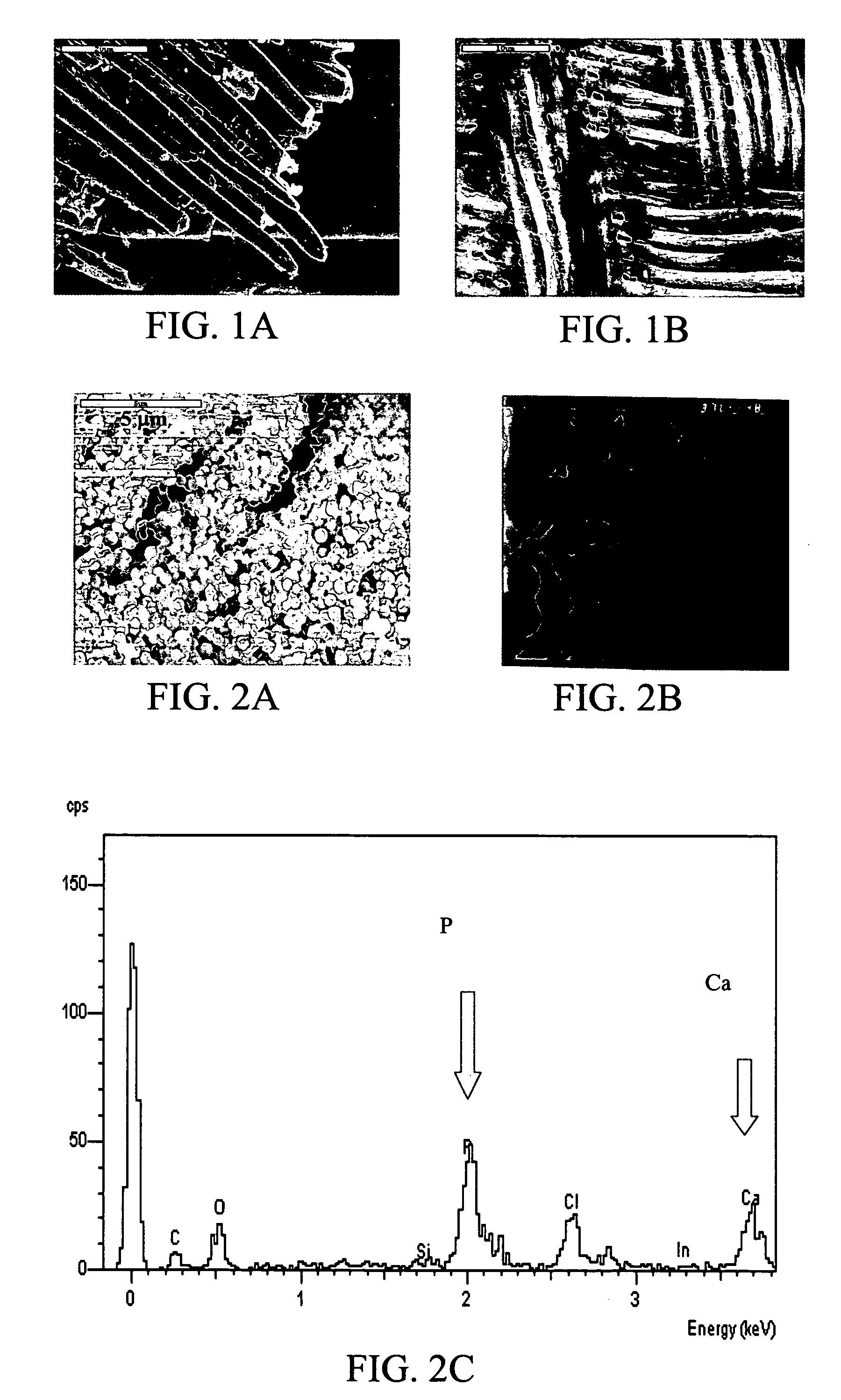
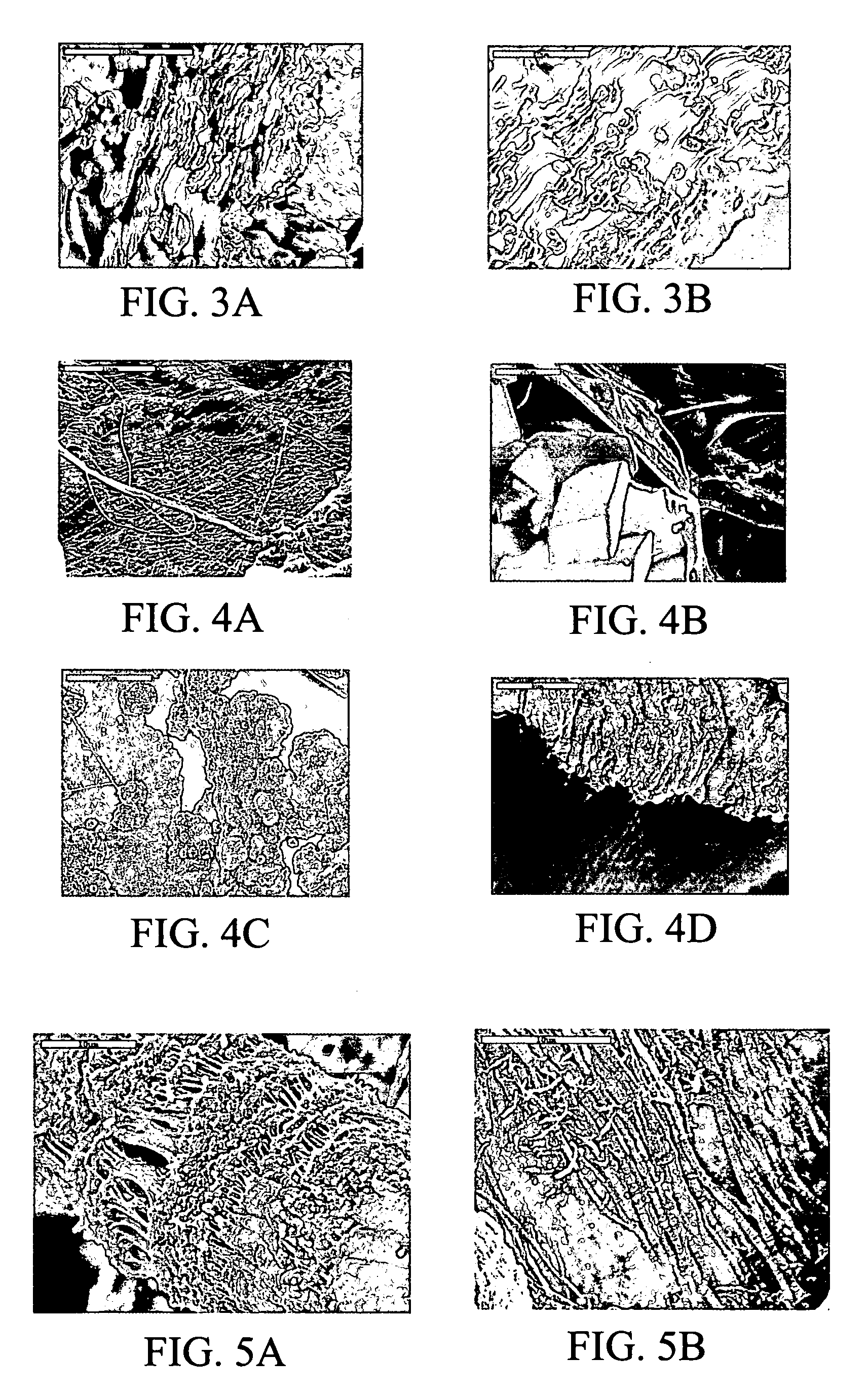
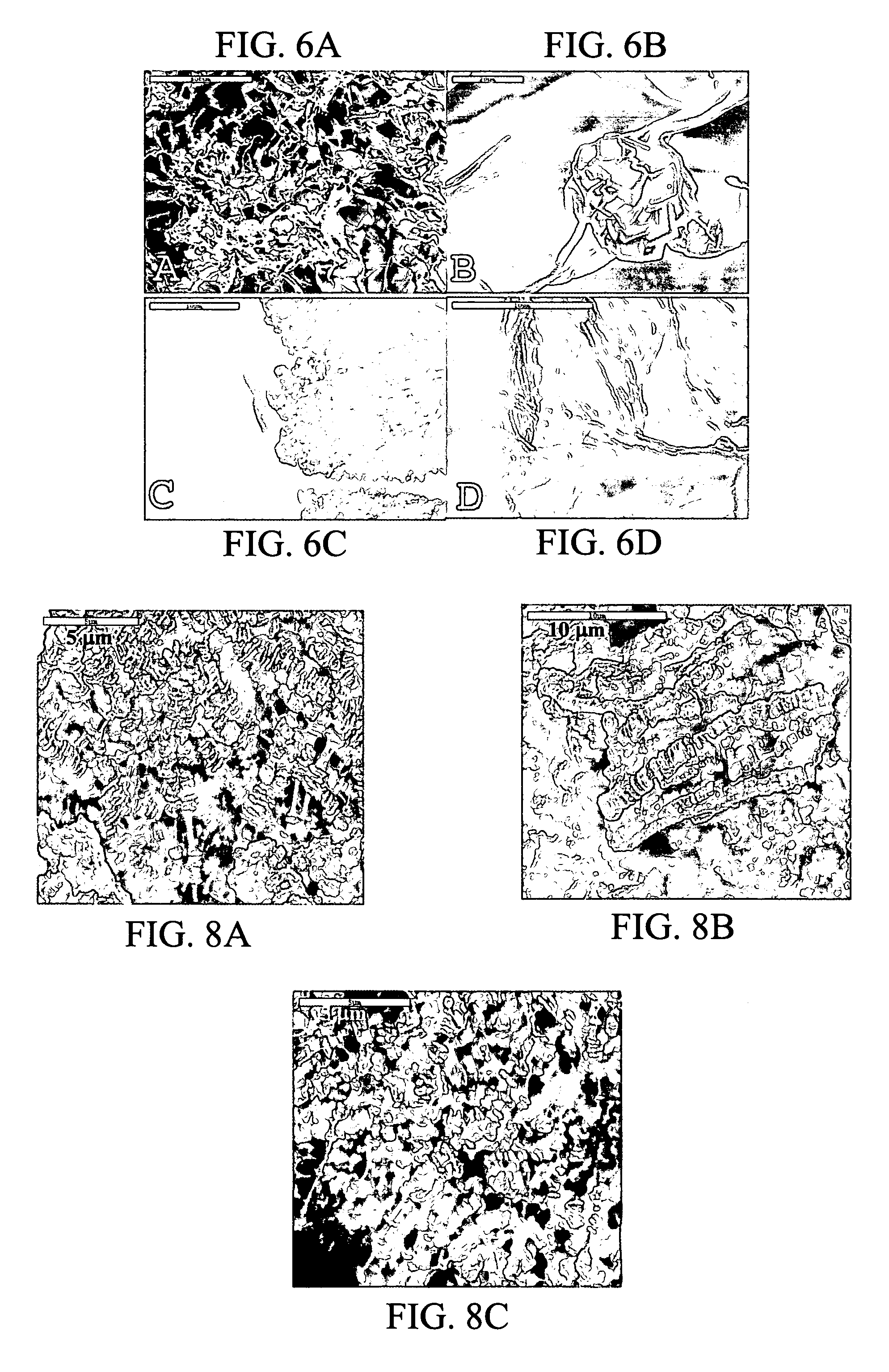
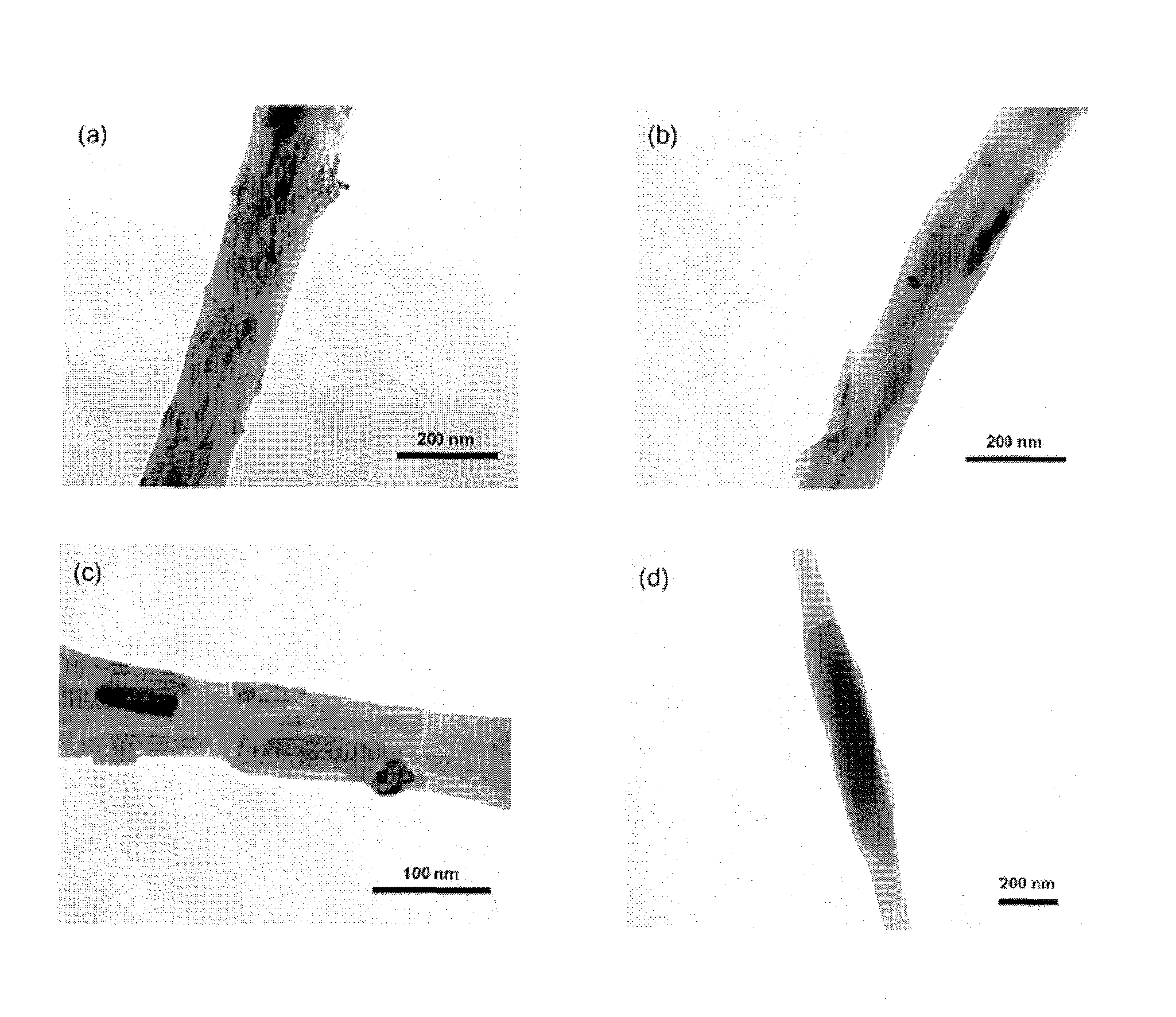
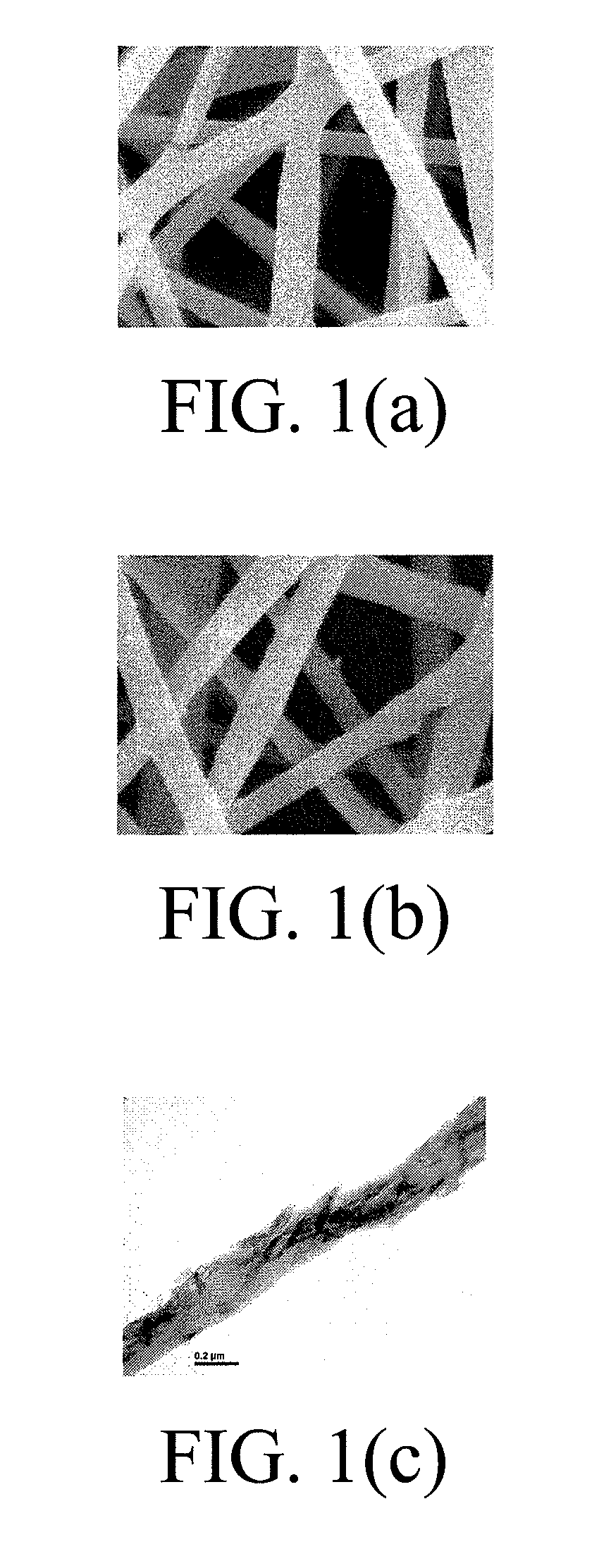
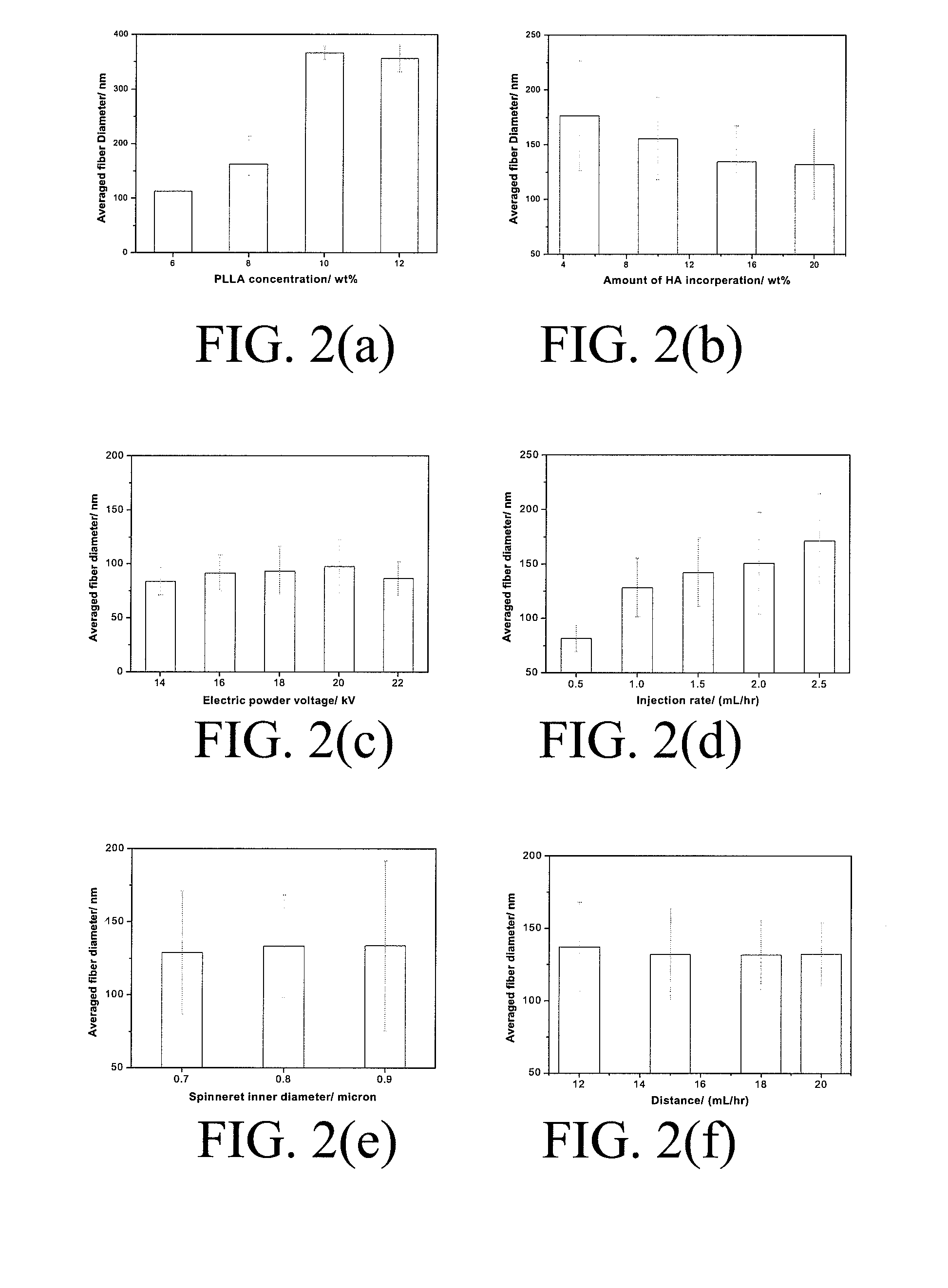
Patents
Literature
153 results about "Fibrous matrix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
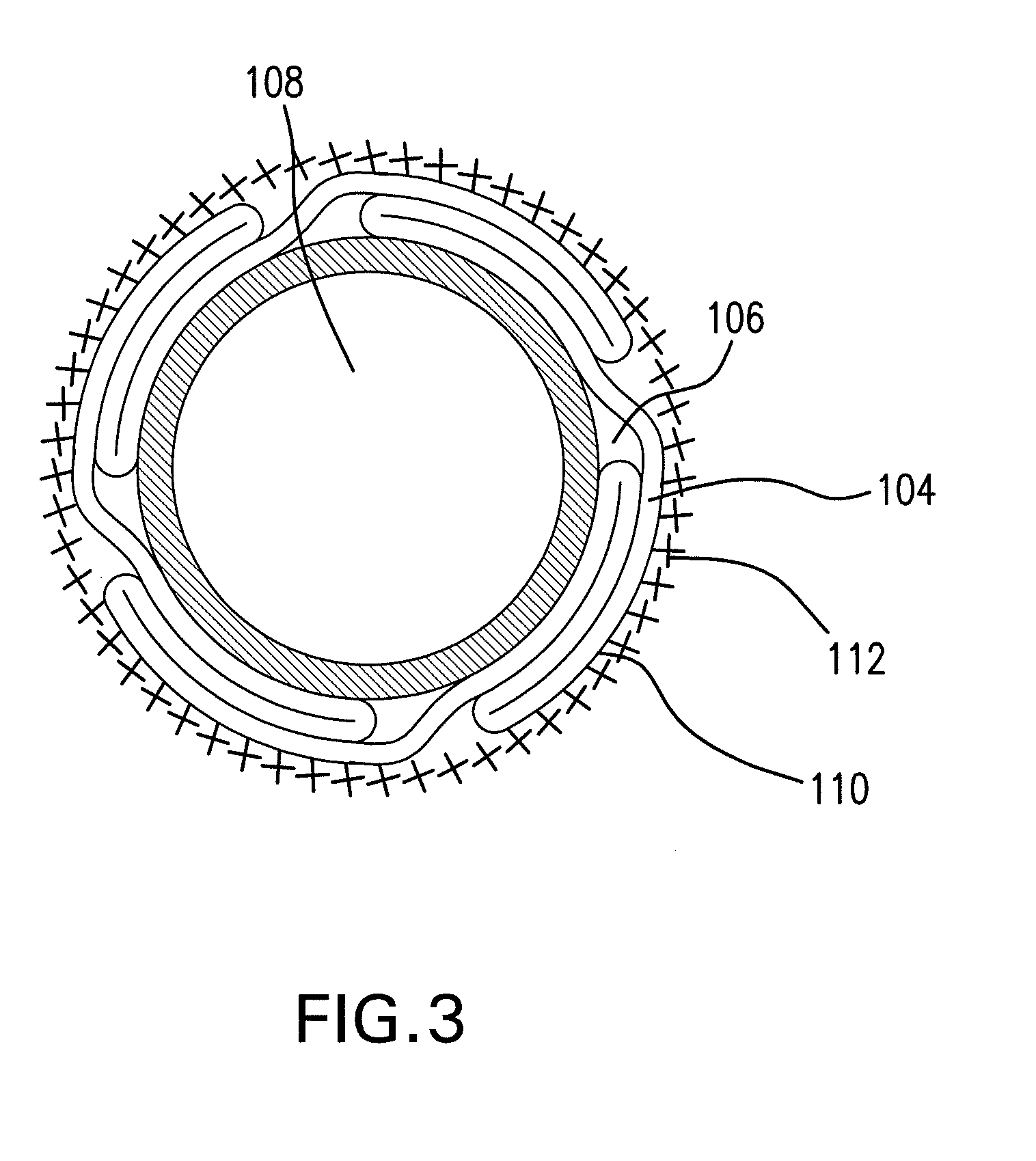
Implants for replacing cartilage, with negatively-charged hydrogel surfaces and flexible matrix reinforcement
ActiveUS9314339B2Strong and durableStrong and secure anchoringFinger jointsWrist jointsFiberChemical agent
A permanent non-resorbable implant allows surgical replacement of cartilage in articulating joints, using a hydrogel material (such as a synthetic polyacrylonitrile polymer) reinforced by a flexible fibrous matrix. Articulating hydrogel surface(s) are chemically treated to provide a negative electrical charge that emulates the negative charge of natural cartilage, and also can be treated with halogenating, cross-linking, or other chemical agents for greater strength. For meniscal-type implants, the reinforcing matrix can extend out from the peripheral rim of the hydrogel, to allow secure anchoring to soft tissue such as a joint capsule. For bone-anchored implants, a porous anchoring layer enables tissue ingrowth, and a non-planer perforated layer can provide a supportive interface between the hard anchoring material and the softer hydrogel material.
