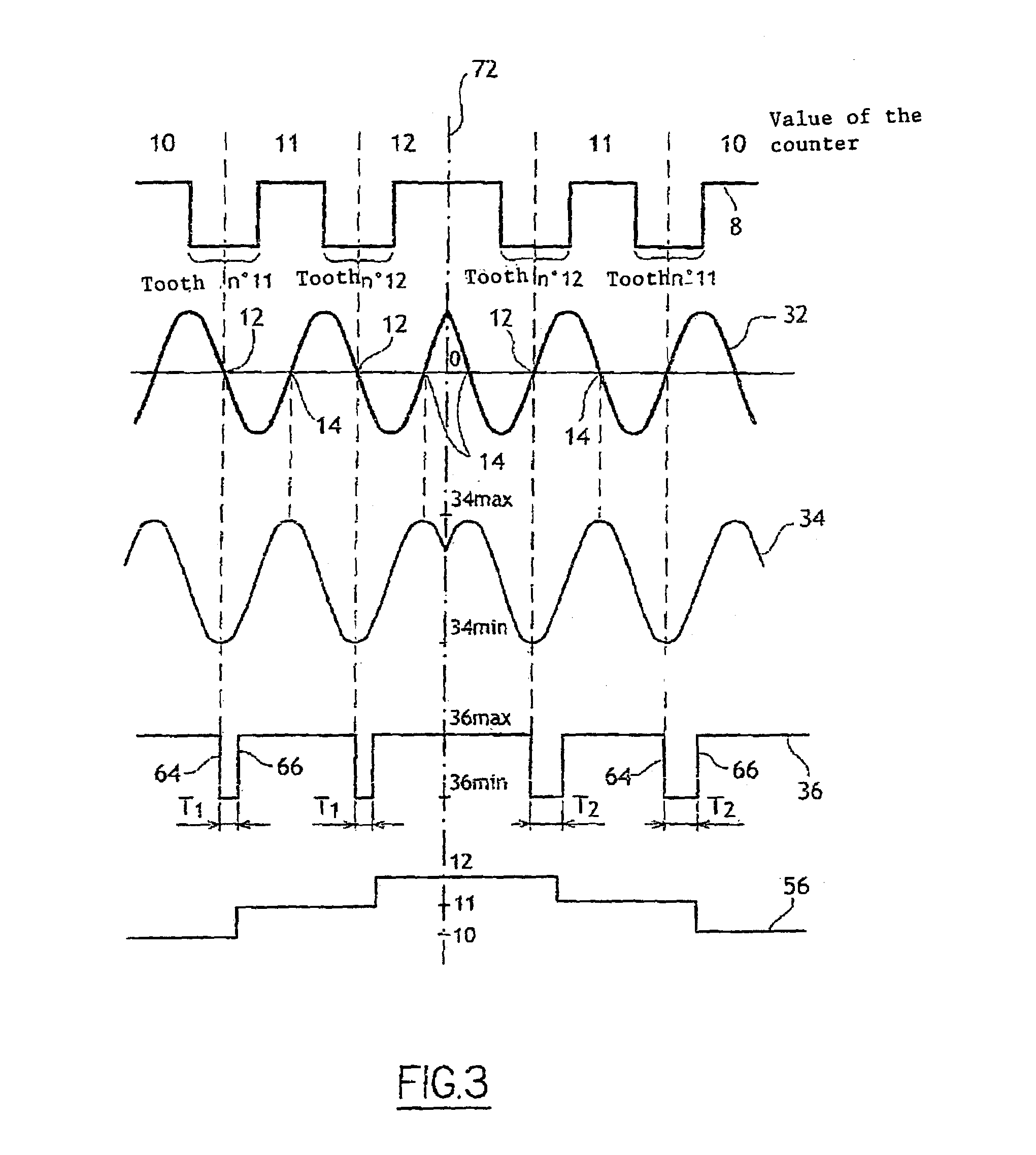
InactiveUS7184876B2Low costDrawback can be obviatedInternal-combustion engine testingAnalogue computers for vehiclesPhase shiftedEngineering
Device for determining the position of an engine includes:a sensor that has a rotary part and a fixed part, whereby said fixed part comprises:elements (for generating a first signal based on the position of the rotary part relative to the fixed part),Second elements for generating a second phase-shifted signal relative to the first signal,elements for comparing the value of the second signal to a reference value,elements for detecting at least one characteristic event on the first signal, for generating a third signal of binary type, and for alternating the binary signal from a first value to a second after detection of at least one of the characteristic events if the result of the comparison is positive,engine control elements that include members for detecting the alternations of third signal and a counter.
