Implantation of cartilage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Osteochondral Plug, Straight, Step, or Dumbbell Shape
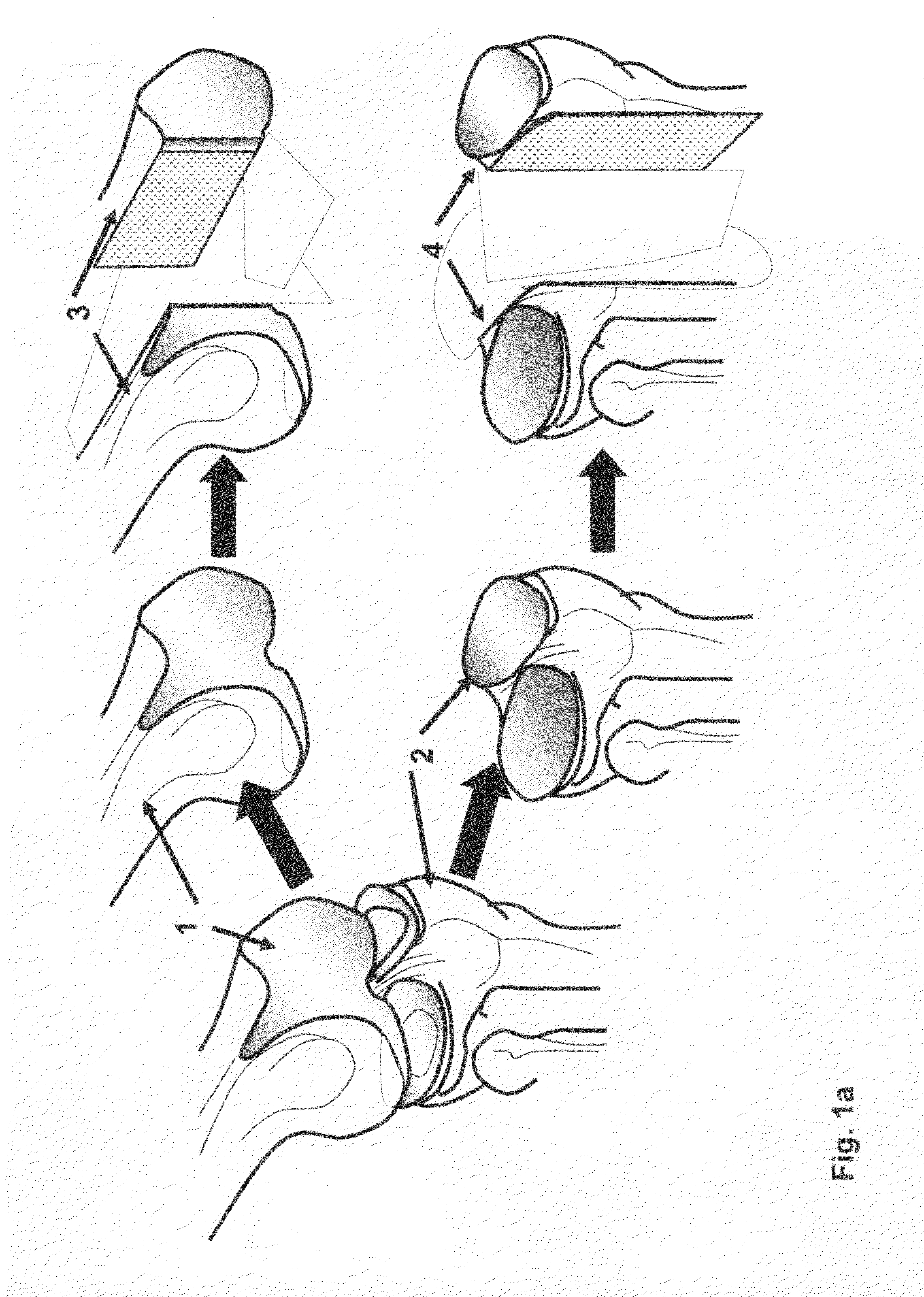
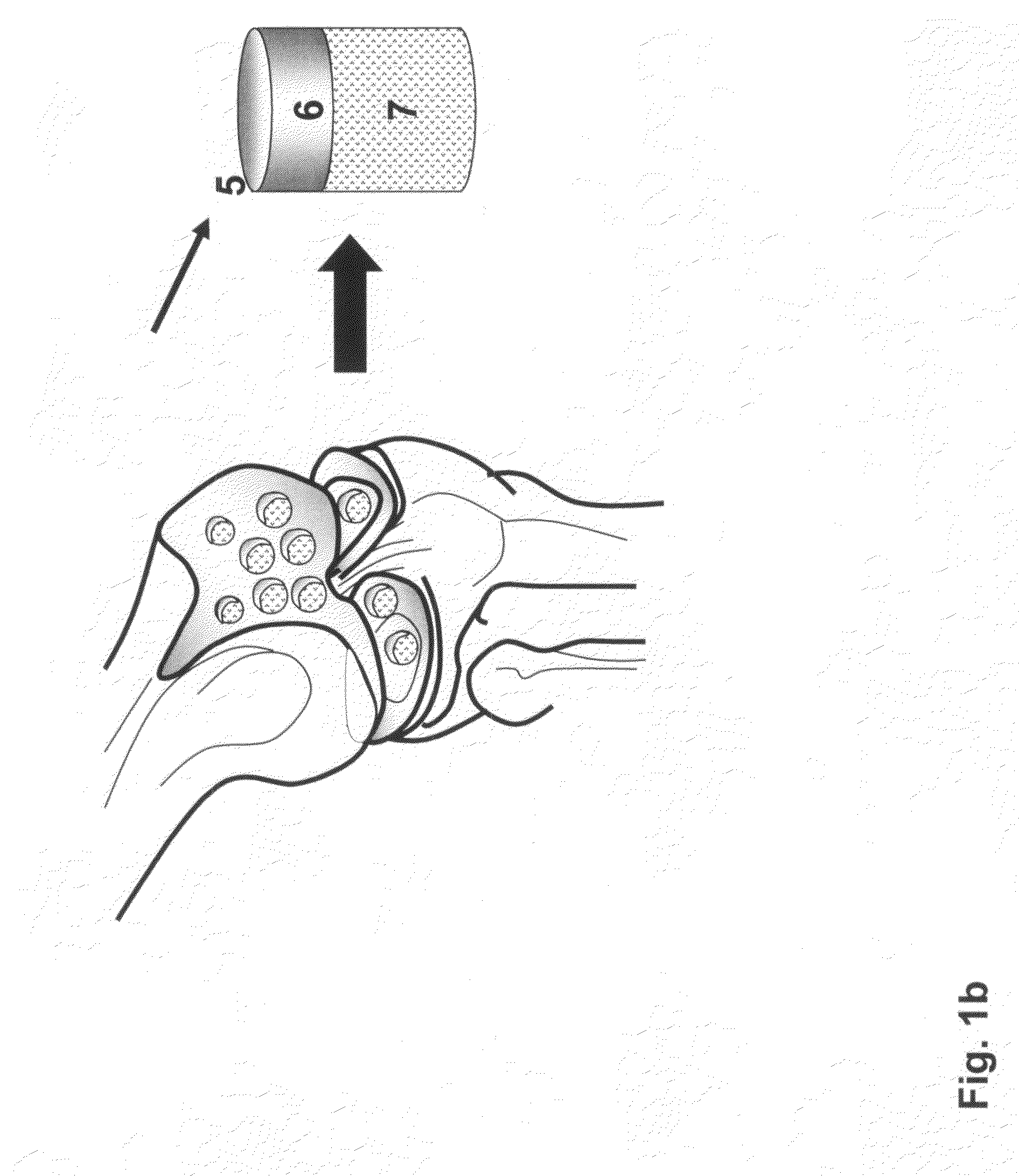
[0133]The distal end of a human femur was procured from a suitable donor, transported on wet ice to the processing facility. A picture was taken and was superimposed on a customer made grid / coordinate system to create a map of the human femoral condyle. The femoral condyle end was “cored” with a coring device or drilled with a hollow cylindrical drill bit to produce multiple cylindrical osteochondral plugs with diameter range from 5-20 mm and the length of the bone portion from 5-20 mm. The coordinate of each individual cylindrical plug was recorded according to the map. The cylindrical plugs were rinsed with isotonic saline. Then one of the cylindrical plugs was inserted into a holder, such as illustrated in FIG. 7, with the cartilage cap positioned face down and supported by the custom made bolt (60) as illustrated in FIG. 7(d, e and f). The length of the bone portion of the osteochondral plug protruding above the top of the hol...