Owner:FORMAE
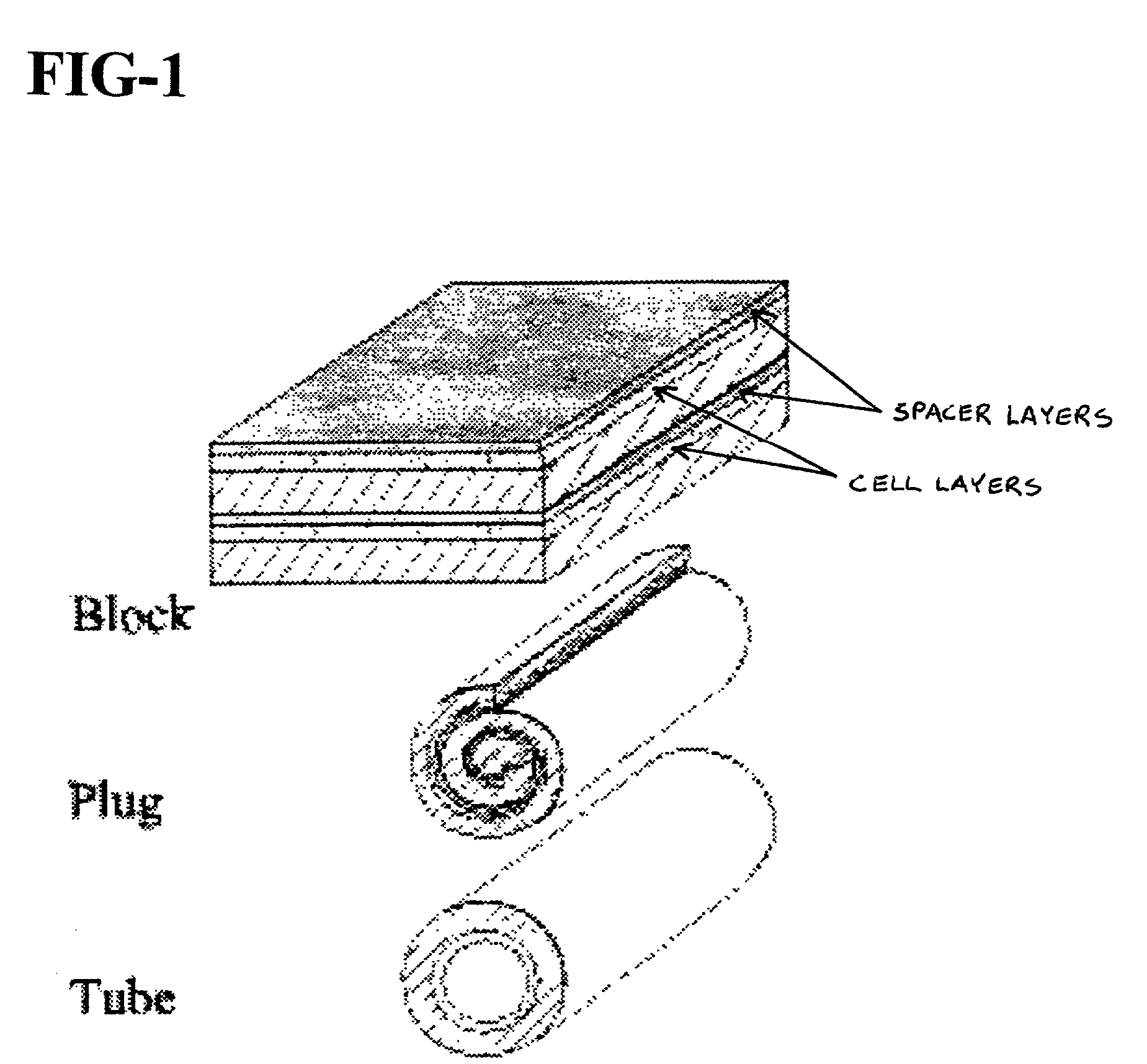
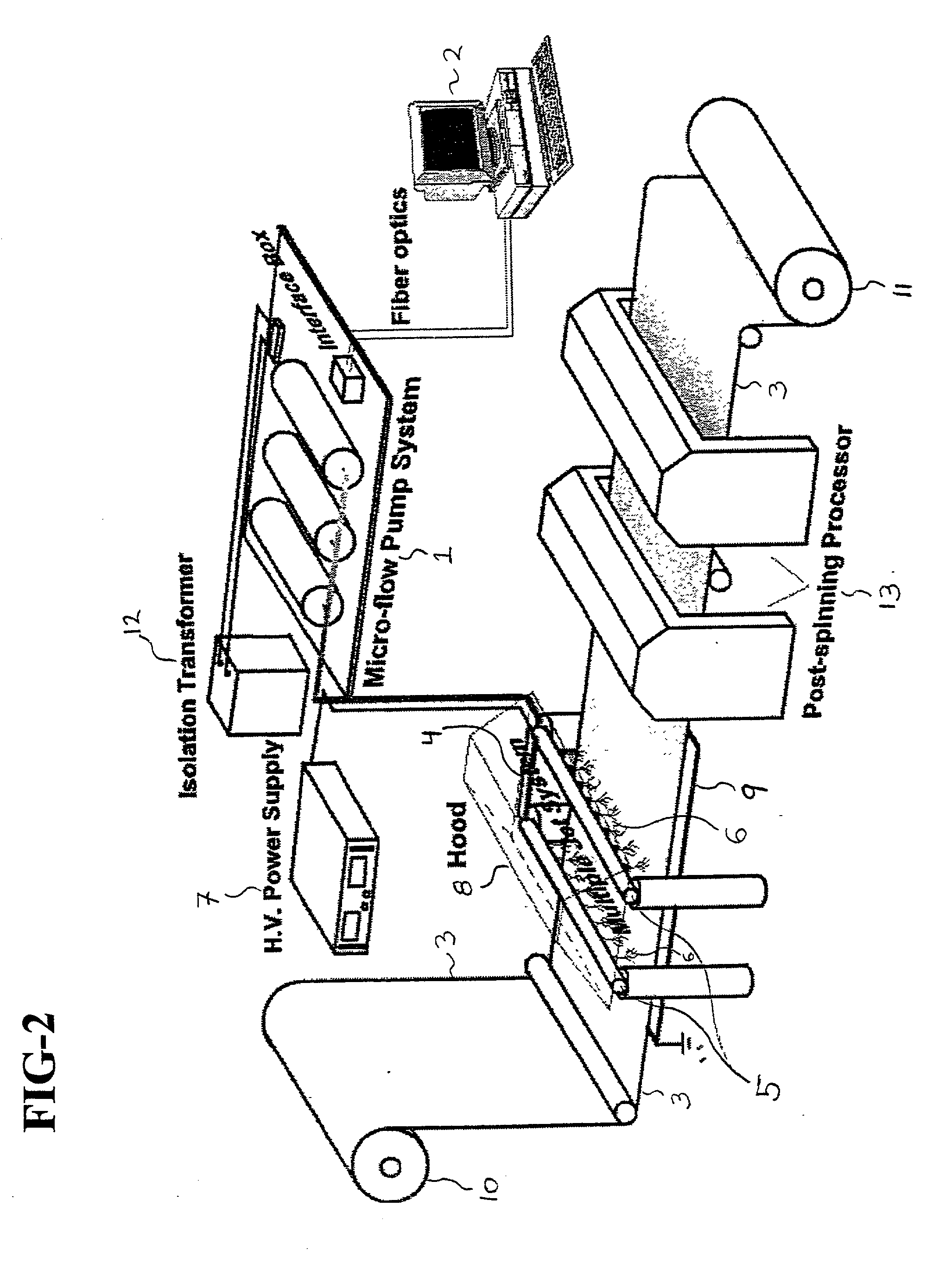
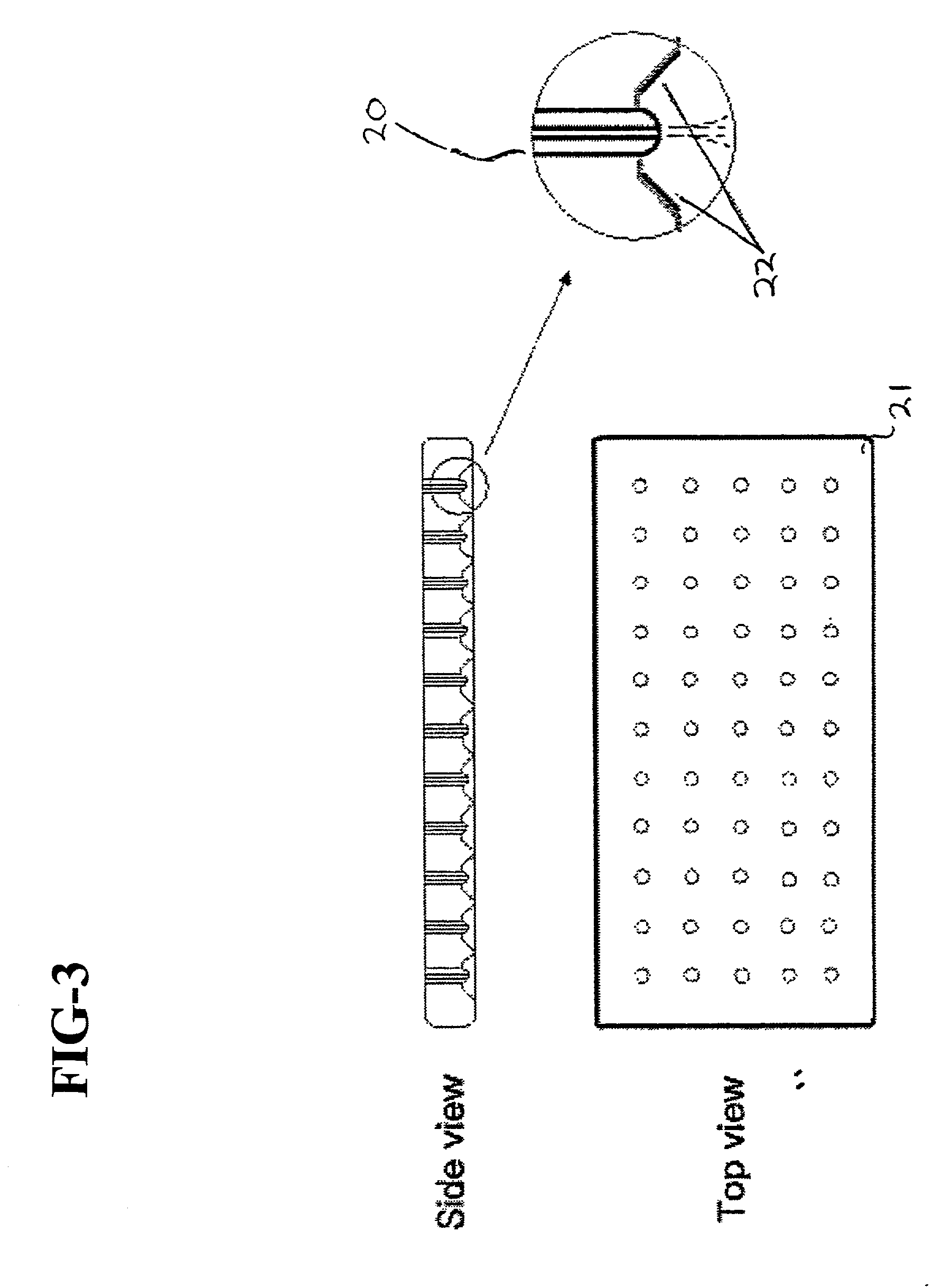
Cell delivery system comprising a fibrous matrix and cells
Cell storage and delivery systems and methods for storing and delivering viable cells to a mammal are disclosed. The cell storage and delivery systems include a biodegradable and / or bioabsorbable fibrous matrix physically associated with viable cells to contain and release the cells at a controlled rate. The biodegradable and / or bioabsorbable matrix can be formed by electrospinning fibers of biodegradable and / or bioabsorbable fiberizable material. The methods include methods for storing viable cells and for delivering viable cells to a mammal using the cell storage and delivery system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Delivery of therapeutic biologicals from implantable tissue matrices