Owner:SIEMENS VDO AUTOMOTIVE CORP
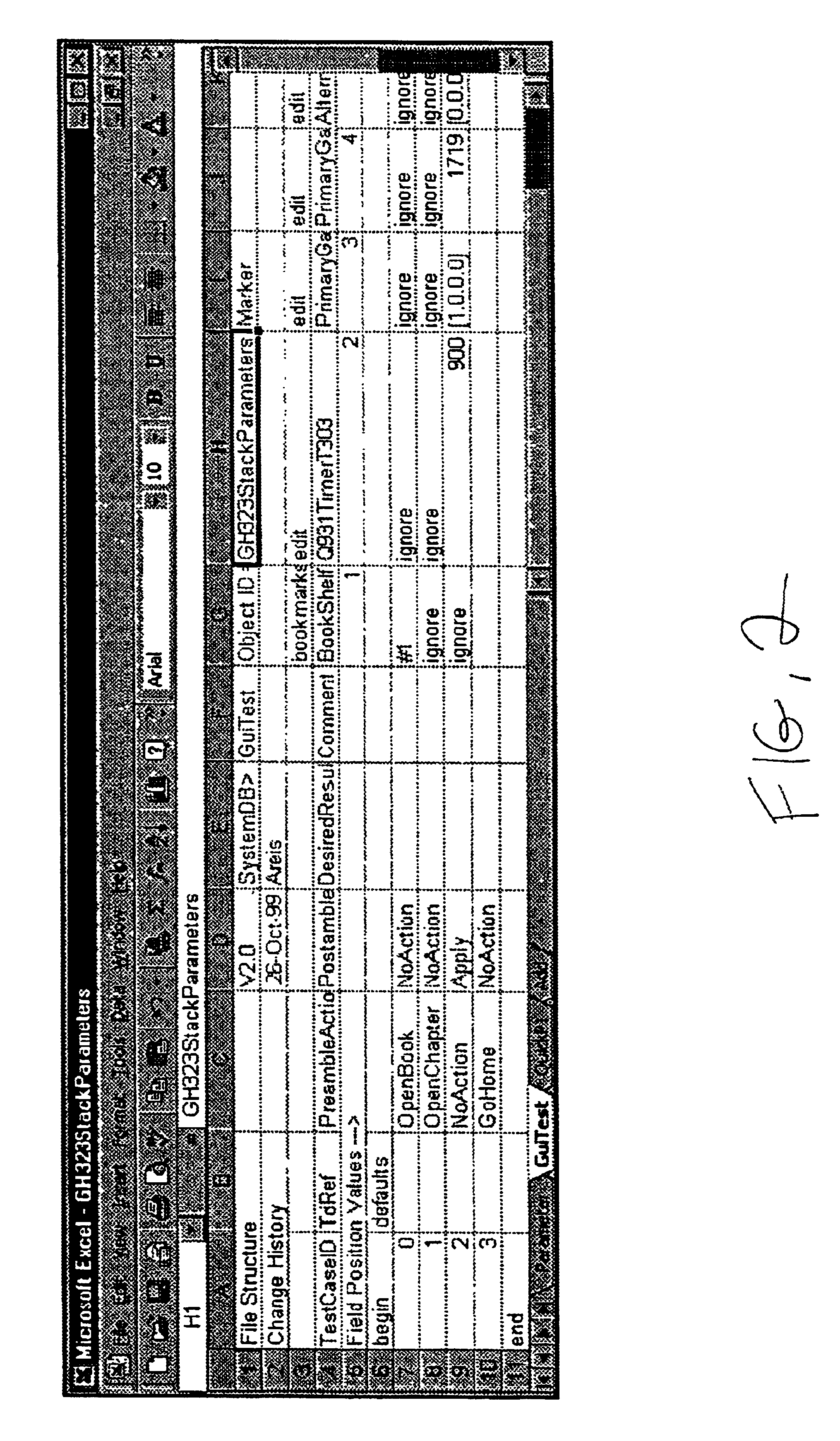
Environment based data driven automated test engine for GUI applications
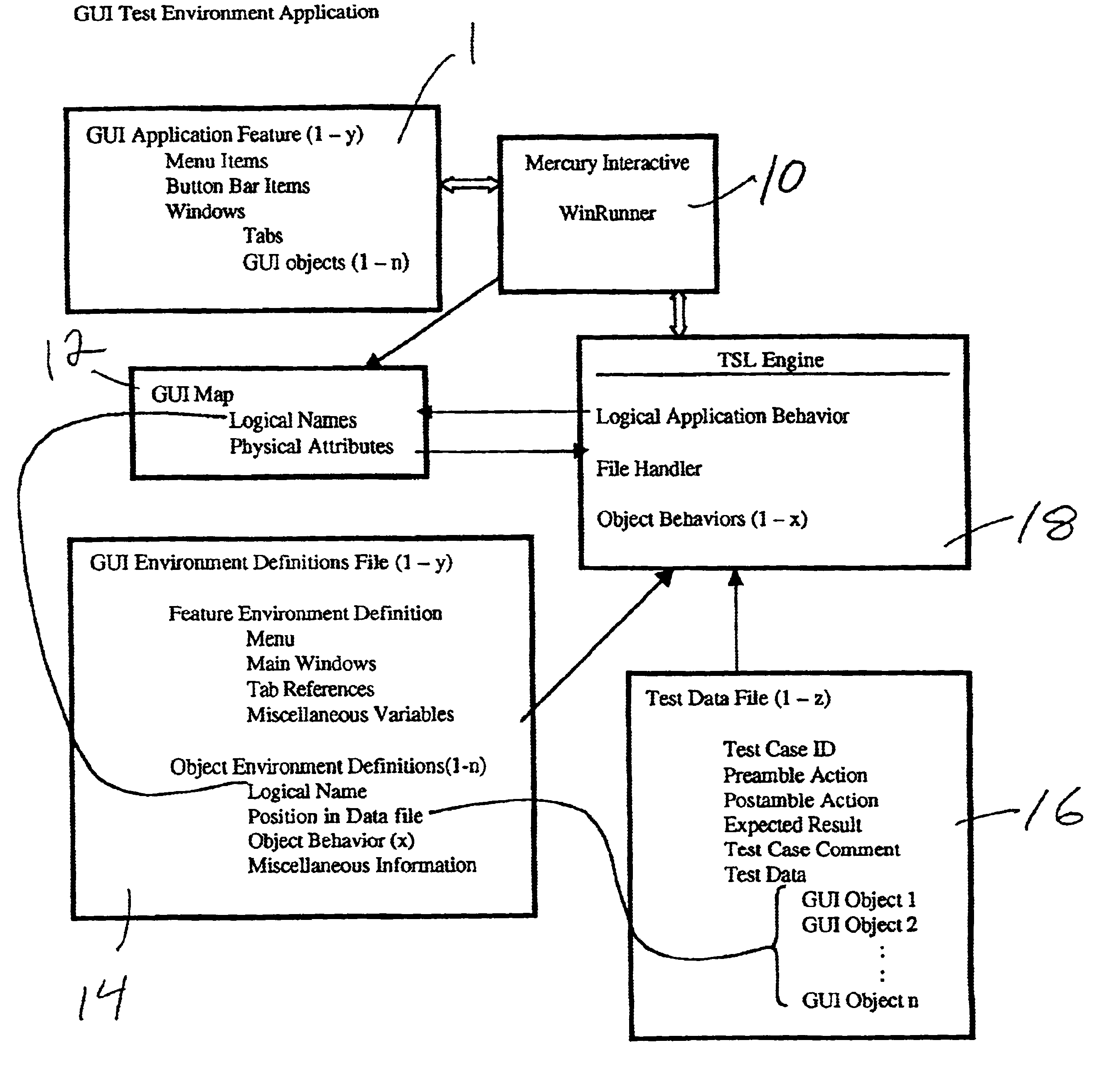
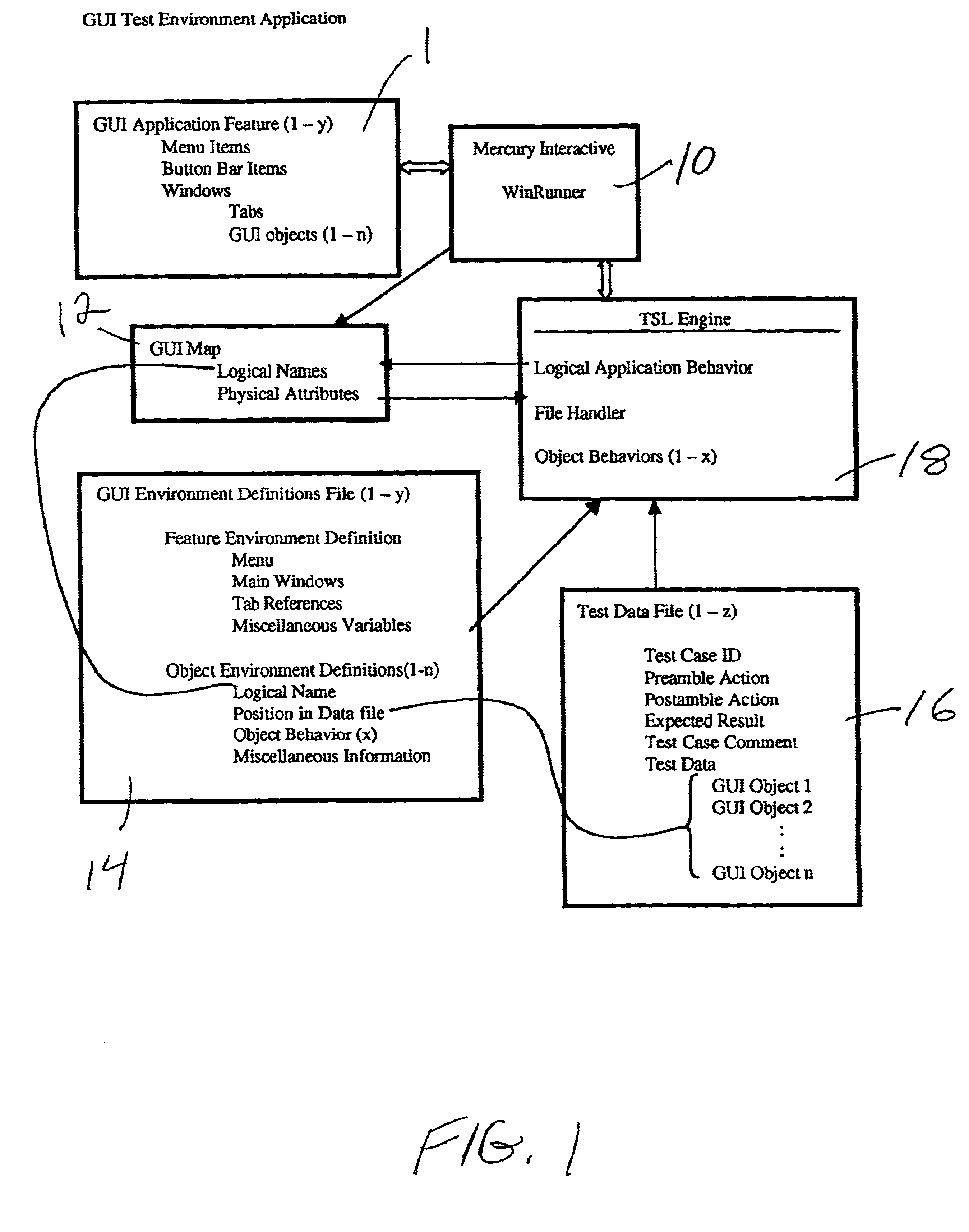
InactiveUS6961873B2Maximum flexibilityReduce investmentSoftware testing/debuggingSpecific program execution arrangementsScripting languageElectronic form
The invention includes a scriptable GUI test tool which generates a GUI map (or which includes a utility which generates a GUI map), at least one environment definition (parameter) file, at least one test data (driver) file, and an automated test engine. A separate environment definition file is provided for each feature of the GUI. Each environment definition file provides the abstract details required by the test engine in order to support common processes for different applications. The test data file is organized into rows of data where each row defines a single test and each column represents a parameter. The automated test engine is composed of a plurality of library modules written in the scripting language of the scriptable GUI test tool. The ATE is driven by the test data file and calls upon the GUI map and environment definition file. According to the presently preferred embodiment, the scriptable GUI test tool is WinRunner®. The environment definition files and the test data files are preferably generated with a spreadsheet program such a Microsoft Excel® and saved as comma delimited text files.