example 2
Osteochondral Plug with Gaps, Hollow Cylinder, or Multiple Small Cylindrical Channels
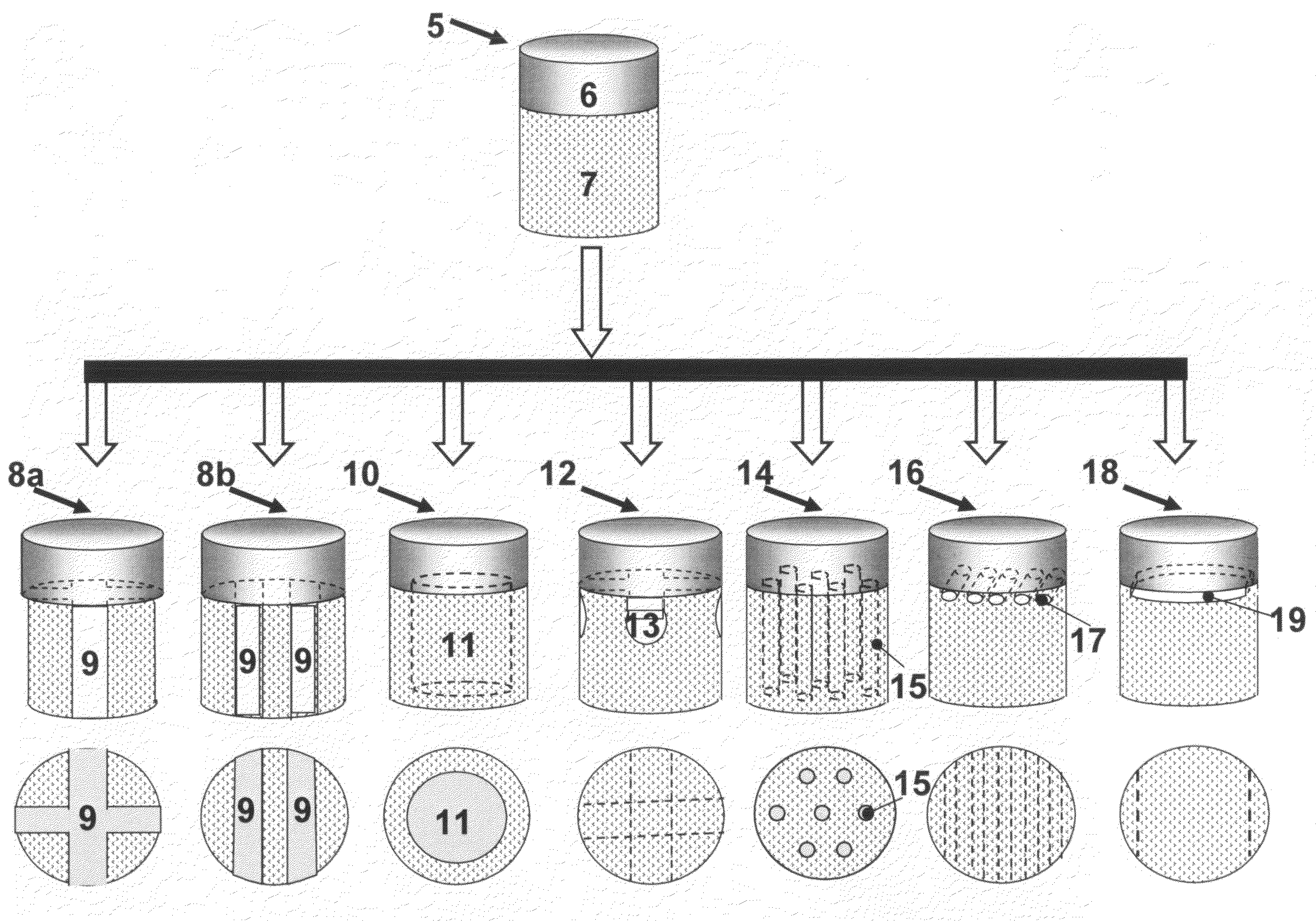
[0135]The osteochondral plugs, crafted to be straight, step cylindrical, or dumbbell shape as illustrated in Example 1 can be further crafted to have channels, gaps, or slots, such as osteochondral plugs (8a, 8b, 10, or 14; 22a, 22b, 23, or 25; 30a, 30b, 31, or 33) illustrated in FIG. 2-FIG. 4. Before being inserted into a holder (63 in FIG. 7), the length of the bone portion of the osteochondral plug was measured. Then, an osteochondral plug, e.g. a dumbbell shape cylindrical plug with 14 mm maximum diameter and 10 mm minimum diameter, was inserted into a holder with the cartilage cap positioned to face down and supported by the custom made bolt (60), as illustrated in FIG. 7(f).
[0136]The length of the bone portion of the osteochondral plug protruding above the top of the holder was adjusted by the custom made bolt (60). Then set screws (57), preferably to be oriented 90 degrees apart, were engaged...
example 3
Osteochondral Plug with Channels at the Cartilage / Bone Interface
[0140]The osteochondral plugs, crafted to be straight, step cylindrical, or dumbbell shape as illustrated in Example 1 can be further crafted to have channels at the cartilage cap and bone portion interface, such as osteochondral plugs (12, 24, or 32) illustrated in FIG. 2-FIG. 4. Before being inserted into a holder, the length of the bone portion of the osteochondral plug was measured. Then, the osteochondral plug, e.g. a step cylindrical plug with 10 mm diameter at the bone portion, was inserted into a holder (61), with the cartilage cap positioned to face up and the bottom of the bone portion was supported by the custom made bolt (60) as illustrated in FIG. 8(e). The length of the osteochondral plug protruding above the top of the holder was adjusted by the custom made bolt. Then four set screws (57), preferably oriented 90 degrees apart, were engaged to further secure the osteochondral plug within the holder (61) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com