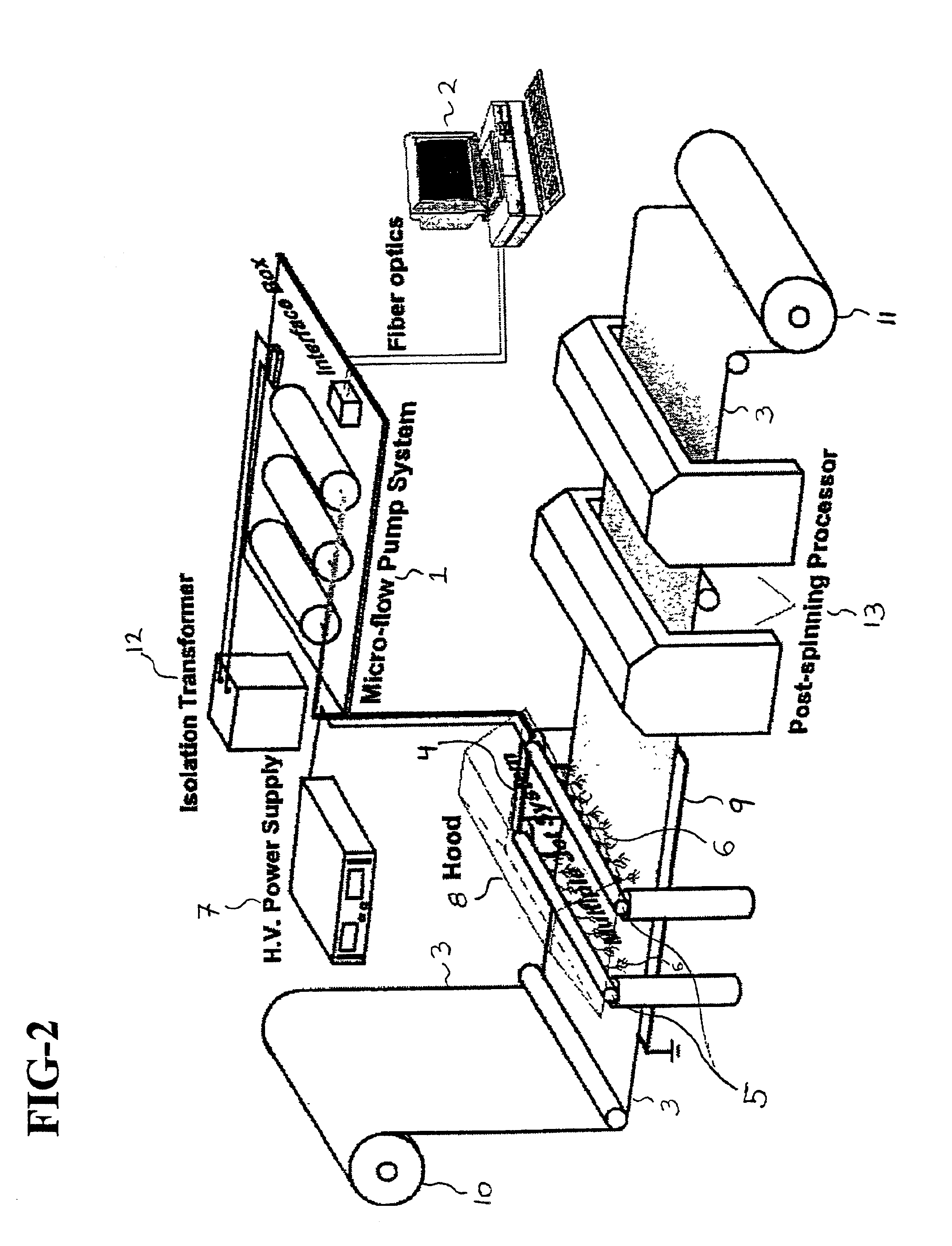
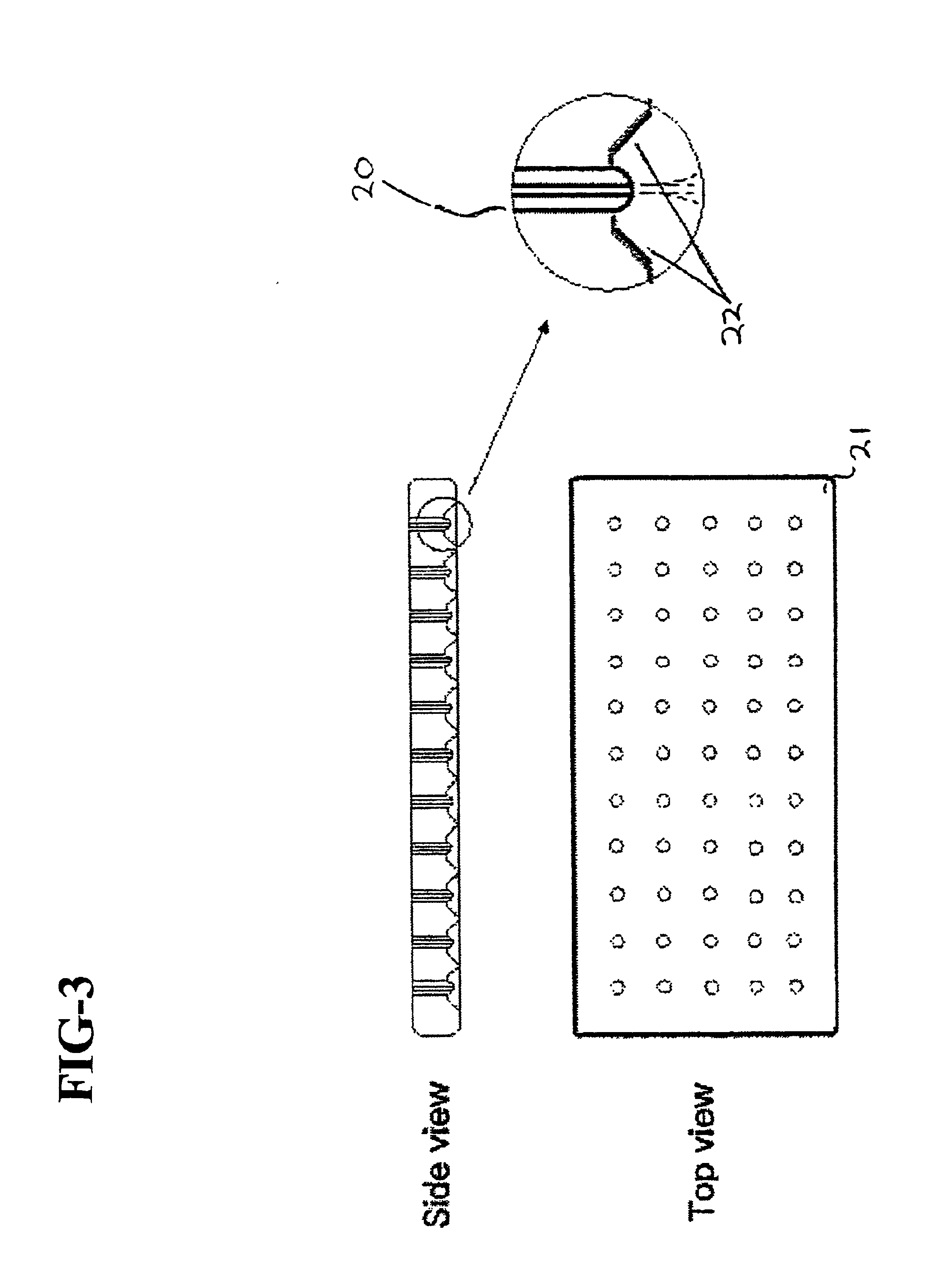
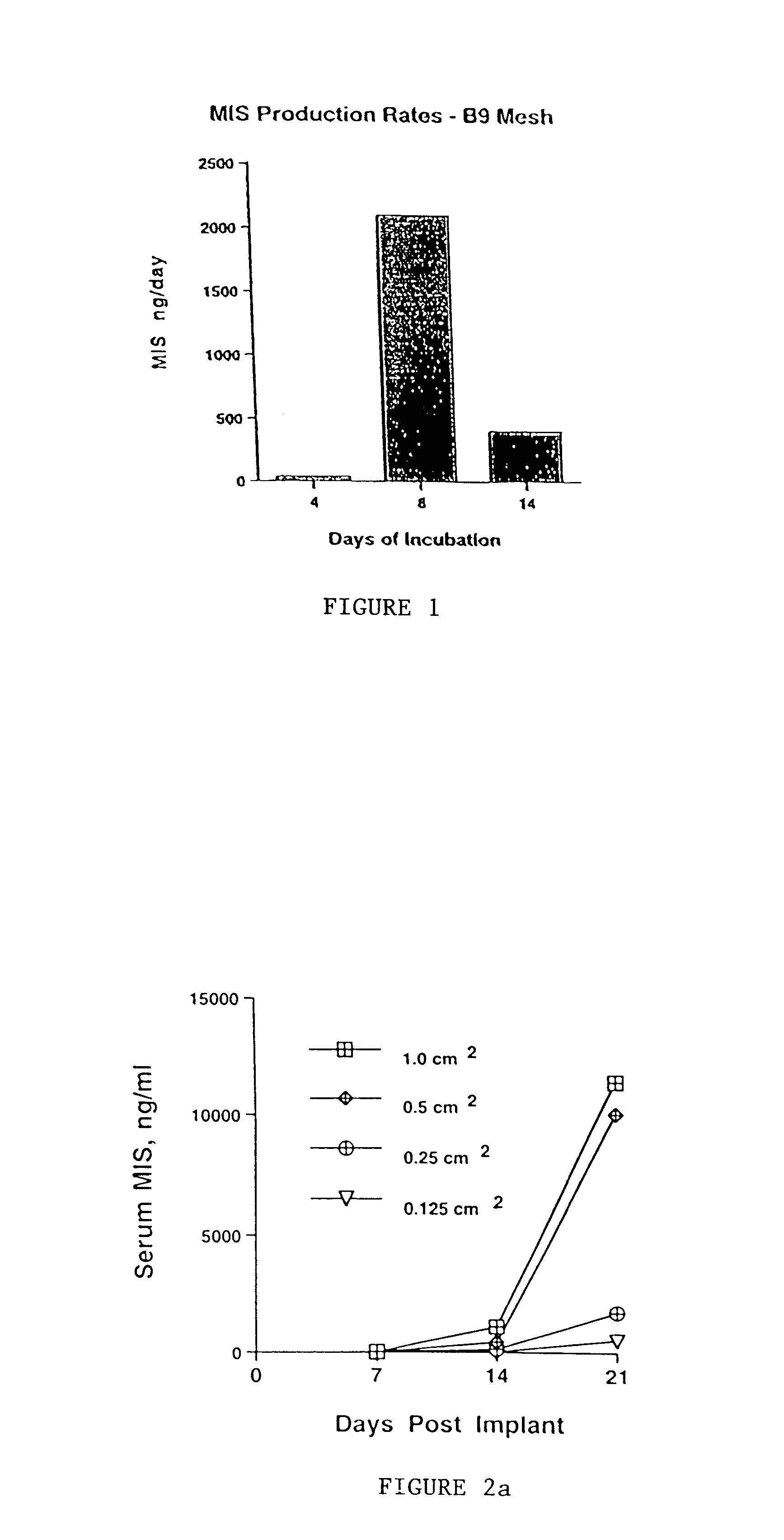
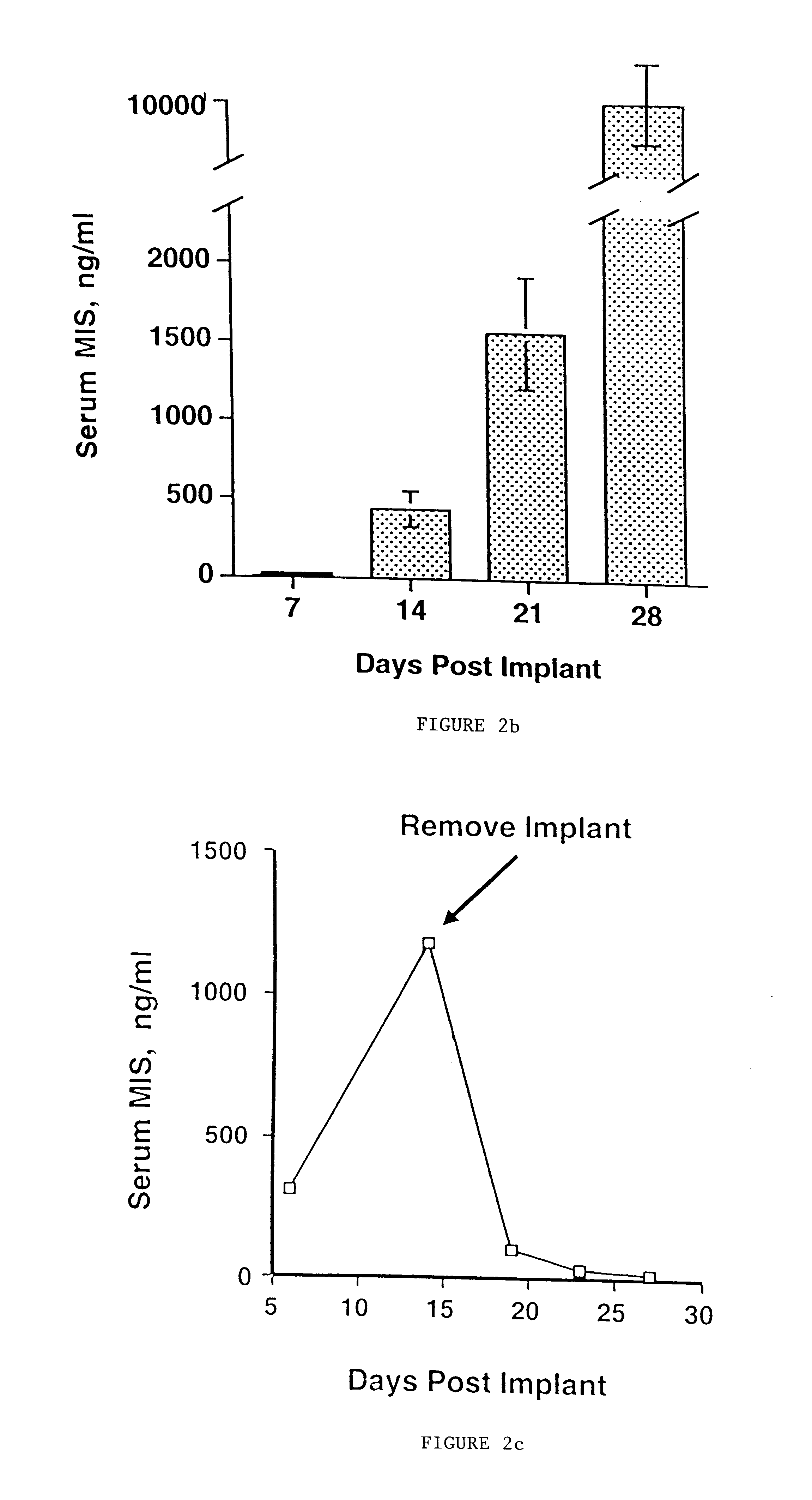
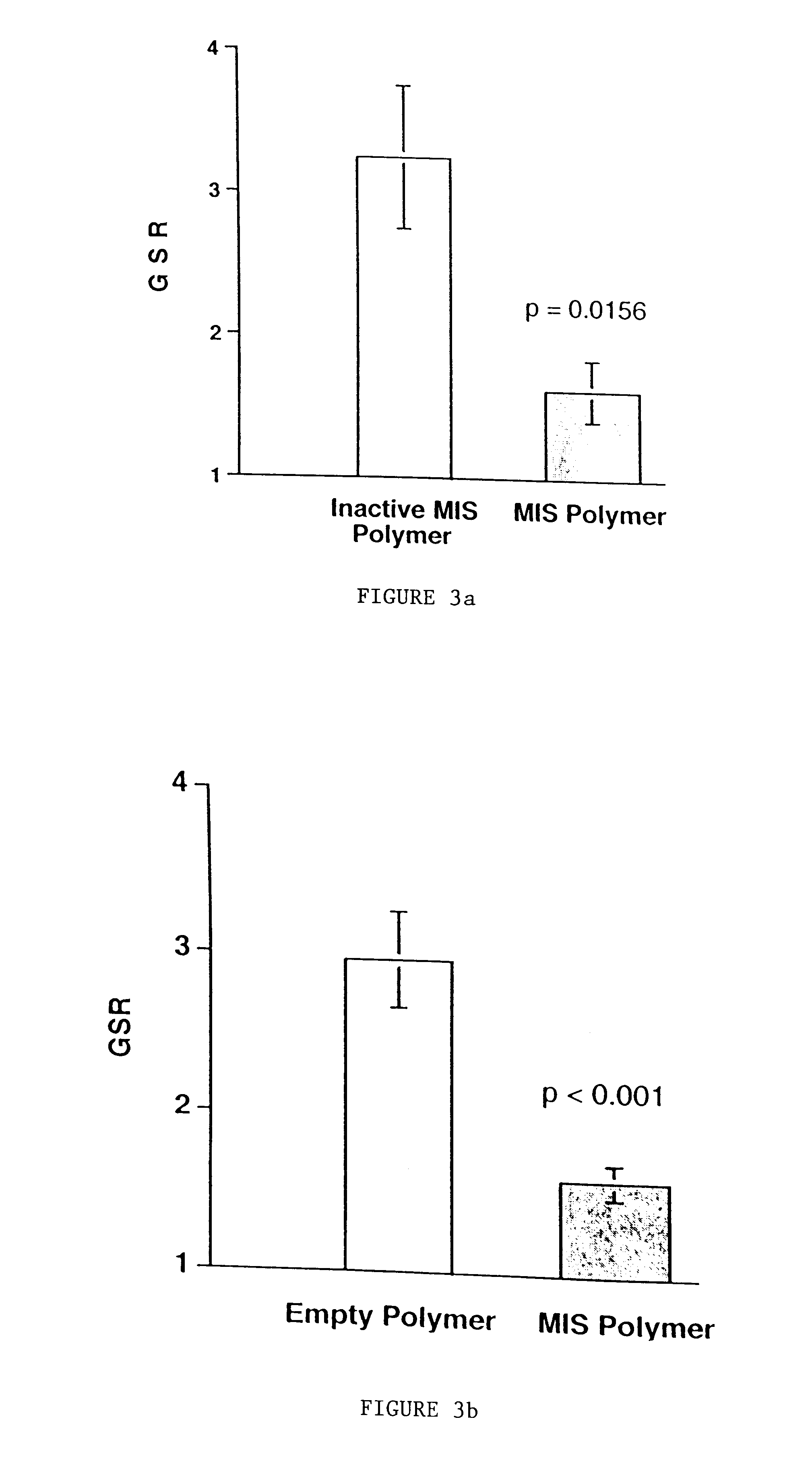
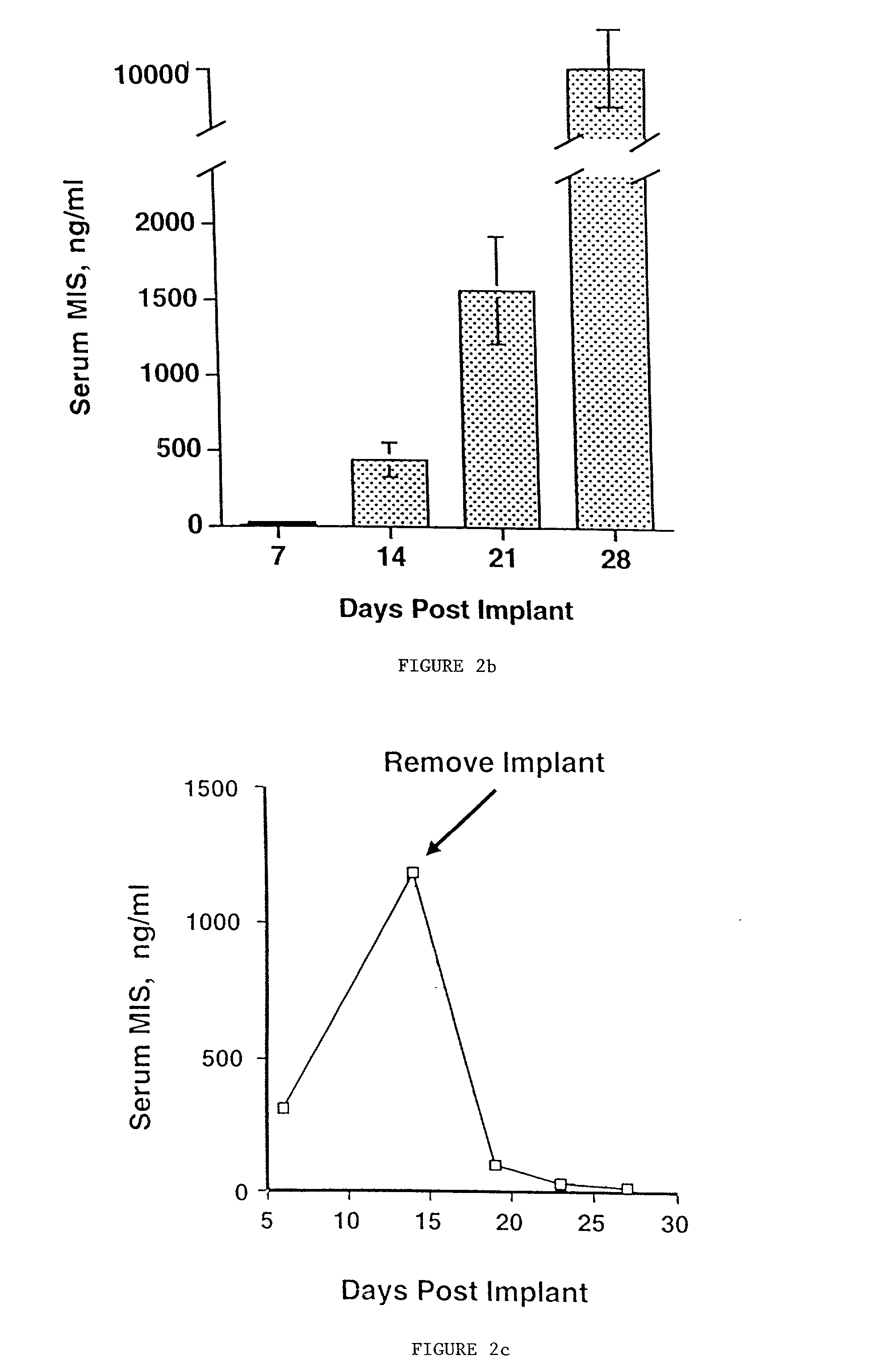
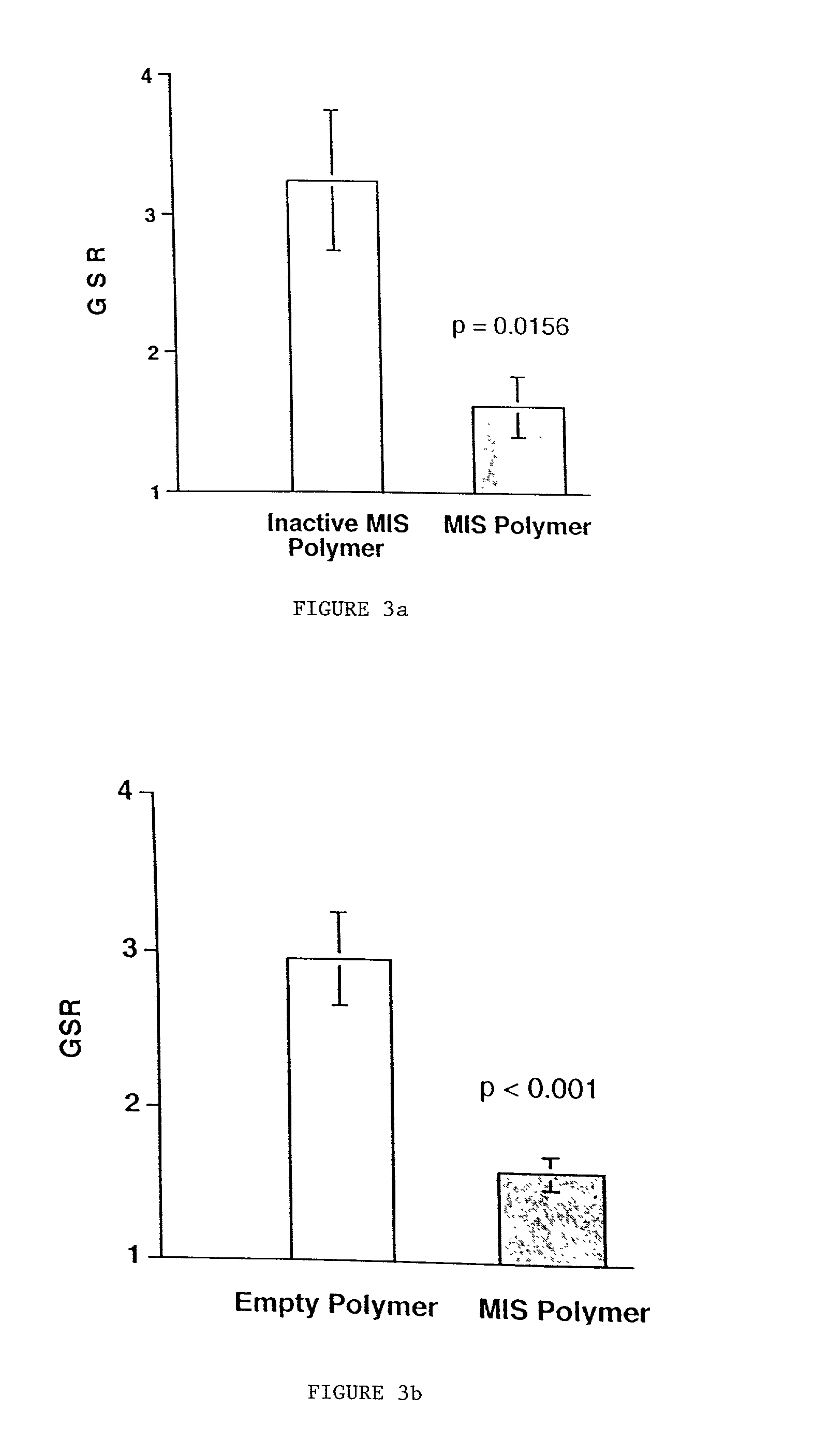
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
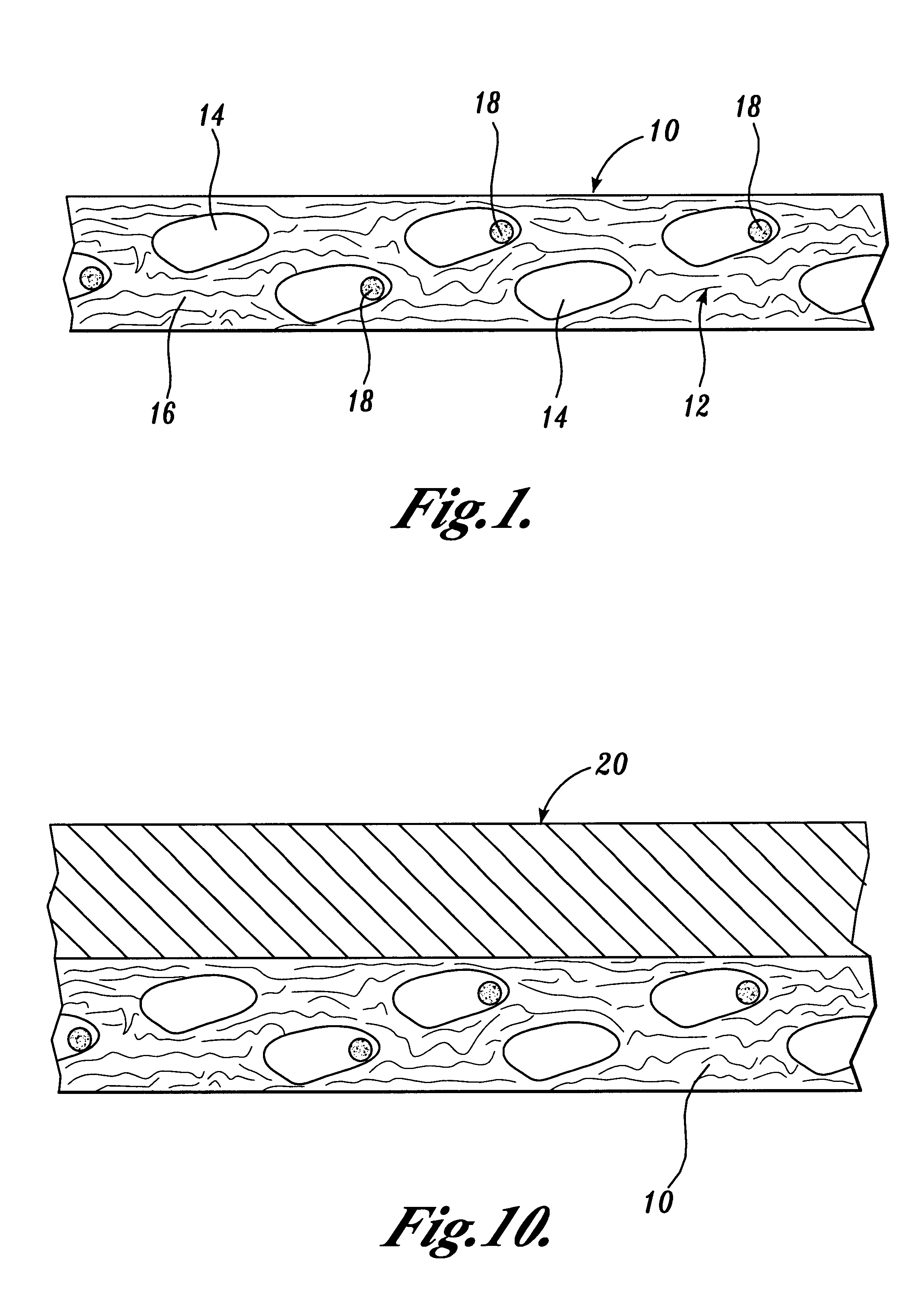
Implantable biodegradable devices for musculoskeletal repair or regeneration
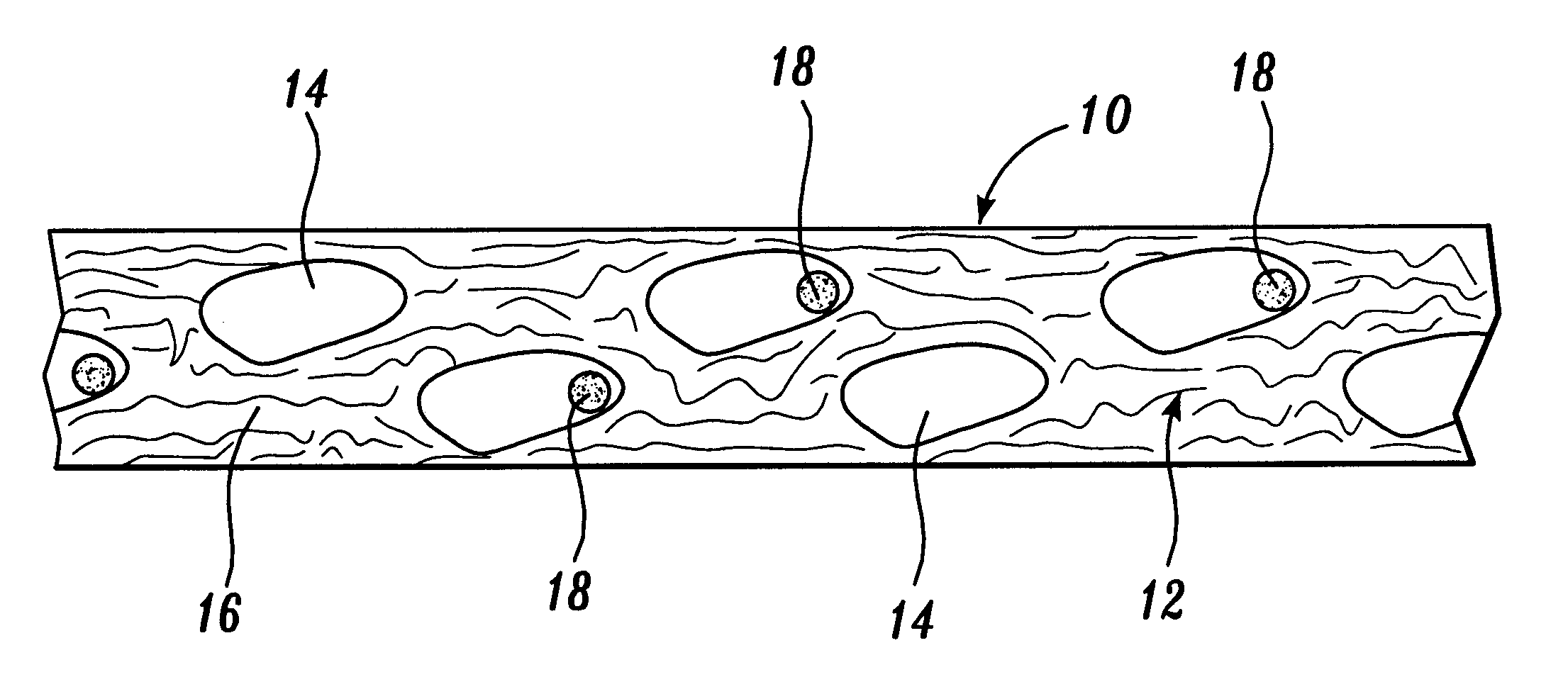
An implantable biodegradable device is disclosed containing a fibrous matrix, the fibrous matrix being constructed from fibers A and fibers B, wherein fibers A biodegrade faster than fibers B, fibers A and fibers B are present in relative amounts and are organized such that the fibrous matrix is provided with properties useful in repair and / or regeneration of mammalian tissue.