Owner:UNIFY INC
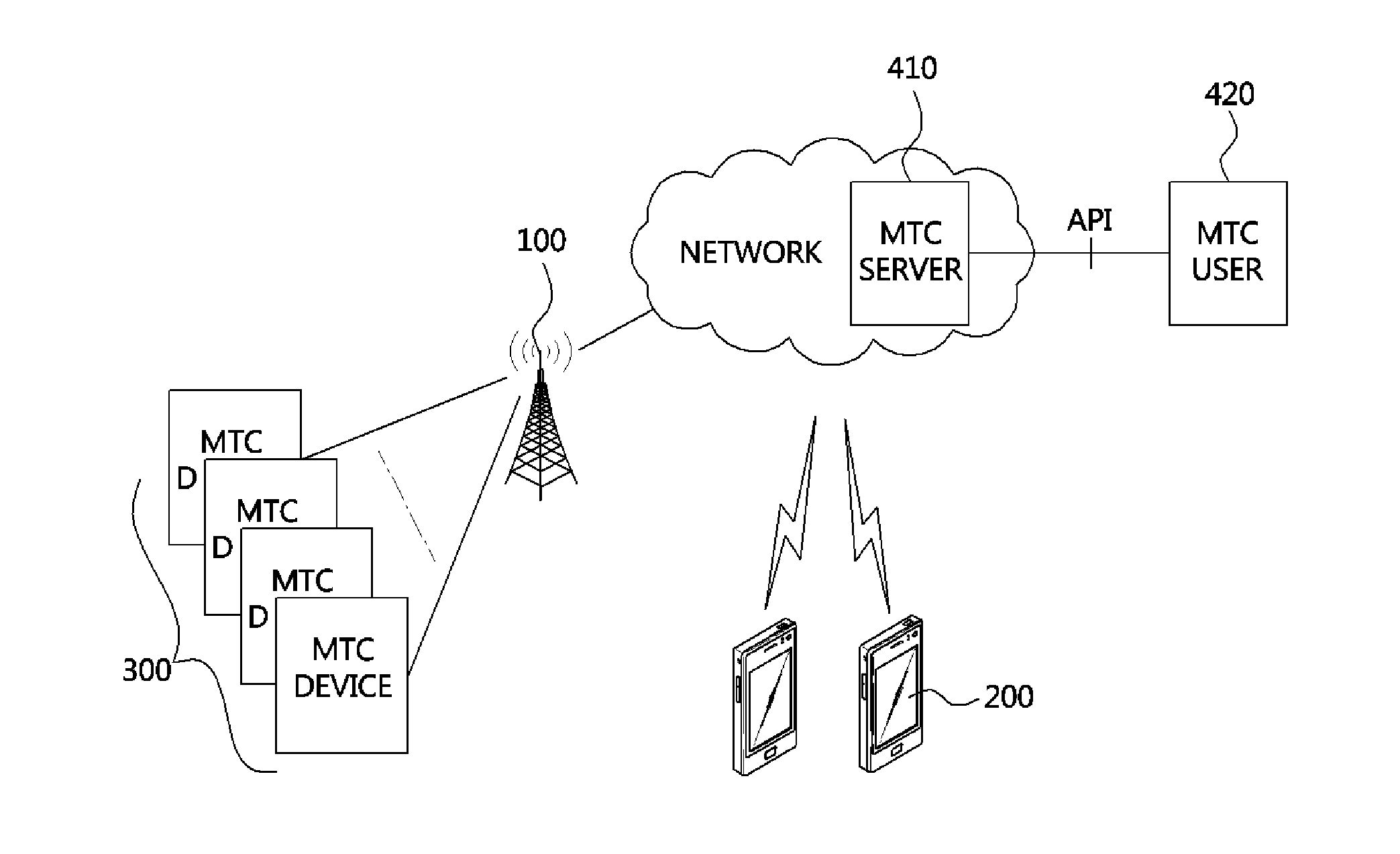
Data transmission and reception method of machine type communication (MTC) device
InactiveUS20130094457A1Maintain compatibilityEfficient serviceWireless commuication servicesMachine-to-machine/machine-type communication serviceFrequency bandData transmission
There are provided a data transmission and reception method of a machine type communication (MTC) device, and an MTC device using the same. The data transmission and reception method of the MTC device includes: extracting information related to an MTC band from a downlink frame received from a base station, searching for an MTC downlink resource region in the downlink frame based on the information related to the MTC band, and extracting MTC data for the corresponding MTC device from the MTC downlink resource region. The MTC band includes a band through which at least a physical broadcast channel and a synchronization channel are transmitted.