Owner:ETHICON INC
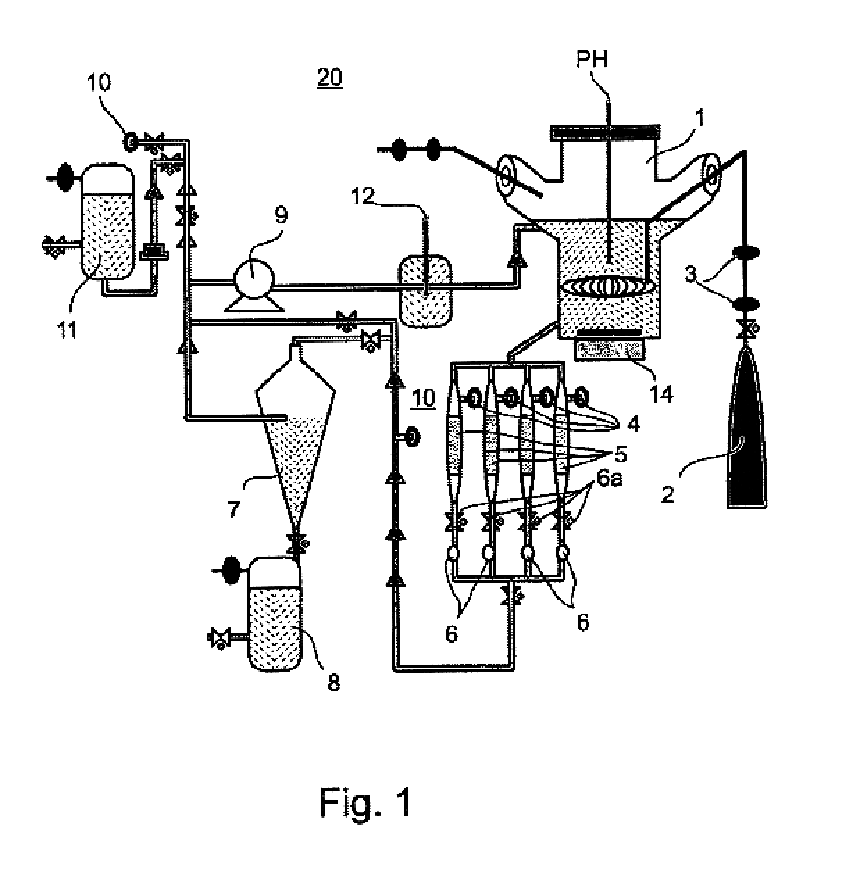
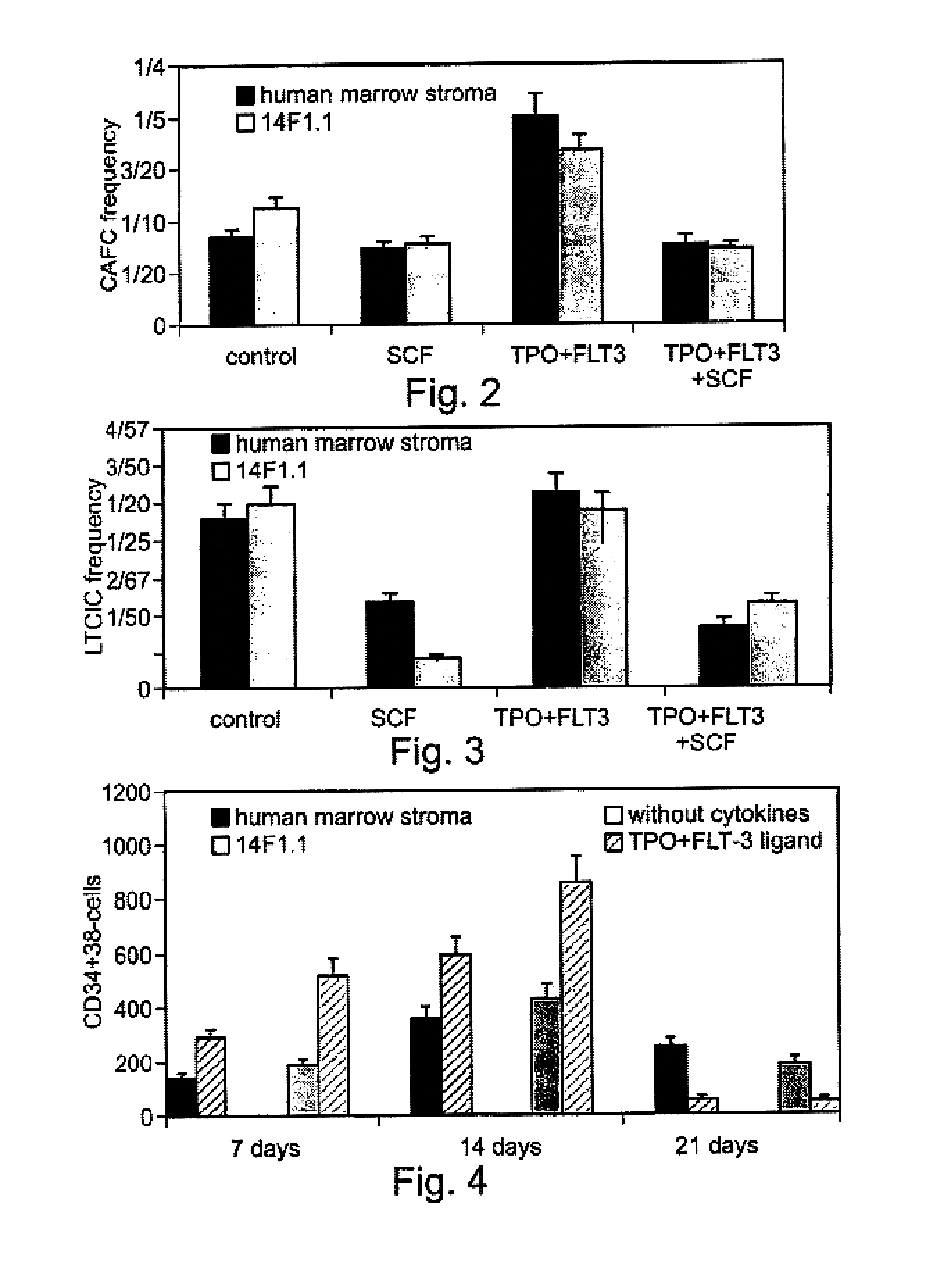
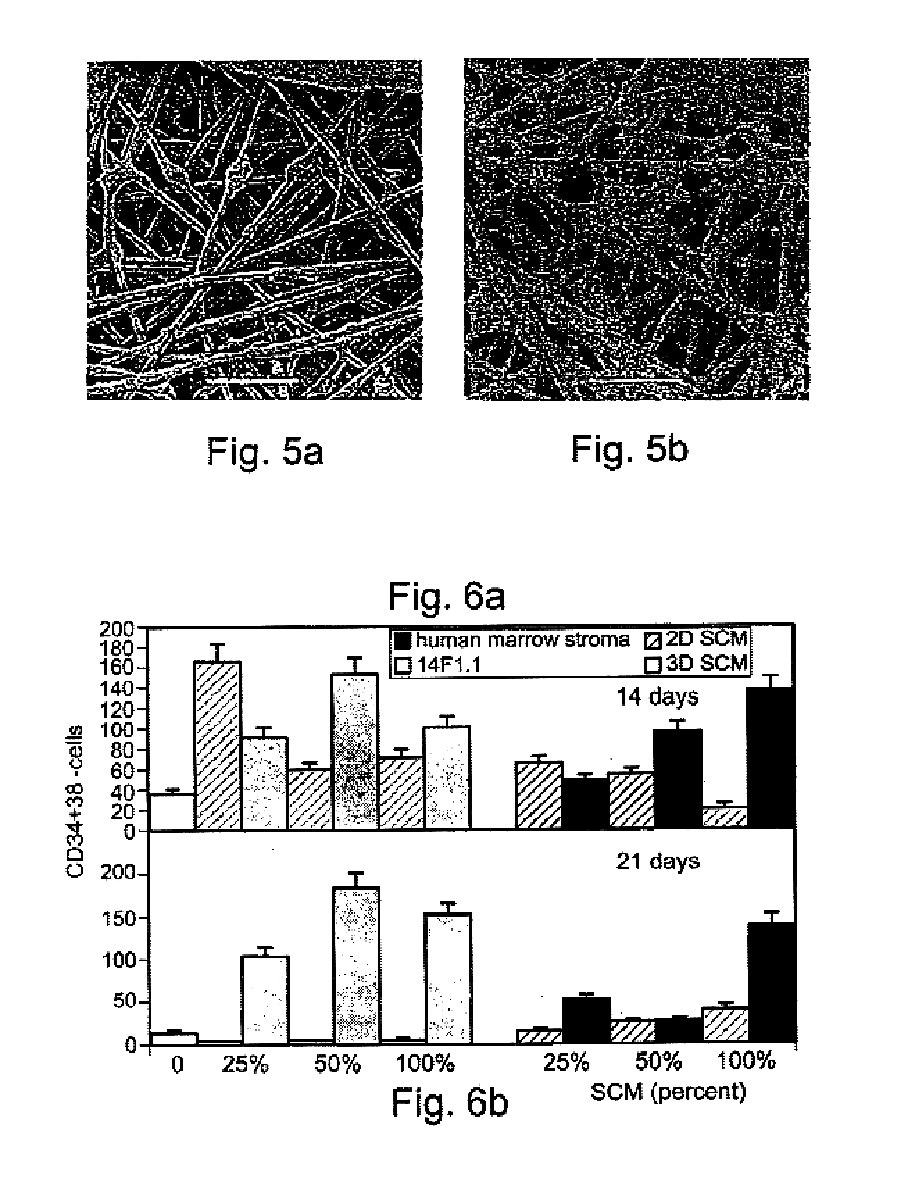
Method of producing undifferentiated hemopoietic stem cells using a stationary phase plug-flow bioreactor
A method of expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells by obtaining undifferentiated hemopoietic stem cells or progenitor cells; and either seeding the undifferentiated hemopoietic stem cells or progenitor cells into a stationary phase plug-flow bioreactor in which a three-dimensional stromal cell culture has been pre-established on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding / maintaining undifferentiated hemopoietic stem cells or progenitor cells, or culturing the undifferentiated hemopoietic stem cells or progenitor cells in conditioned medium obtained from such a reactor.
Owner:PLURISTEAM LTD +1
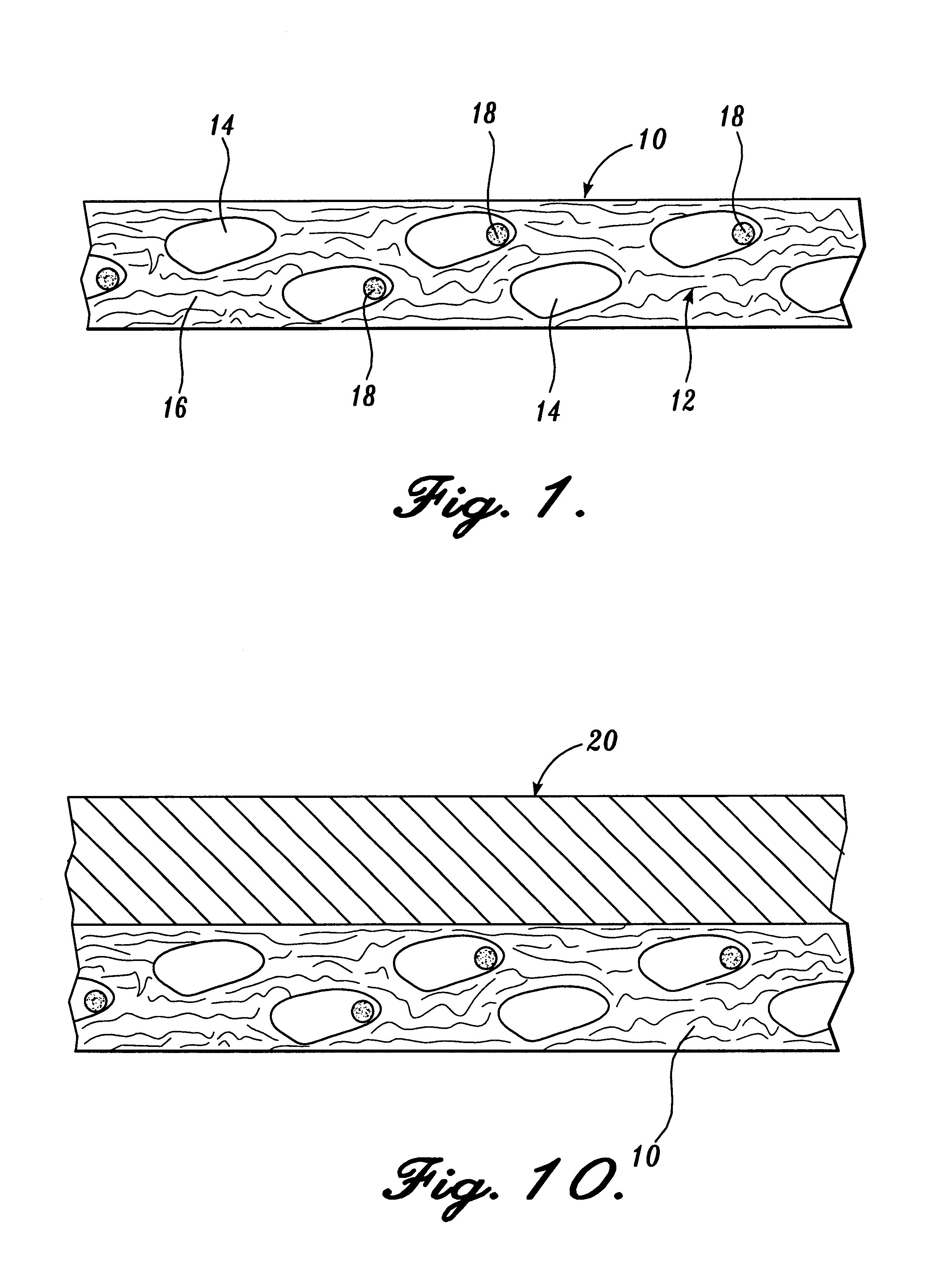
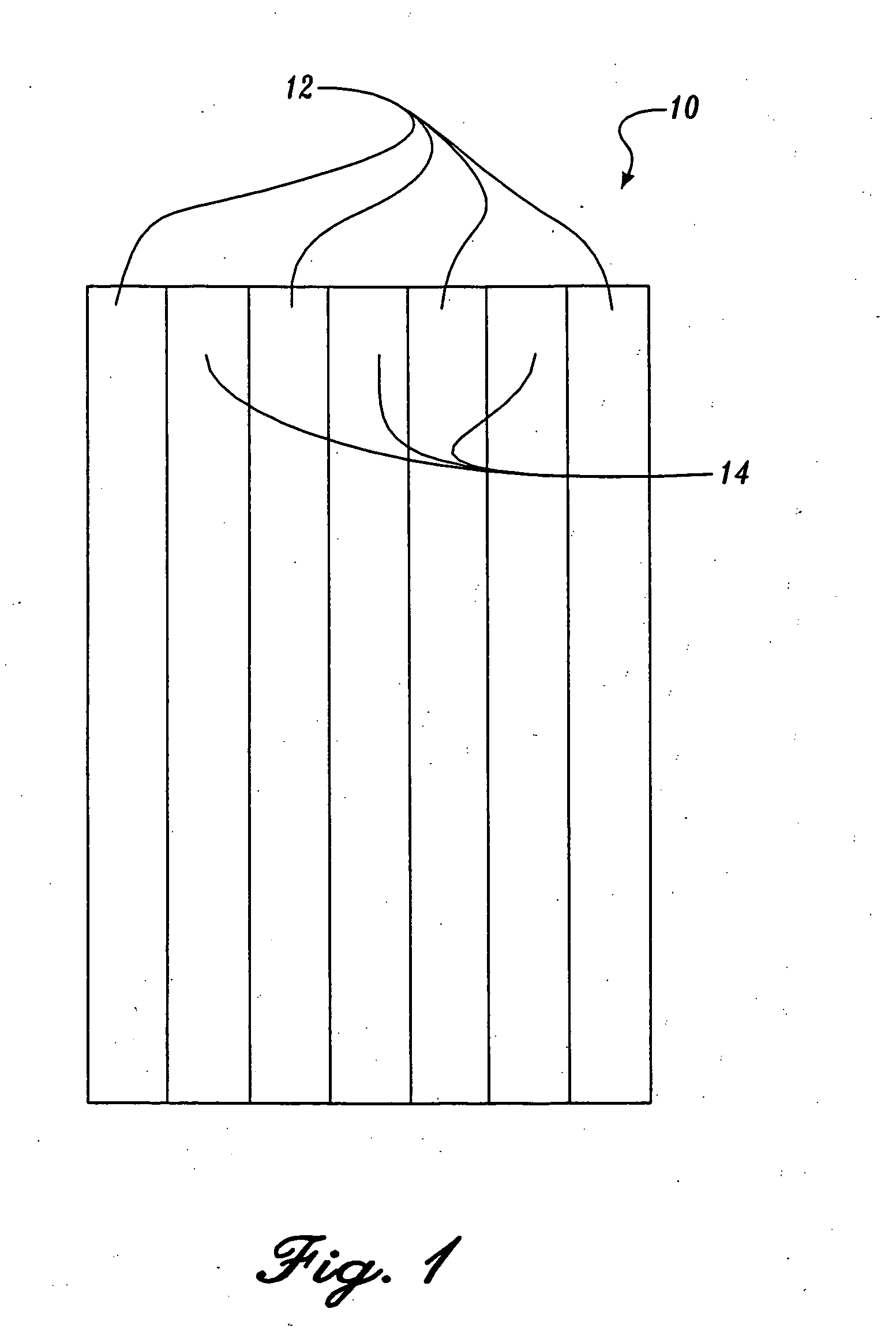
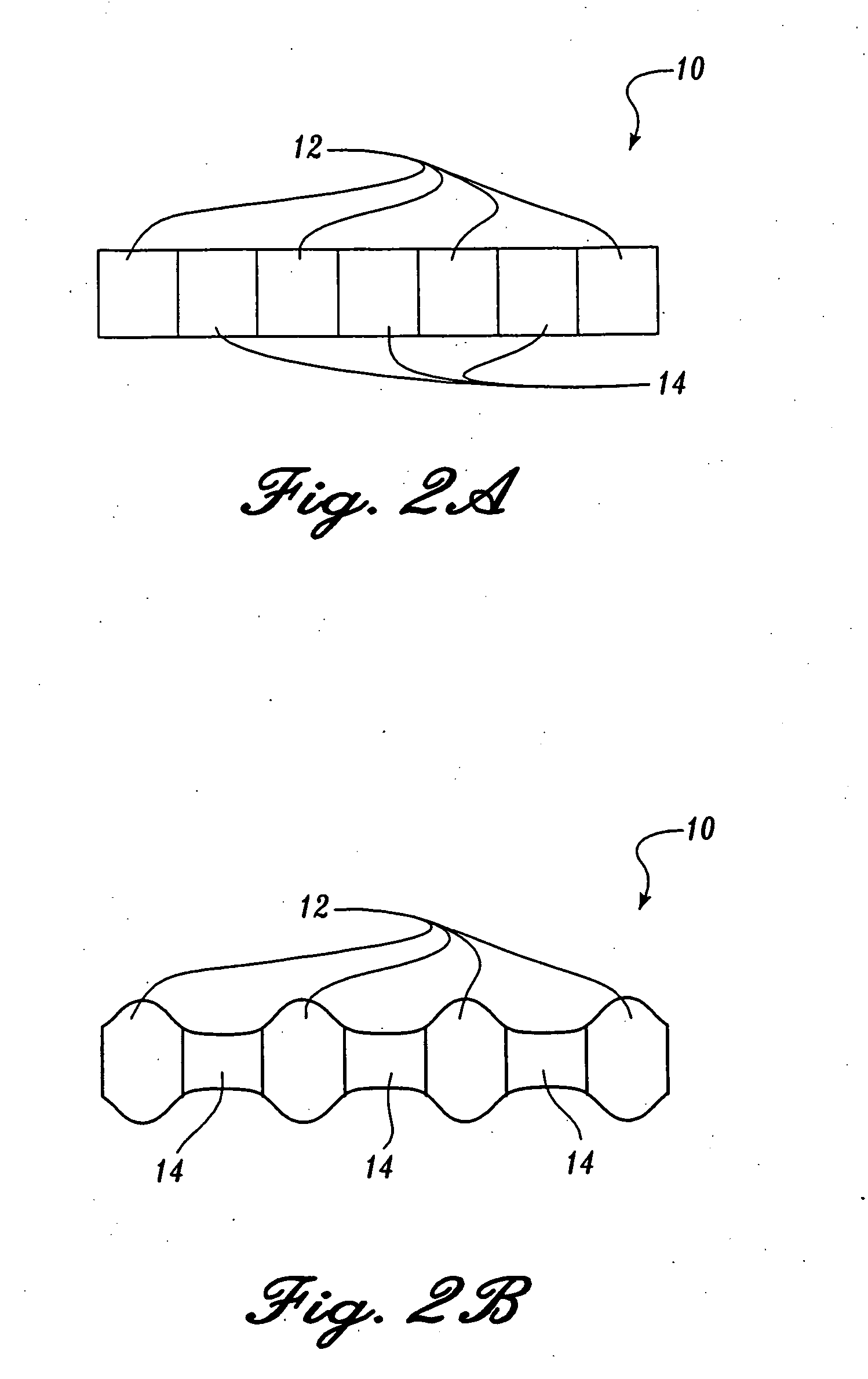
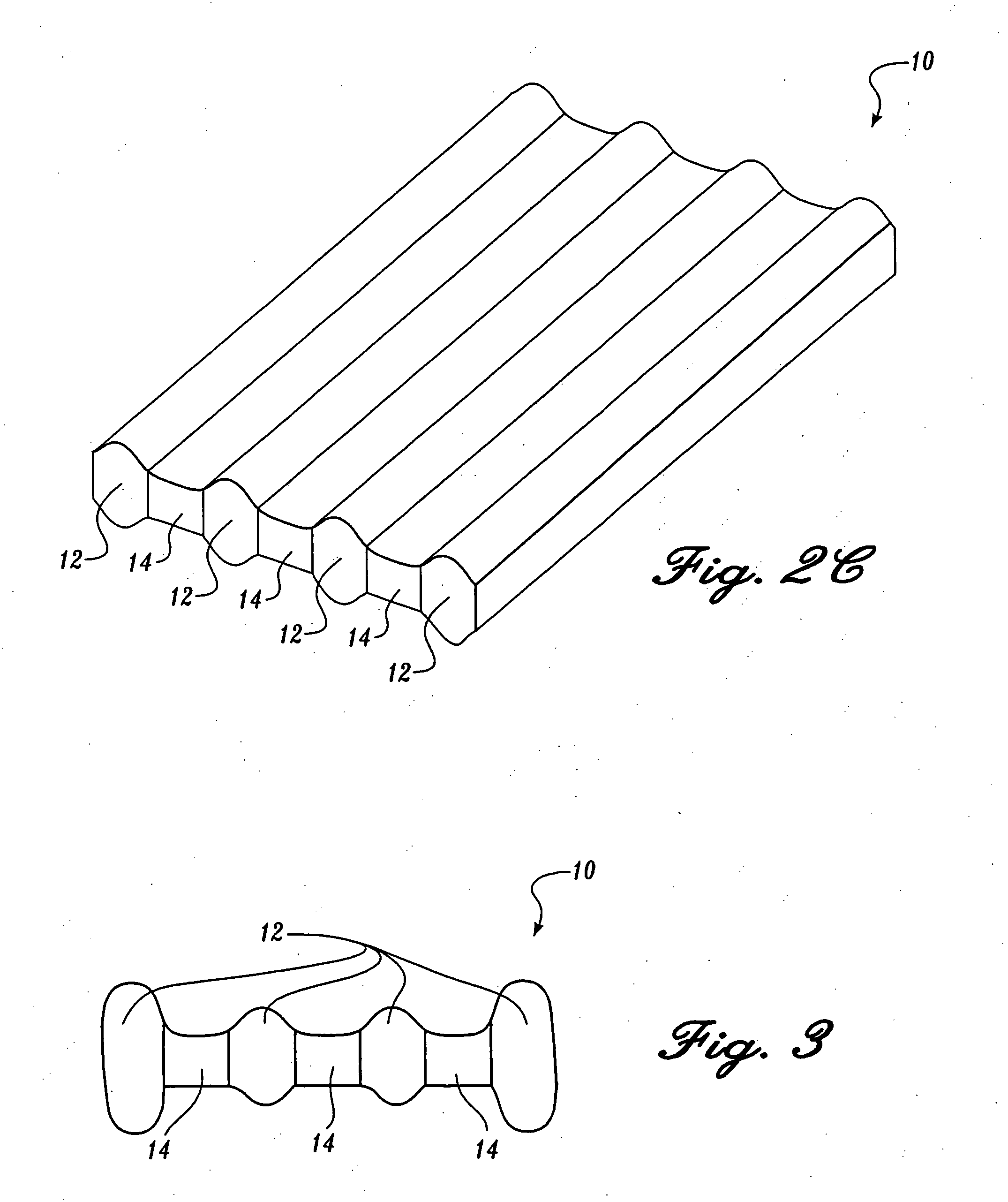
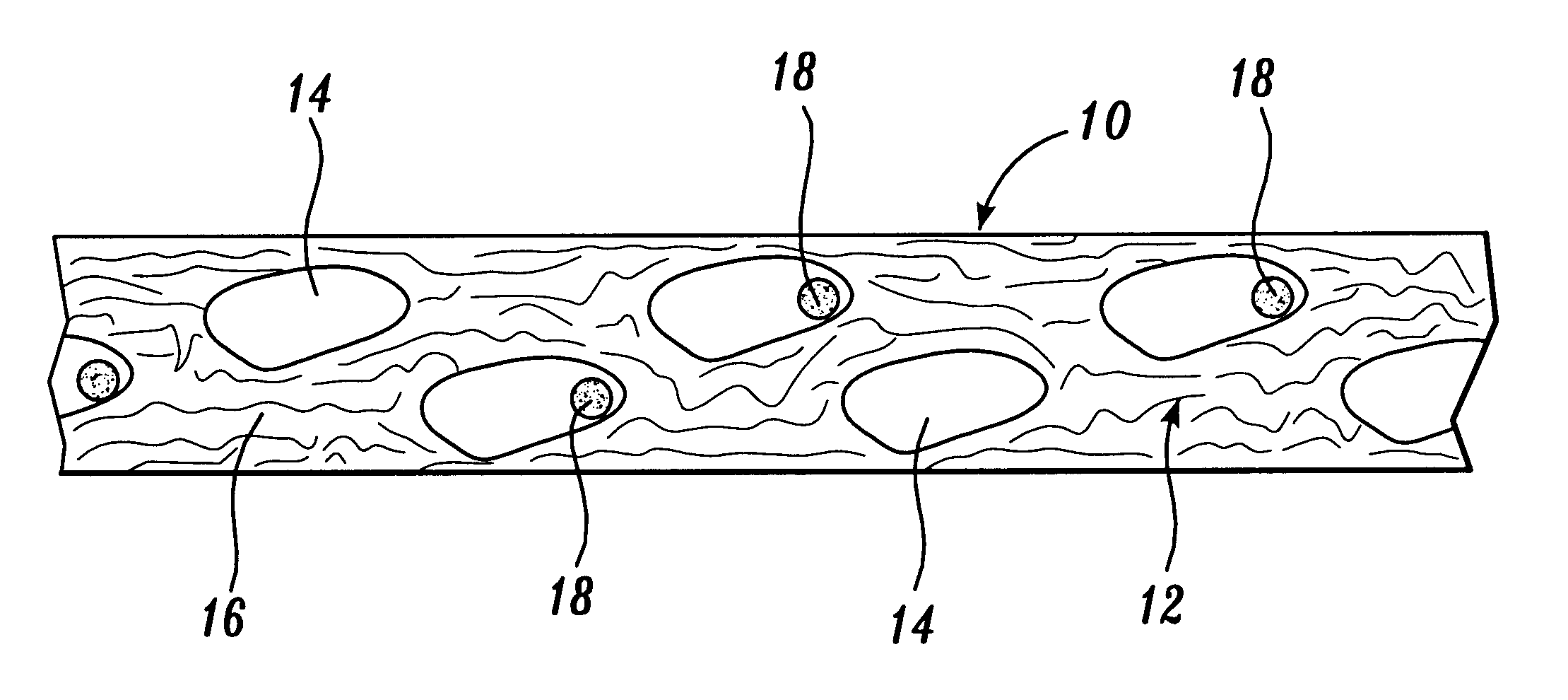
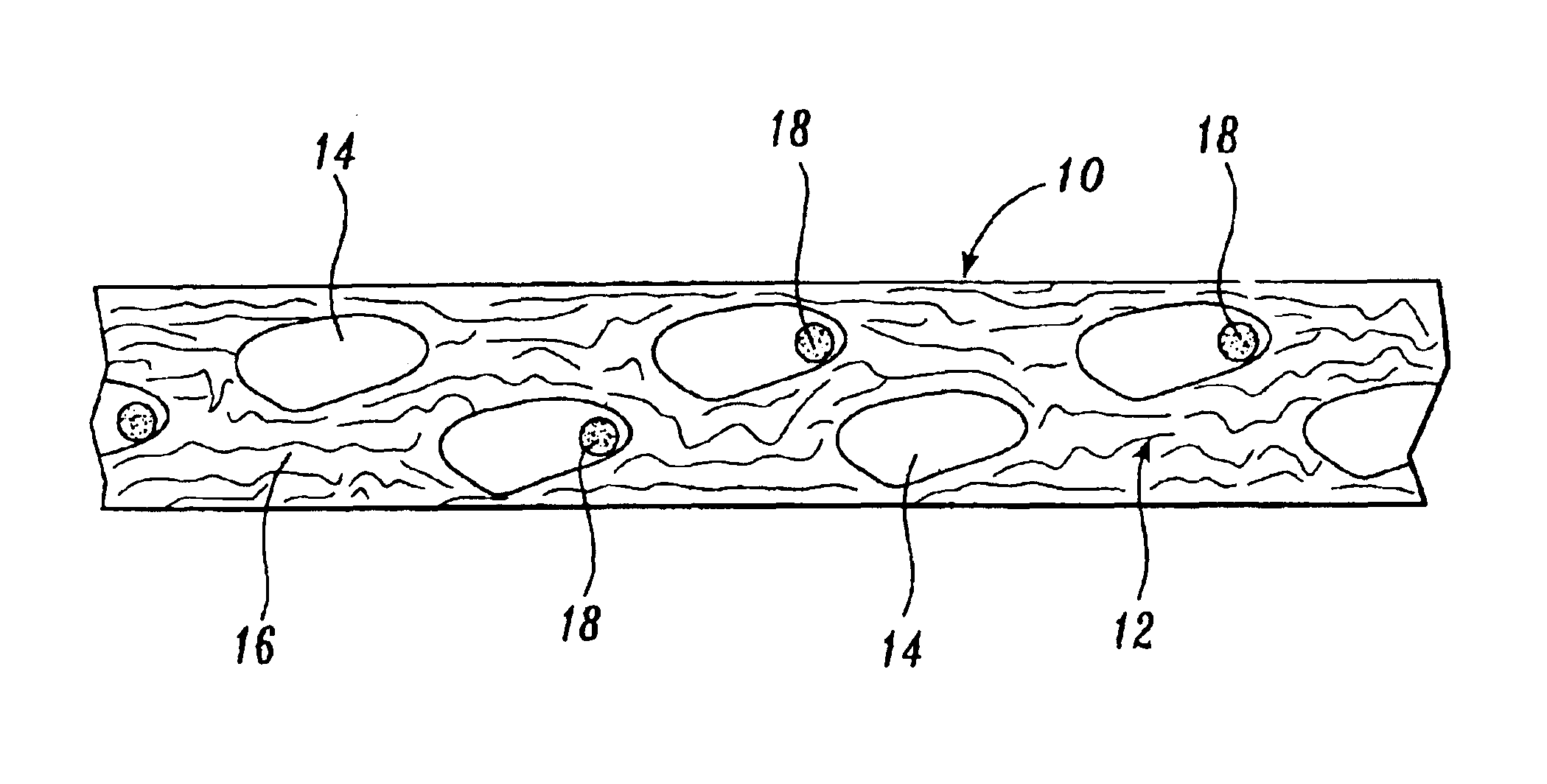
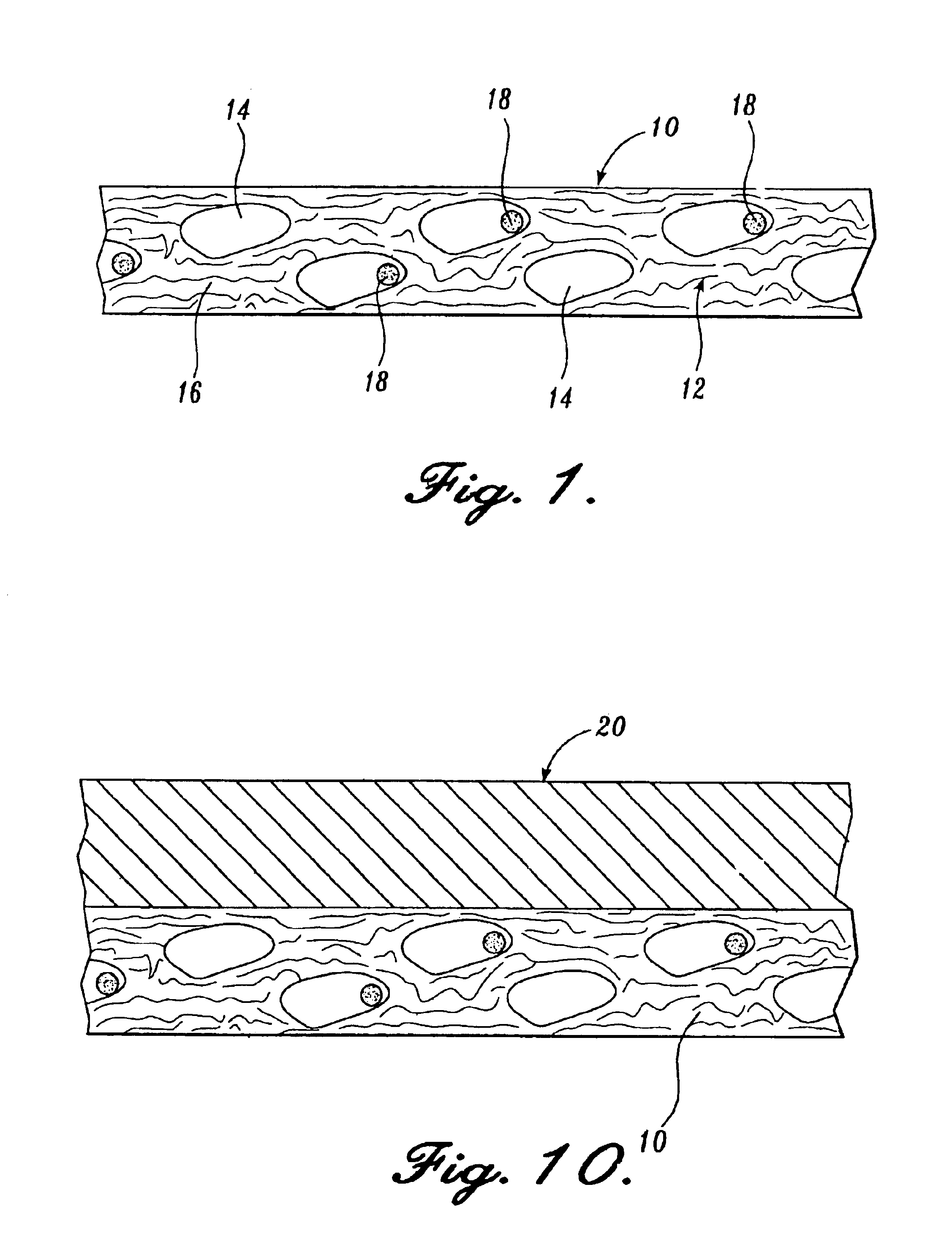
Reticulated absorbent composite
InactiveUS6969781B2Promote absorptionImprove acquisitionLayered productsBaby linensElastic fibresFiber
An absorbent composite (10) having a fibrous matrix that includes absorbent material is disclosed. The fibrous matrix defines voids (14) and passages between the voids, which are distributed throughout the composite. Absorbent material (18) is located within some of the voids (14). Absorbent material located in these voids is expandable into the void. In a preferred embodiment, the composite's fibrous matrix includes resilient and matrix fibers (16). The composite optionally includes a wet strength agent.