Owner:ELECTRONICS & TELECOMM RES INST
Wireless communication method for simultaneous data transmission, and wireless communication terminal using same
ActiveUS20170202026A1Increase overall resource utilization ratioImprove wireless performanceError prevention/detection by using return channelNetwork topologiesData transmissionHigh density
The present invention relates to a wireless communication method for simultaneous data transmission and a wireless communication terminal using the same, and more particularly, a wireless communication method in which a plurality of terminals simultaneously transmit data for improving a data throughput in a high density environment, and a wireless communication terminal using the same. To this end, the present invention provides a wireless communication method for a terminal including transmitting a trigger frame indicating simultaneous uplink data transmission of multi-users, receiving uplink data transmitted by a plurality of terminals in response to the trigger frame, and transmitting a block acknowledgement for the plurality of terminals having transmitted the uplink data, and a wireless communication terminal using the same.
Owner:WILUS INST OF STANDARDS & TECH +1
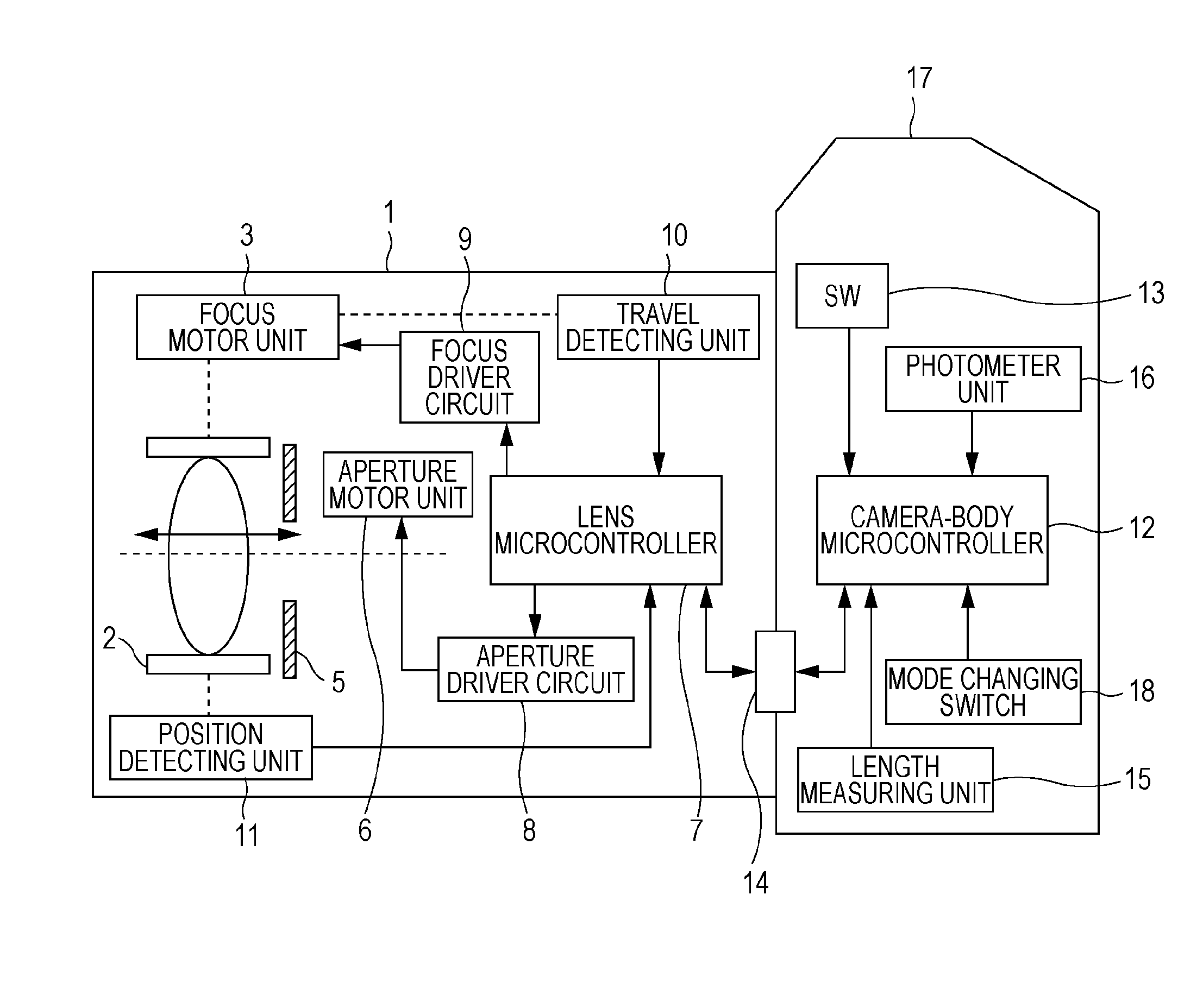
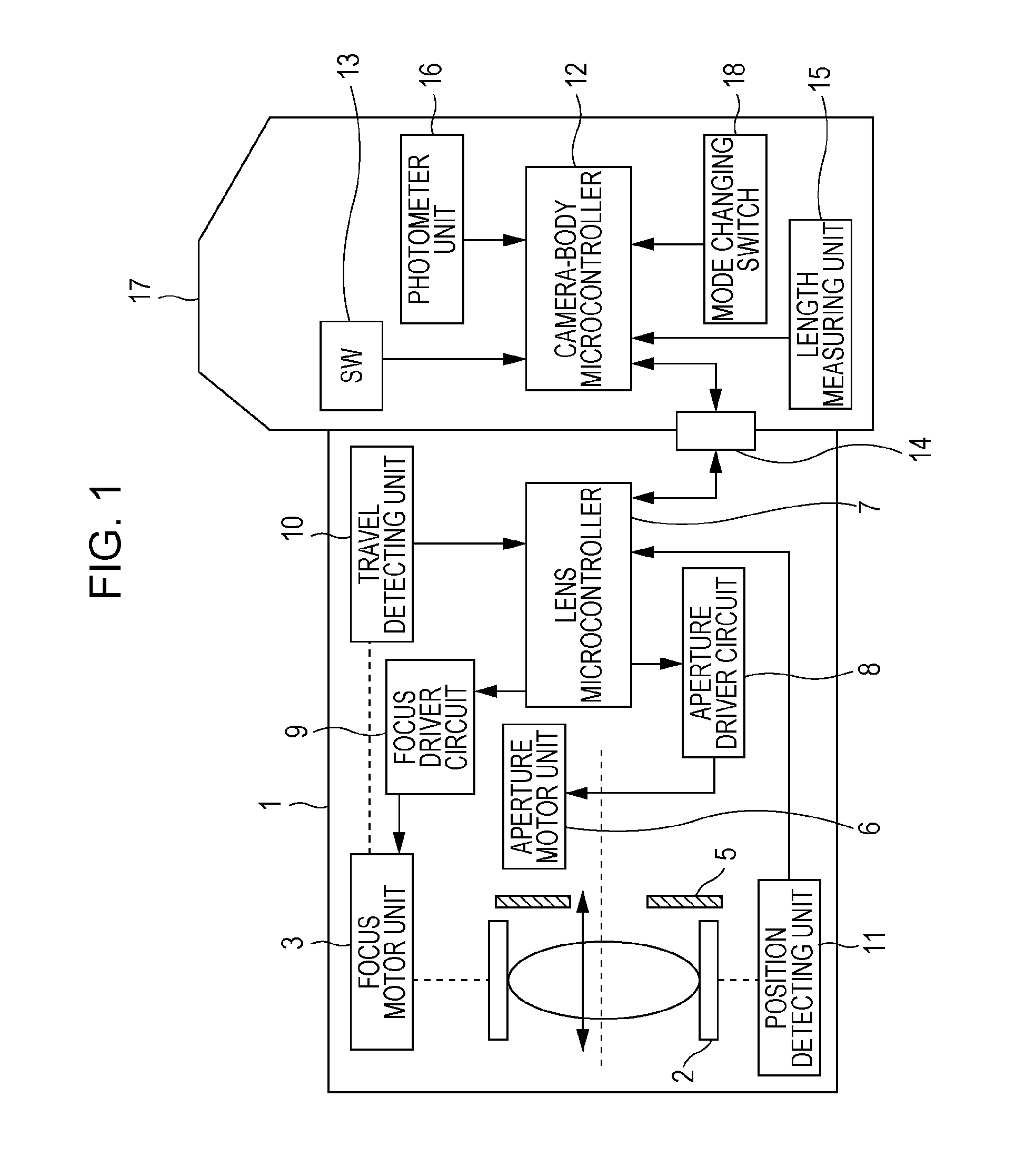
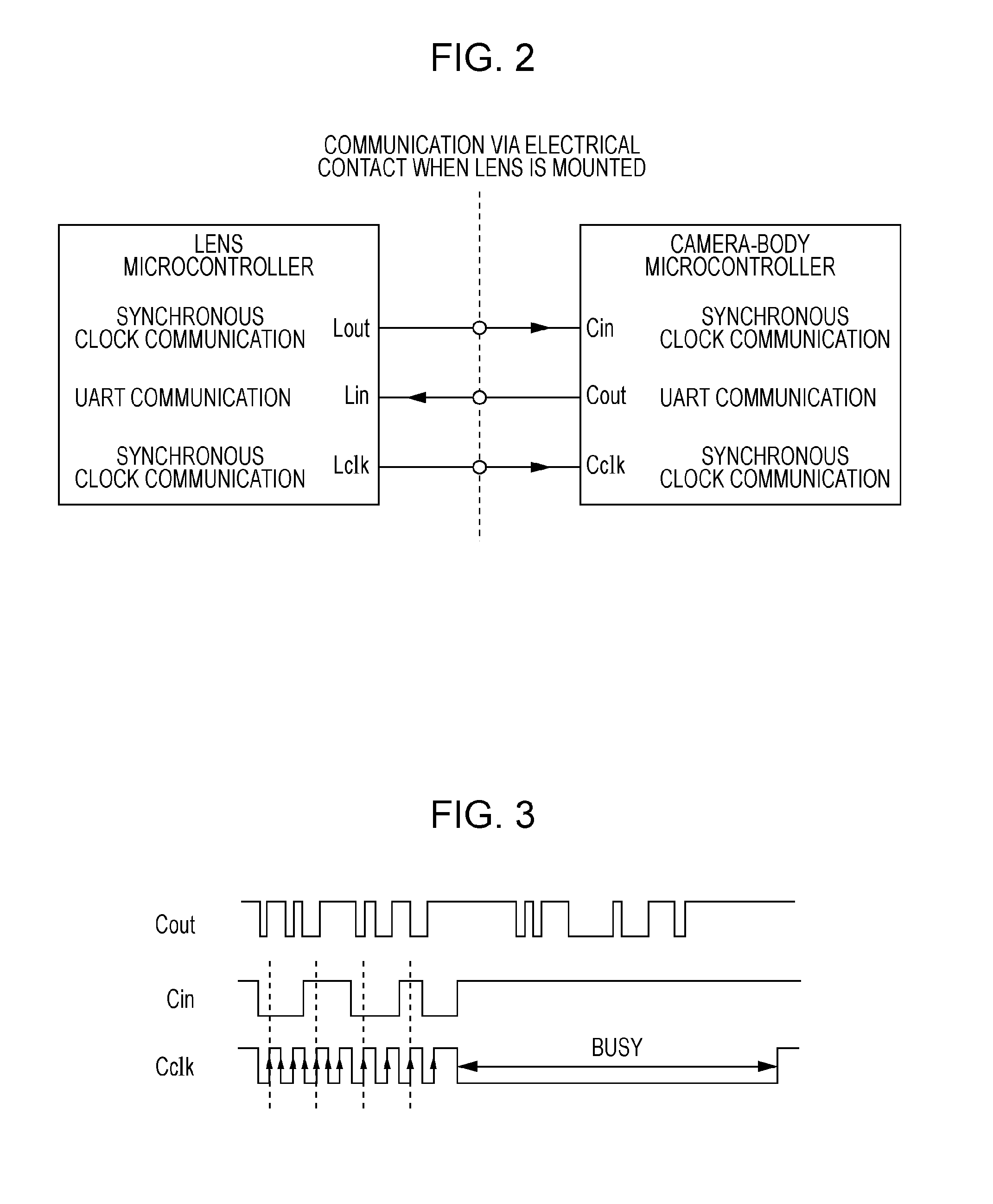
Lens-interchangeable camera body and camera system capable of changing communication method with selected shooting mode
ActiveUS20110044682A1Maintain compatibilityAccurate focusTelevision system detailsCamera body detailsCamera lensCommunication unit
The present invention relates to a camera system.The system includes a selector configured to select one of a still image shooting mode and a movie shooting mode, anda communication unit employing a plurality of communication methods by which communication between an interchangeable lens and a camera body is performed. If the still image shooting mode is selected by the selector and a lens information request command is sent from the camera body to the interchangeable lens, the communication unit operates such that information on the interchangeable lens is sent from the interchangeable lens to the camera body. If the movie shooting mode is selected by the selector, the communication unit operates such that the information on the interchangeable lens is sent from the interchangeable lens to the camera body even without the lens information request command being sent from the camera body to the interchangeable lens.
Owner:CANON KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com