Owner:NAT INST FOR STRATEGIC TECH ACQUISITIONS & COMMLIZATION
Fluted composite and related absorbent articles
InactiveUS20060081348A1Improve acquisitionImprove distributionNon-fibrous pulp additionNatural cellulose pulp/paperFiberFlute
An absorbent composite that includes a fibrous matrix having absorbent material dispersed in bands along the composite's length is disclosed. The bands define liquid distribution zones. On liquid contact, absorbent material swelling occurs and produces a wetted composite having flutes that include swollen absorbent material separated by distribution zones, regions of the composite that are substantially free of absorbent material. Absorbent articles that include the composite are also disclosed.
Owner:GRAEF PETER A +6
Cell storage and delivery system
Cell storage and delivery systems and methods for storing and delivering viable cells to a mammal are disclosed. The cell storage and delivery systems include a biodegradable and / or bioabsorbable fibrous matrix physically associated with viable cells to contain and release the cells at a controlled rate. The biodegradable and / or bioabsorbable matrix can be formed by electrospinning fibers of biodegradable and / or bioabsorbable fiberizable material. The methods include methods for storing viable cells and for delivering viable cells to a mammal using the cell storage and delivery system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Absorbent composite having fibrous bands
InactiveUS6867346B1Promote absorptionImprove acquisitionLayered productsBaby linensFiberPolymer science
An absorbent composite having fibrous bands is described. The composite includes one or more fibrous bands in a fibrous base. The base includes a fibrous matrix and absorbent material. The fibrous bands are substantially free of absorbent material. Absorbent articles that include the composite and methods for forming the composite are also disclosed.
Owner:NAT INST FOR STRATEGIC TECH ACQUISITIONS & COMMLIZATION
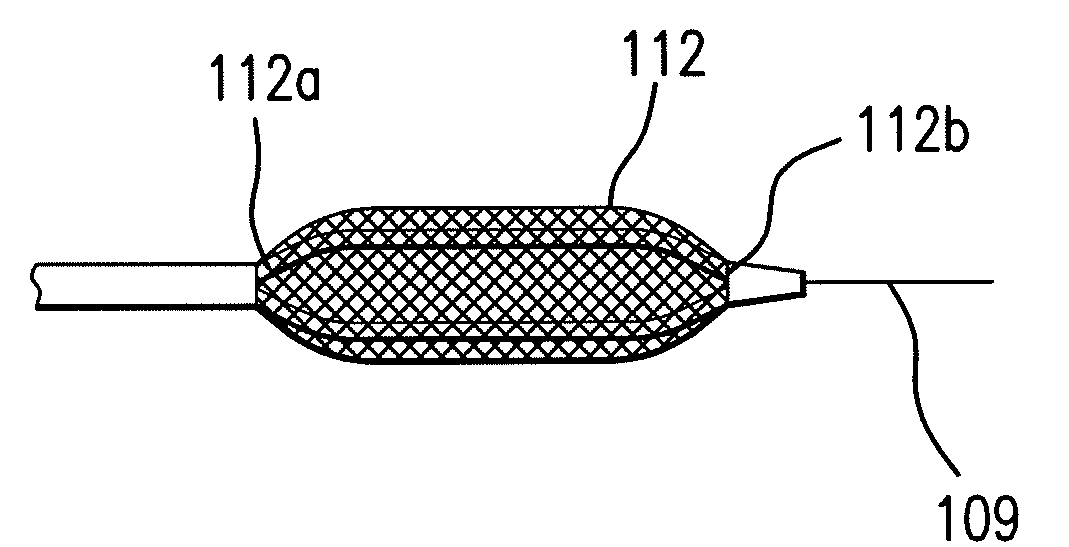
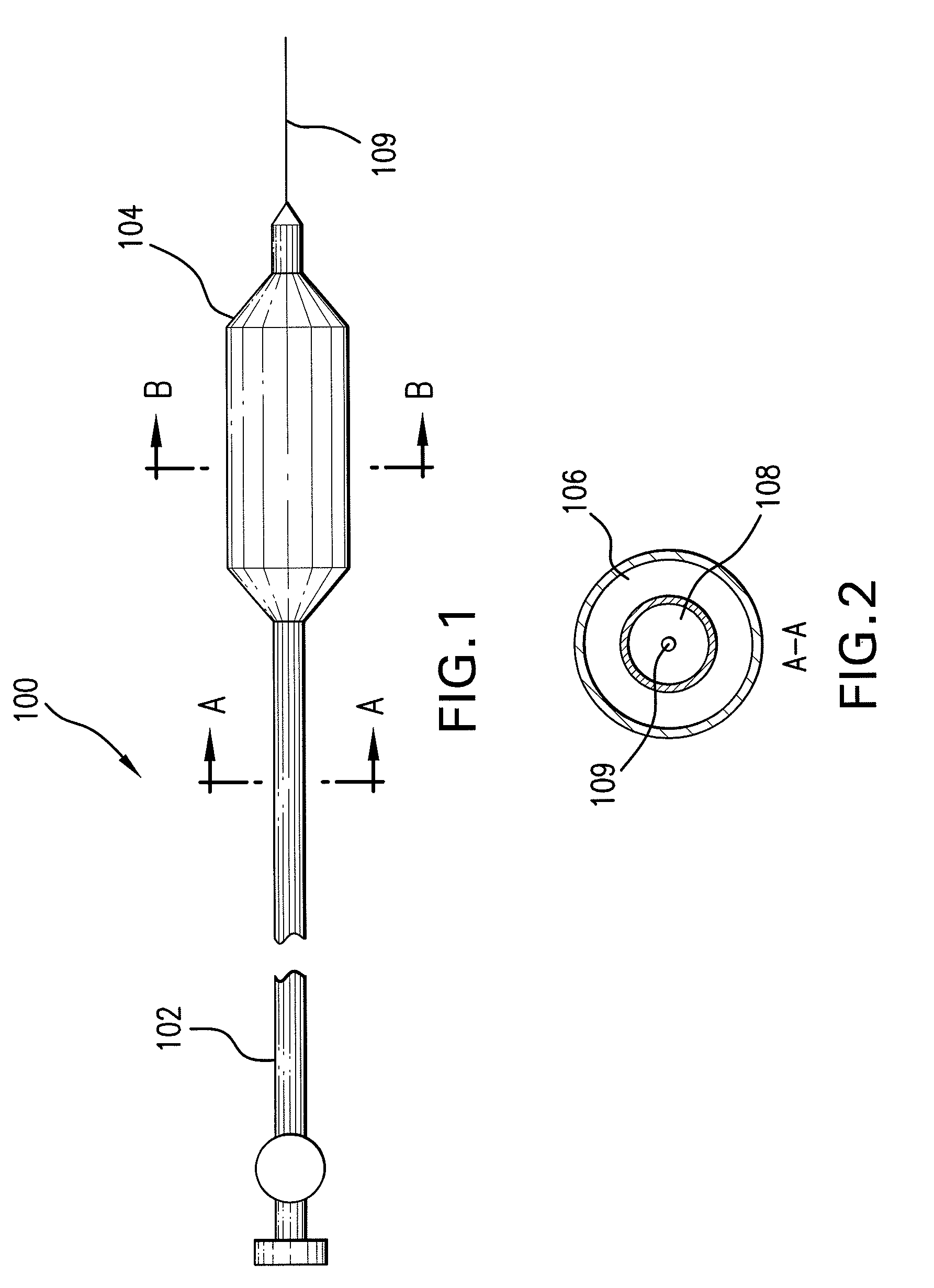
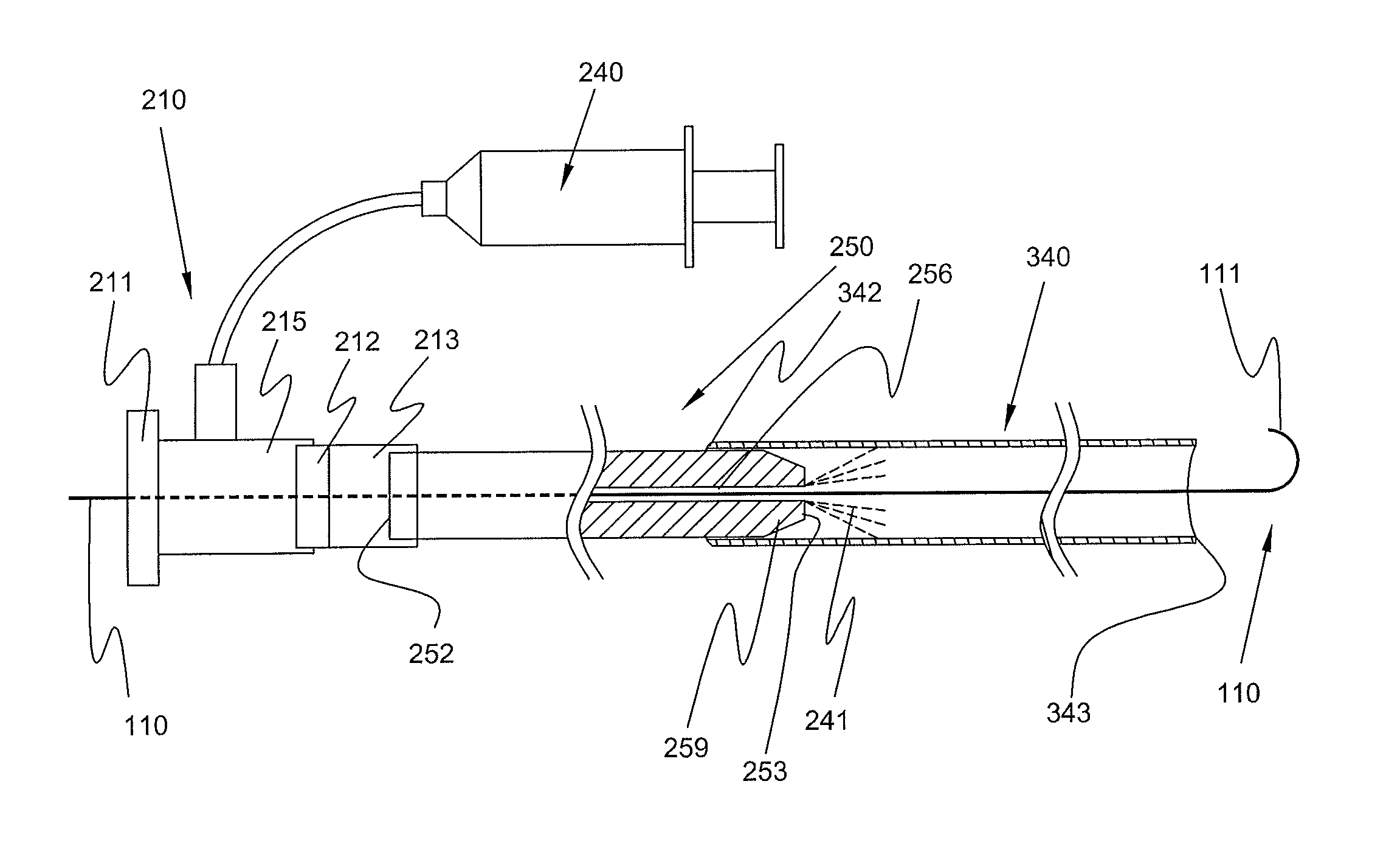
Expandable Member Having A Covering Formed Of A Fibrous Matrix For Intraluminal Drug Delivery
The present invention generally relates to an intraluminal catheter device for use in angioplasty and delivery of a therapeutic agent. Particularly, the present invention is directed to a catheter having an expandable member having a therapeutic agent disposed thereon and a fibrous matrix covering for delivering a therapeutic agent and methods of using the same.
Owner:ABBOTT CARDIOVASCULAR
Reticulated absorbent composite
InactiveUS6962645B2Promote absorptionImprove acquisitionCellulosic pulp after-treatmentNon-fibrous pulp additionElastic fibresFiber
An absorbent composite (10) having a fibrous matrix that includes absorbent material is disclosed. The fibrous matrix defines voids (14) and passages between the voids, which are distributed throughout the composite. Absorbent material (18) is located within some of the voids (14). Absorbent material located in these voids is expandable into the void. In a preferred embodiment, the composite's fibrous matrix includes resilient and matrix fibers (16). The composite optionally includes a wet strength agent.
Owner:NAT INST FOR STRATEGIC TECH ACQUISITIONS & COMMLIZATION
Expandable Member Formed Of A Fibrous Matrix For Intraluminal Drug Delivery
An intraluminal catheter device having an expandable member formed of a matrix of fiber elements with a therapeutic agent incorporated therein. The therapeutic agent can be coated on the fiber elements in a co-axial configuration. The fiber elements may also have a second coating including a protective substance surrounding the therapeutic agent. The matrix of fiber elements can be formed by electrospinning. A process of delivering a therapeutic agent to a target site includes providing an intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member having a therapeutic agent dispersed therein, and advancing the catheter device at a desired treatment site. Once at the desired treatment site, fluid is introduced into the inflation lumen to expand the expandable member from a first profile to a second profile, and the therapeutic agent is delivered to the desired treatment site.
Owner:ABBOTT CARDIOVASCULAR
Expandable Member Formed Of A Fibrous Matrix Having Hydrogel Polymer For Intraluminal Drug Delivery
An intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent incorporated therein. The hydrogel polymer can be coated on the fiber elements in a co-axial configuration. The fiber elements may also have a second coating including a protective substance surrounding the hydrogel polymer having a therapeutic agent therein. The matrix of fiber elements can be formed by electrospinning. A process of delivering a therapeutic agent to a target site includes providing an intraluminal catheter device having an expandable member formed of a matrix of fiber elements, the expandable member including a hydrogel polymer having a therapeutic agent dispersed therein, and advancing the catheter device at a desired treatment site. Once at the desired treatment site, fluid is introduced into the inflation lumen to expand the expandable member from a first profile to a second profile, and the therapeutic agent is released from the hydrogel polymer and delivered to the desired treatment site.
Owner:ABBOTT CARDIOVASCULAR
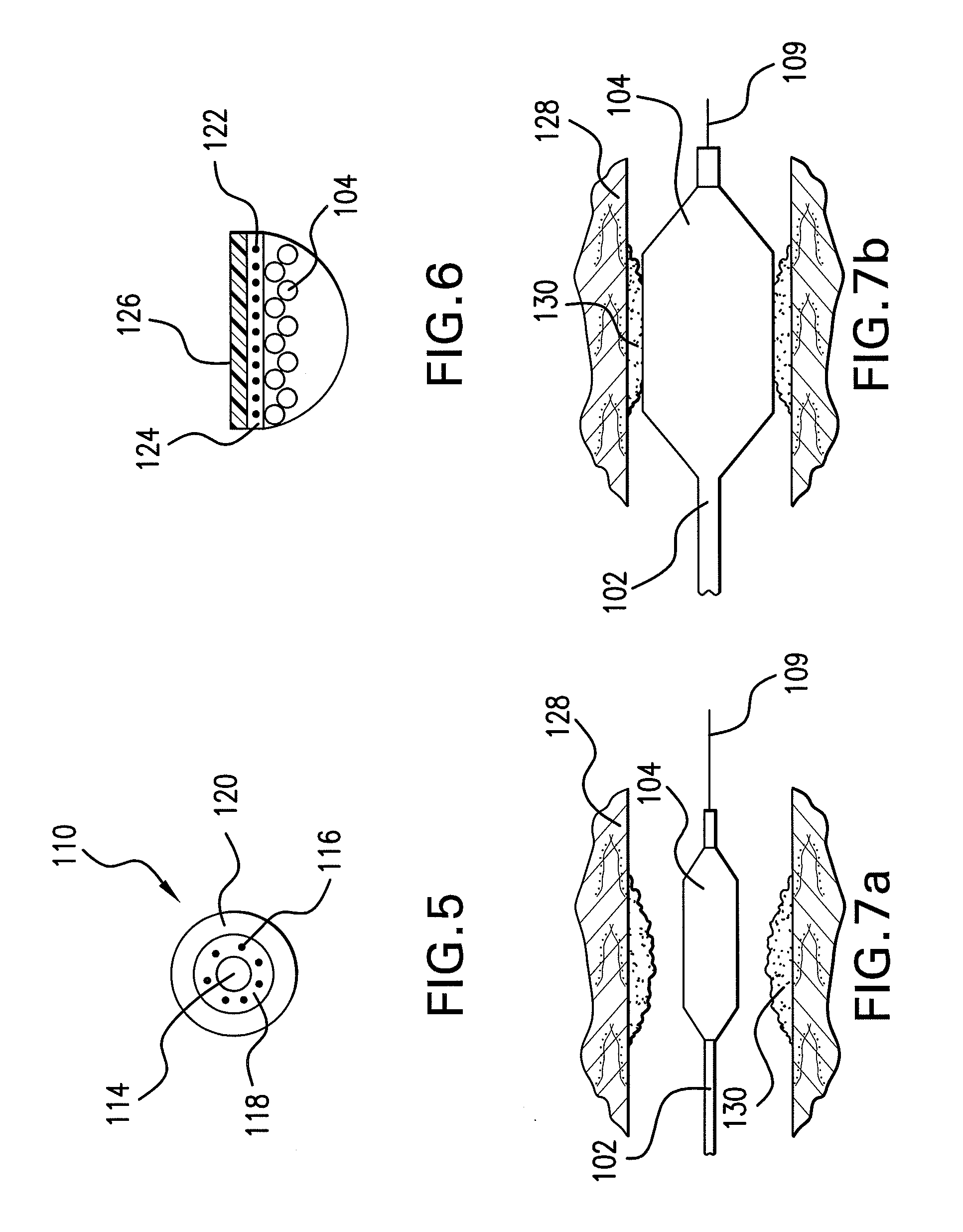
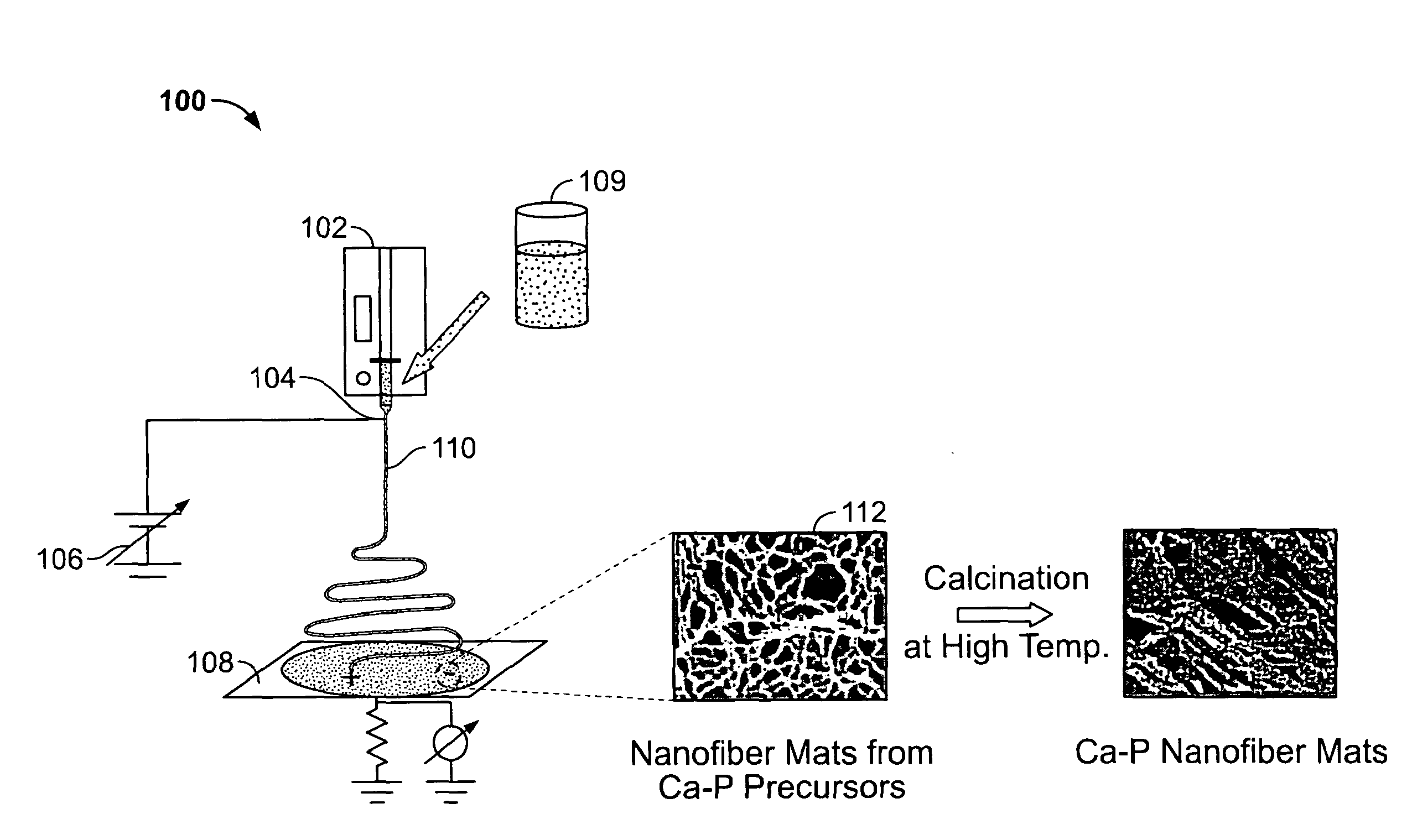
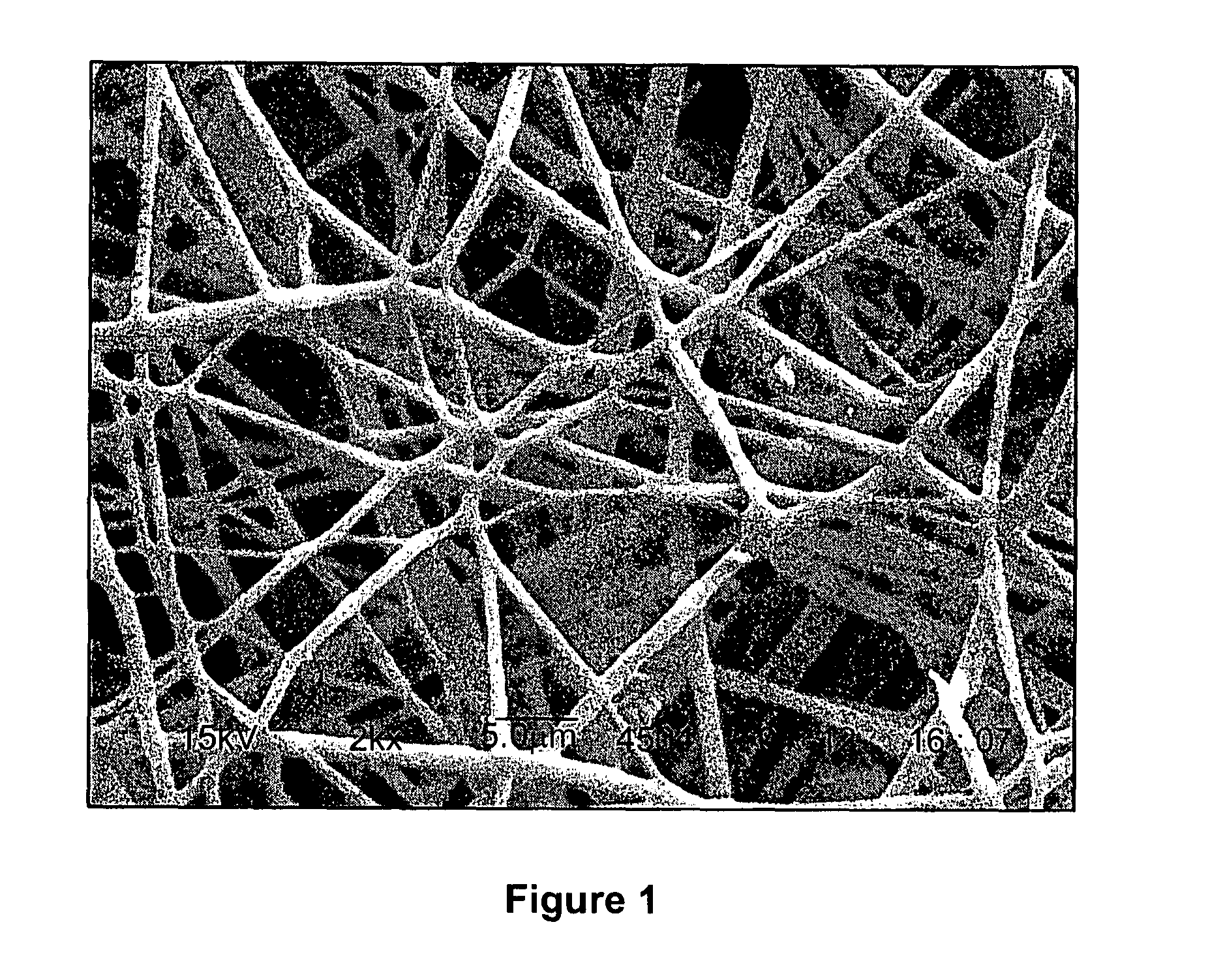
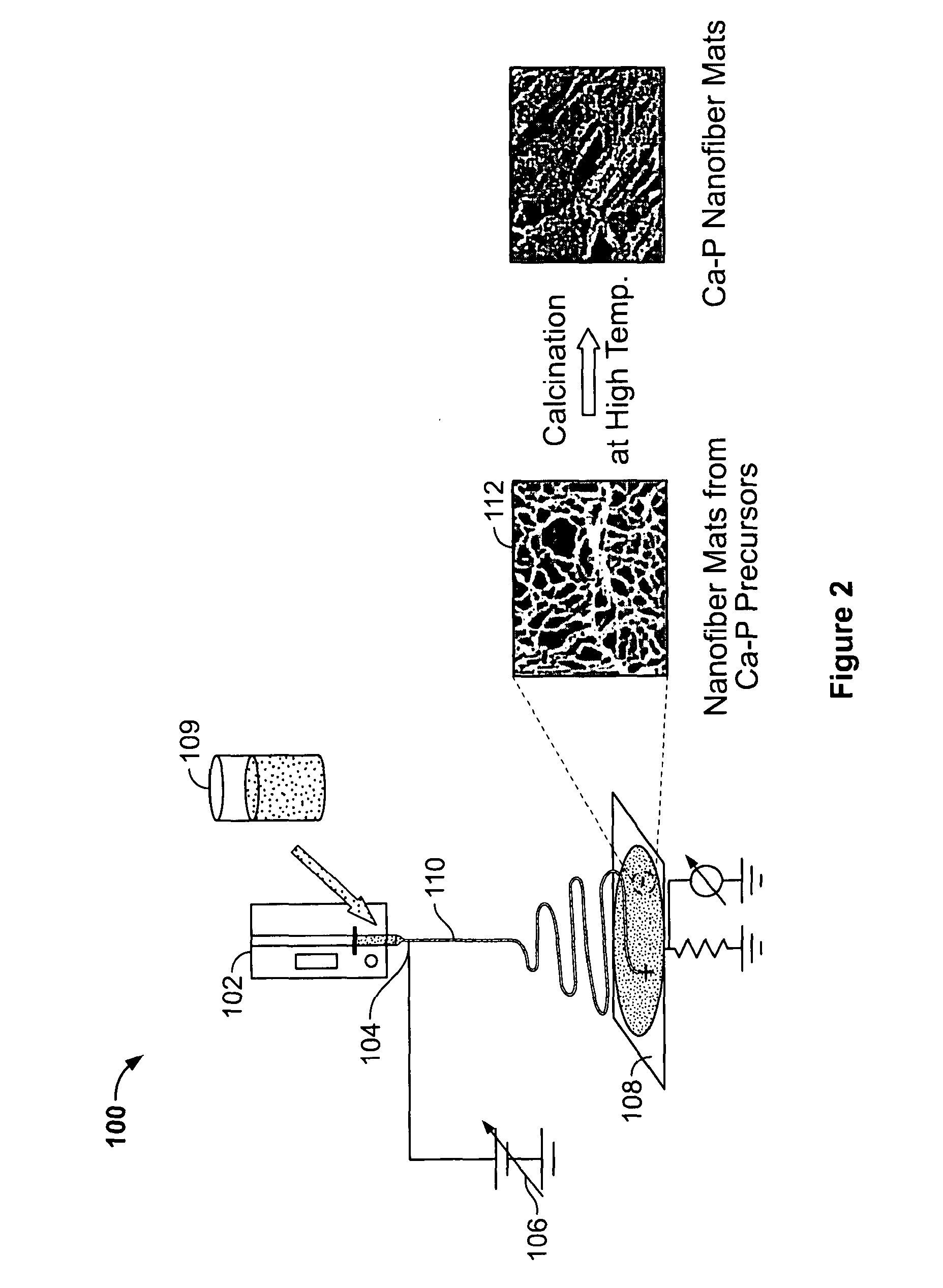
Calcium Phosphate Nanofibers
ActiveUS20090317446A1Suitable environmentMaterial nanotechnologyDental implantsCalcium biphosphateFiber
Calcium-phosphate nanofiber matrices comprising randomly dispersed crystalline calcium-phosphate nanofibers are provided. The nanofibers are synthesized using sol-gel methods combined with electrospinning. The nanofibers may be hollow, solid or may comprise a calcium-phosphate shell surrounding a polymer containing inner core to which biologically functional additives may be added. The nanofiber matrices may be used to culture bone and dental cells, and as implants to treat bone, dental or periodontal diseases and defects.
Owner:CORNELL RES FOUNDATION INC
Biomimetic organic/inorganic composites, processes for their production, and methods of use
The subject invention concerns a composite comprising an organic fluid-swellable, fibrous matrix, such as collagen, and a mineral phase, such as calcium carbonate or phosphate mineral phase, for use as a biomimetic of bone. In another aspect, the subject invention concerns a process for making a composite involving the inclusion of acidic polymers to a supersaturated mineralizing solution, in order to induce an amorphous liquid-phase precursor to the inorganic mineral, which is then absorbed (pulled by capillary action) into the organic matrix. Advantageously, once solidified, a high mineral content can be achieved, with the inorganic mineral crystals embedded within the collagen fibers (intrafibrillarly) and oriented such that they are aligned along the long axes of the fibers of the organic matrix, thereby closely mimicking the natural structure of bone. The present invention further concerns a method of treating a patient suffering from a bone defect by applying a biomimetic composite to the bone defect site.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Carbon-carbon composite article manufactured with needled fibers
InactiveUS20060177663A1Manufacturing EaseReduce layeringLayered productsTextiles and paperCarbon compositesCarbon fibers
Method of making a carbon-carbon composite article such as an aircraft brake disc. The method includes: selecting carbon fiber precursors, having limited shrinkage in the axial direction when carbonized, in the form of individualized chopped or cut fibers; placing the selected chopped or cut carbon fiber precursors into a preform mold configured in the form of a brake disc to form a fibrous matrix; and then needling the molded fibrous matrix to provide it with three-dimensional structural integrity and to reduce layering. The carbon fiber precursor matrix may subsequently be infused with liquid carbon matrix precursor, the impregnated matrix may be carbonized; e.g., at 600-1800° C. for 1-10 hours to provide a preform having a density of at least about 1.1 g / cc, and the carbonized preform may be further densified to a density of at least about 1.6 g / cc by known liquid resin infiltration techniques and / or by conventional CVI / CVD processing.
Owner:HONEYWELL INT INC
Multiple function, self-repairing composites with special adhesives
InactiveUS7811666B2Fast chemical reactionExtended shelf lifeLayered productsConstructions elementsFiberAdhesive
A system for self-repairing matrices such as concrete or cementitous matrices, polymeric matrices, and / or fibrous matrices, including laminates thereof. The system includes repair agents retained in and / or on vessels, such as hollow fibers, within the matrix. Upon impact, the vessel rupture, releasing the chemicals. For multi-layer laminates, the systems provides a total dynamic energetic circulation system that functions as an in situ fluidic system in at least one layer or area. The energy from the impact ruptures the vessels to release the chemical(s), and mixes the chemical(s) and pushes the chemical(s) and / or resulting compound through the matrix. The repair agents can withstand high temperatures, such as the heat of processing of many laminates, e.g., 250-350° F.
Owner:DRY CAROLYN
Delivery of therapeutic biologicals from implantable tissue matrices
InactiveUS20020031500A1Many of effectMany of inconveniencePowder deliveryBiocideProgenitorActive agent
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
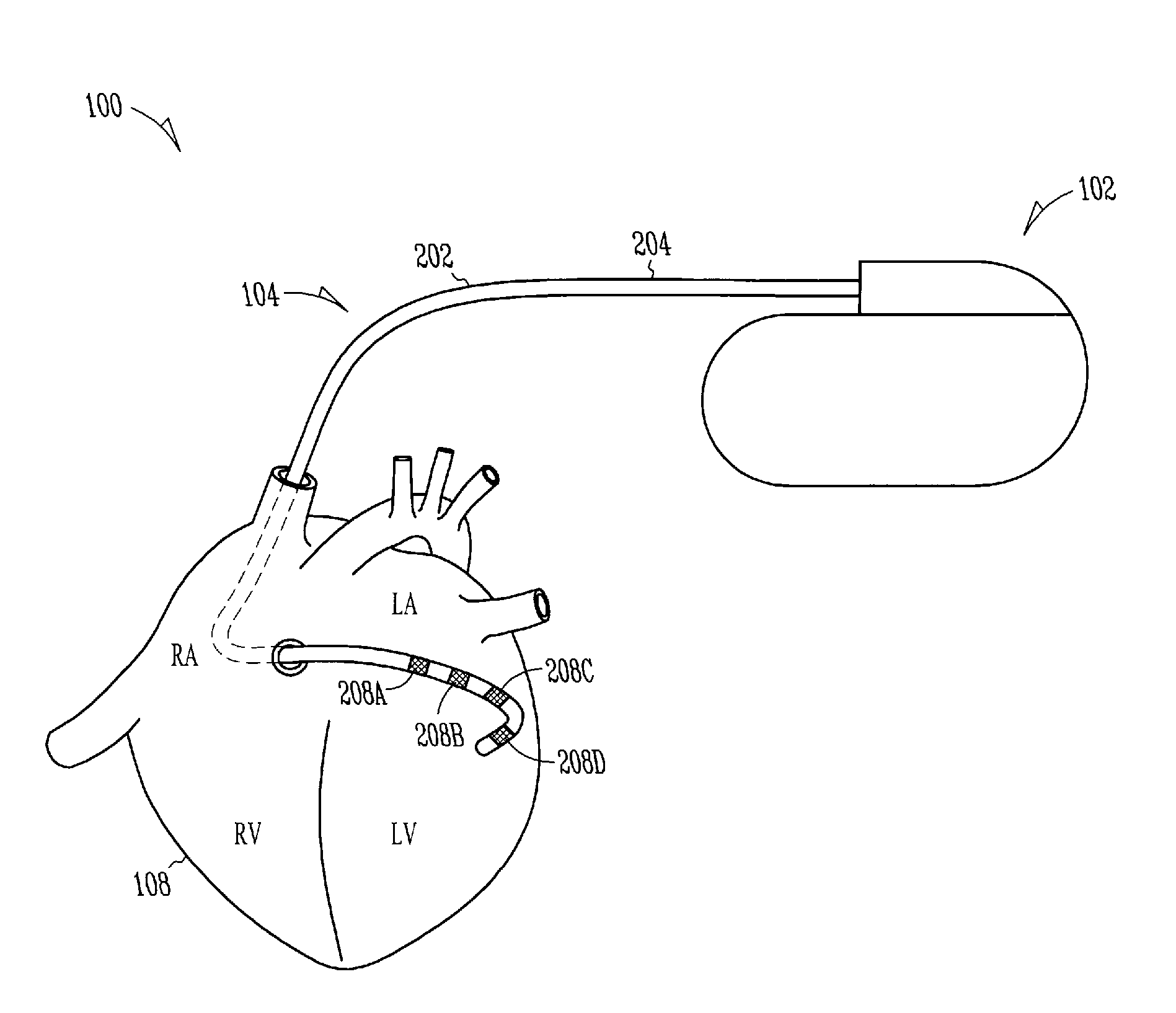
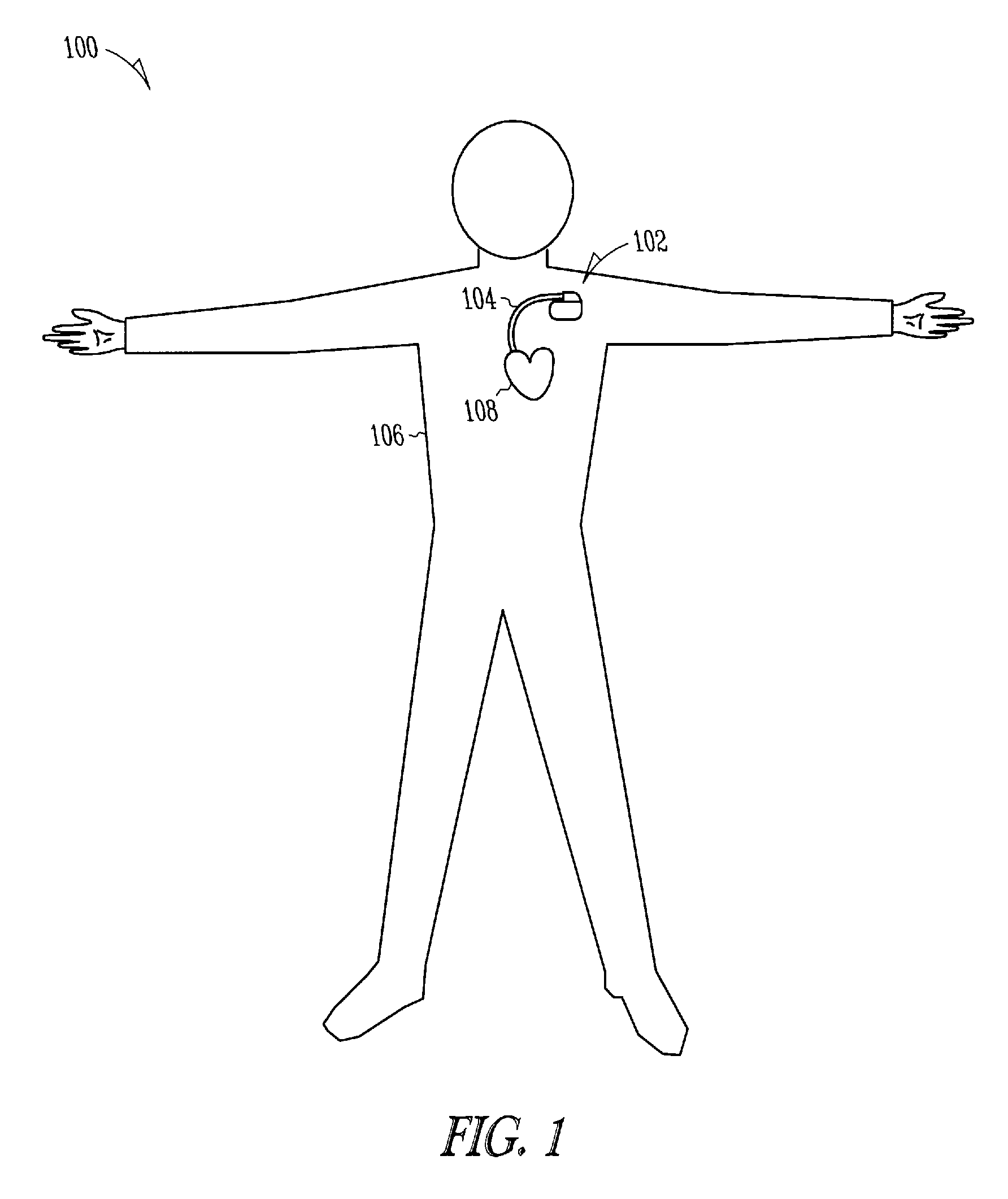
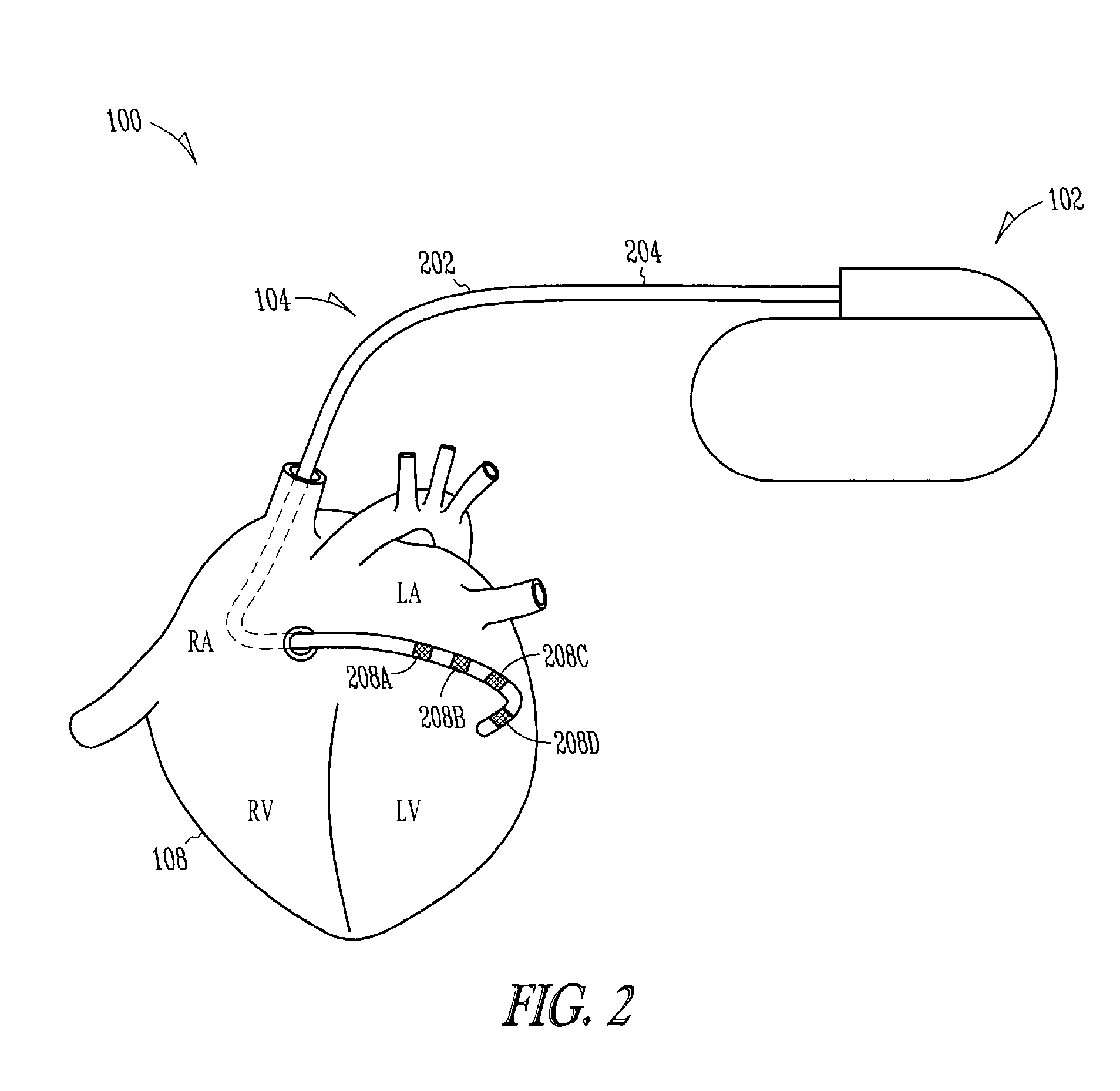
Lead with fibrous matrix coating and methods related thereto
A lead includes a lead body extending from a lead proximal end portion to a lead distal end portion and having an intermediate portion therebetween, one or more tissue sensing / stimulation electrodes disposed along the lead body, one or more terminal connections disposed along the lead proximal end portion. The lead further includes one or more conductors contained within the lead body extending between the tissue sensing / stimulation electrodes and the terminal connections, and a fibrous matrix coating is disposed onto at least a portion of the lead body and / or sensing / stimulation electrodes.
Owner:CARDIAC PACEMAKERS INC
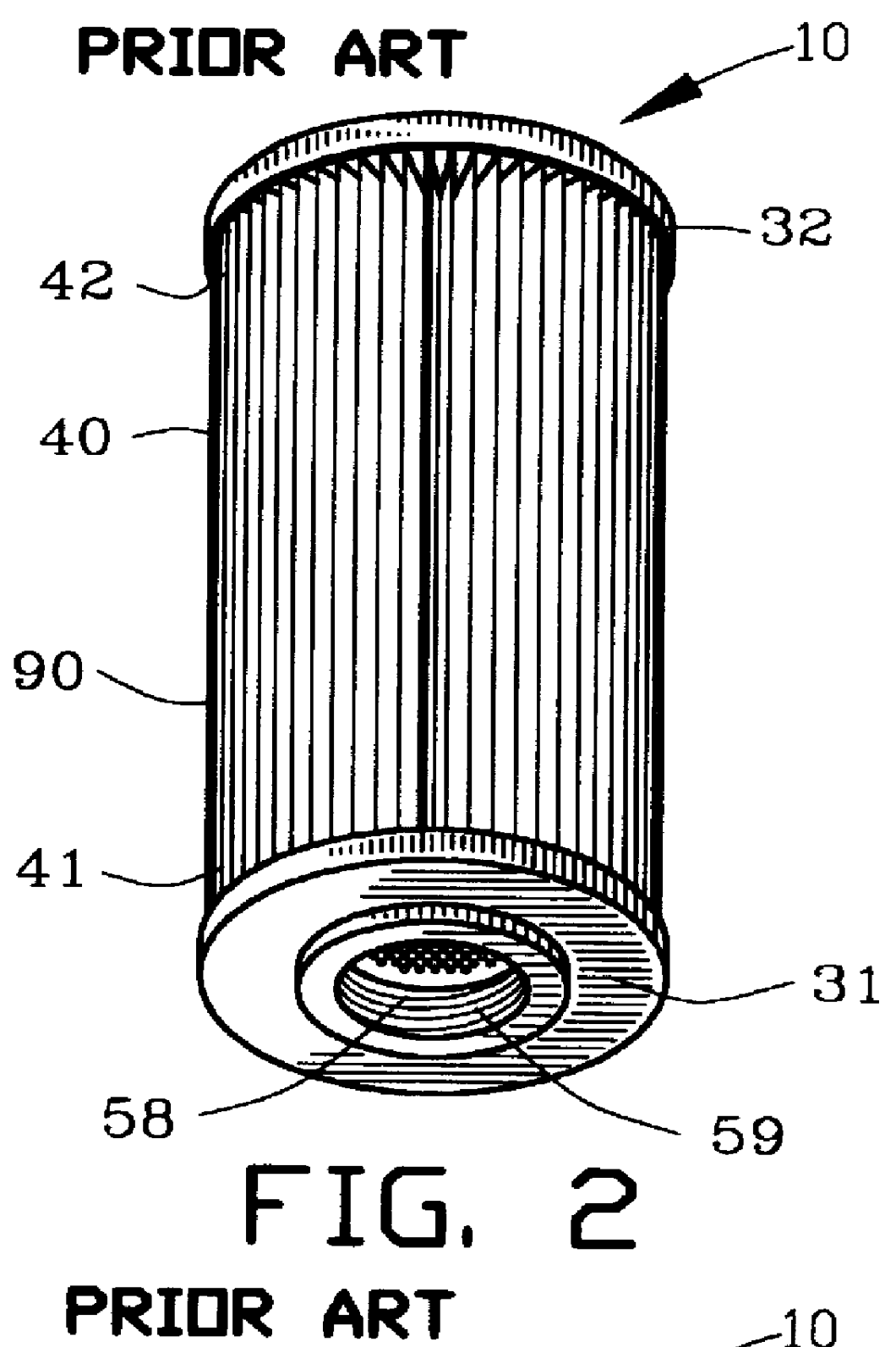
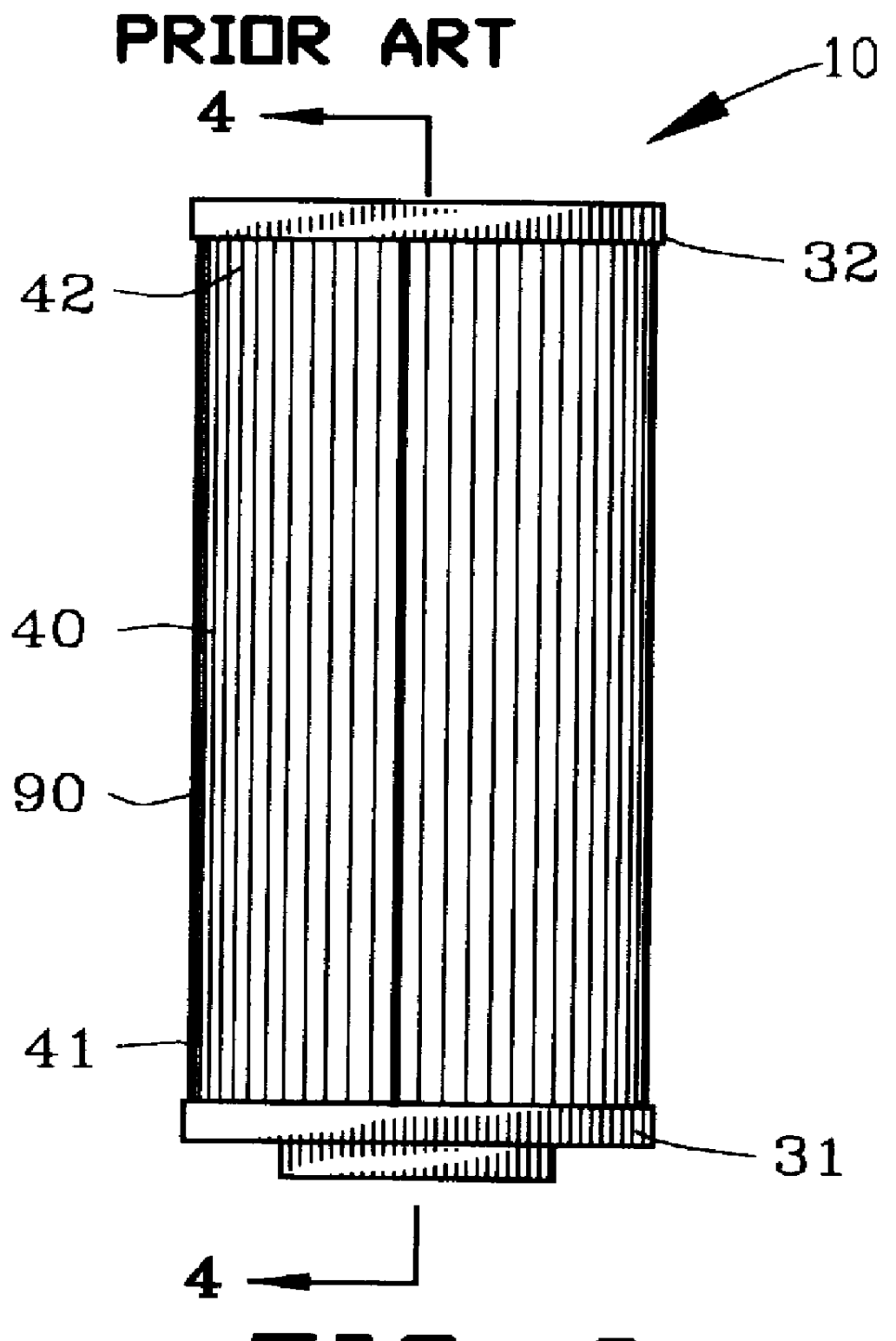
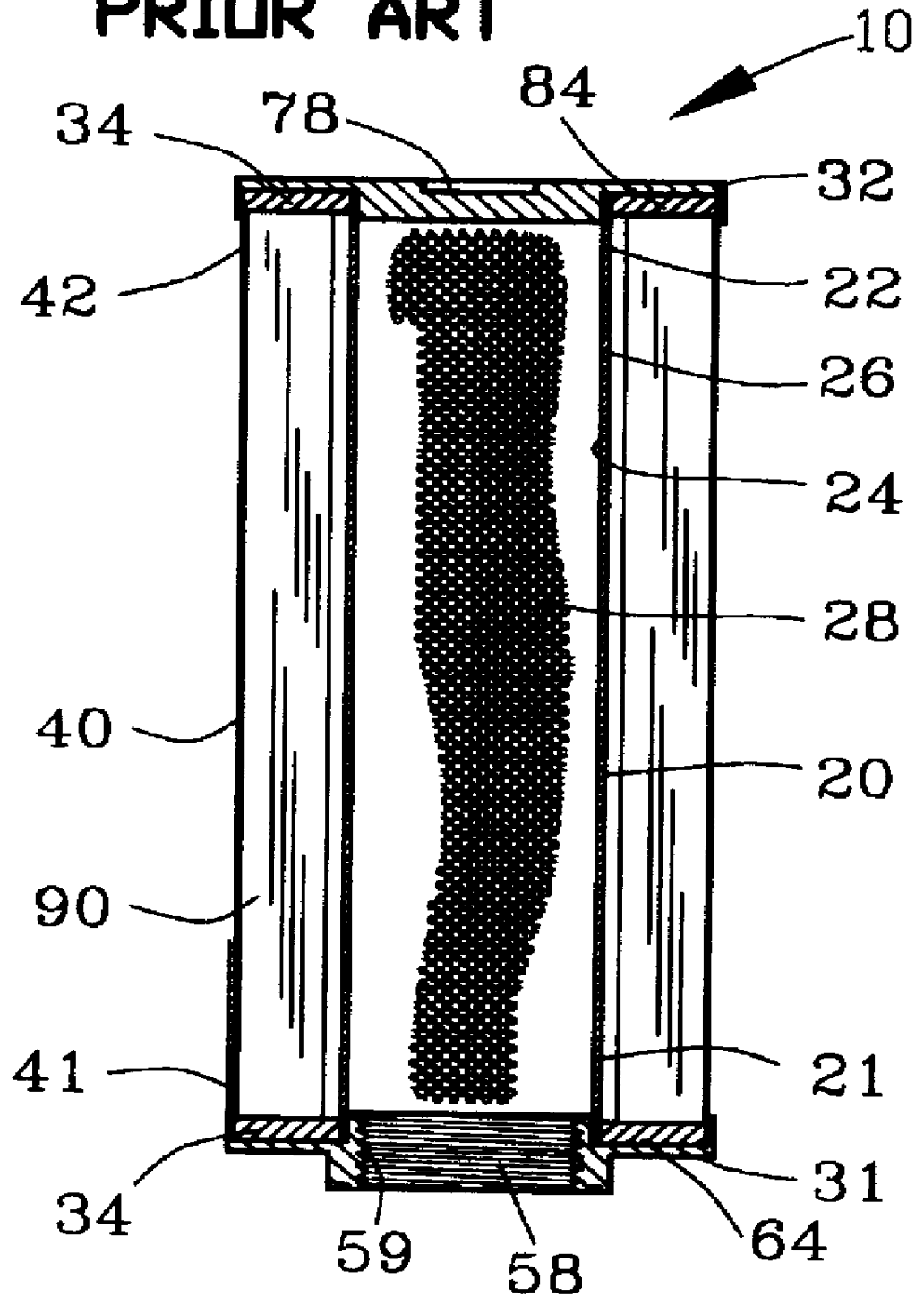
Fluid filter and method of making
InactiveUS6096212AIncrease surface areaIncrease capacityLoose filtering material filtersCartridge filtersFilter mediaMetal fibers
A fluid filter assembly and method of making is disclosed for filtering a fluid. The fluid filter assembly comprises a first filter component including a filter media comprising a matrix of metallic fibers. A second filter component includes a filter support. A sinter bond bonds the matrix of metallic fibers of the filter media to the second filter component.
Owner:USF FILTRATION & SEPARATIONS GROUP
Aerogel Mat and Manufacturing Method Thereof
ActiveUS20120025127A1Enhanced couplingAvoid damageStuffed mattressesSpring mattressesFiberSilane compounds
The present invention relates to a method of manufacturing a mat containing aerogel and to a mat manufactured using this method. A method of manufacturing a mat containing silica aerogel according to an aspect of the invention includes: (S1) producing a wet gel by mixing water glass and alcohol in a reactor; (S2) modifying a surface of the wet gel by adding an organic silane compound and an organic solvent to the reactor and mixing; (S3) separating a upper liquid from a solution in the reactor and impregnating a fibrous matrix with the upper liquid; and (S4) drying the fibrous matrix impregnated with the upper liquid. According to an aspect of the invention, a mat containing silica aerogel can be manufactured using only water glass as raw material, even when applying the drying process in an ambient environment, without using expensive materials or supercritical apparatus.
Owner:DAEHYUP TECH
Rotating element sheet material with generalized containment structure
The present invention relates to rotating element sheet material with a generalized containment structure and methods of fabricating such rotating element sheet material, where the rotating element sheet material comprises a fibrous matrix, a plurality of rotatable elements, and an enabling fluid, and where the plurality of rotatable elements are disposed within the fibrous matrix and are in contact with the enabling fluid. In addition, rotating element sheet material with a generalized containment structure, and methods of fabricating such rotating element sheet material, includes rotating element sheet material which comprises a fibrous matrix and a plurality of micro-capsules, and where the micro-capsules define a hollow space therein, and the hollow space contains a subset of a plurality of rotatable elements and an enabling fluid, and where the plurality of micro-cavities are disposed within the fibrous matrix.
Owner:E INK CORPORATION
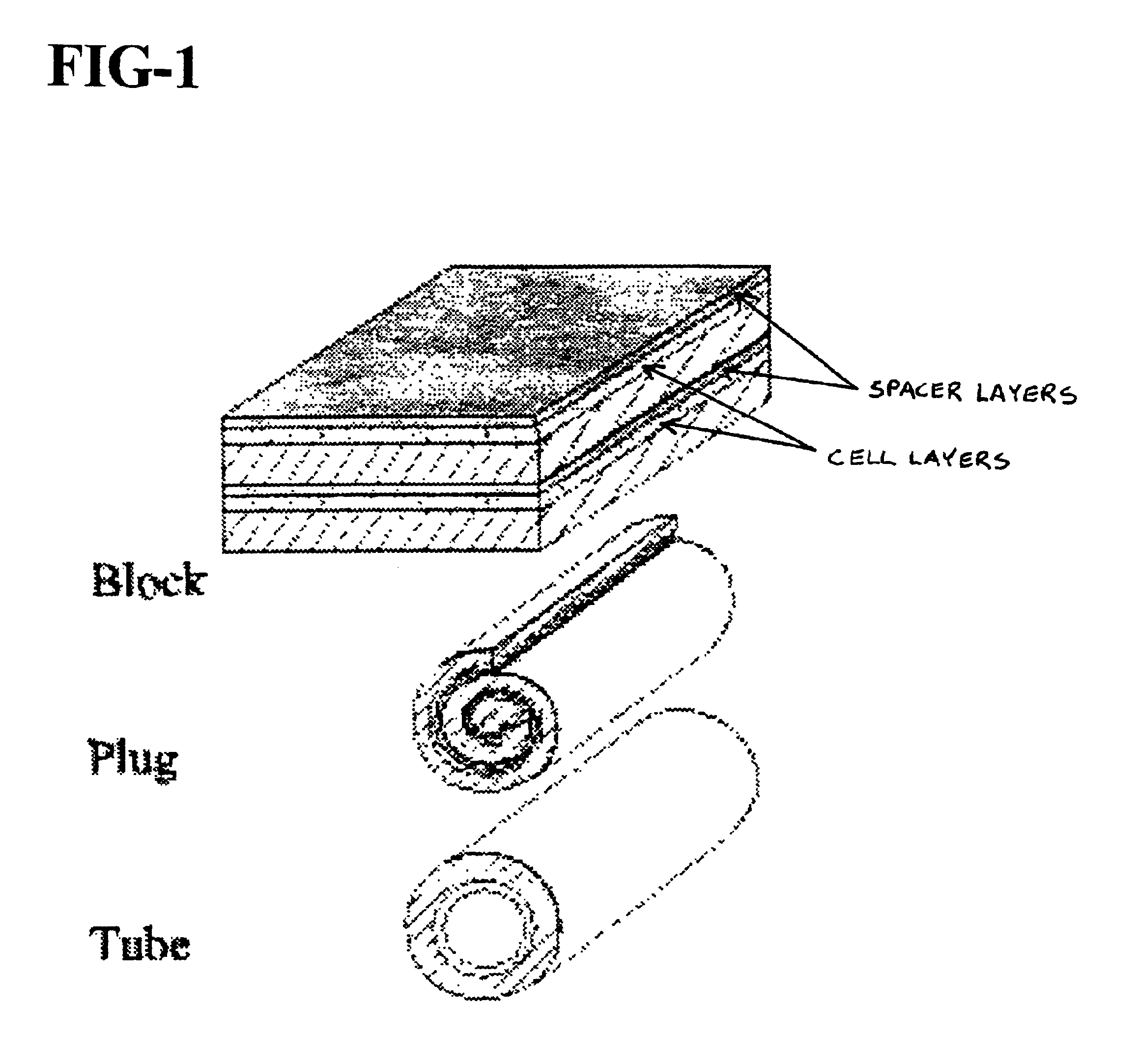
Manufacture of thick preform composites via multiple pre-shaped fabric mat layers
Preform for carbon-carbon composite part (55) comprising multiple layers of fibrous mats (51, 52, 53) wherein each mat (51, 52, 53) comprises random carbon-containing fibrous matrix (11) having polymeric binder distributed therein and wherein adjacent mats (51, 52, 53) are bound together by additional polymer binder, stitching, and interlocking tabs. Also, method of manufacturing thick multi-layer composite preform, by: providing optionally reconfigurable tool including perforated screen through which vacuum can be drawn; delivering chopped fibers (b) to the tool while drawing vacuum therethrough to form fibrous object; delivering binder (c) to the fibrous object; melting or curing the binder (d) to make a fibrous mat (51, 52, 53); assembling plurality of the fibrous mats (51, 52, 53) and additional binder into the shape of a preform (e); and heat-pressing the resulting mat assembly (f) into finished thick preform (55).
Owner:HONEYWELL INT INC +2
Electrospun apatite/polymer nano-composite scaffolds
InactiveUS7879093B2Convenient and versatile fabrication techniqueElectric discharge heatingBone implantFiberApatite
Owner:UNIV OF CONNECTICUT
Fibrous substrate containing fibers and nanofibrillar polysaccharide
A single-layer fibrous substrate comprising, by dry weight compared with the weight of the substrate:between 39.9 and 87.9% natural fibers refined to above 50° SR;between 12 and 60% nanofibrillar polysaccharide; andbetween 0.1 and 4% of at least one retention agent.
Owner:AHLSTROM MUNKSJO OYJ
Fabrics with high thermal conductivity coatings
ActiveUS20050277350A1Improve thermal conductivityHigh thermal conductivity materialGlass/slag layered productsNatural mineral layered productsFiberCarbide
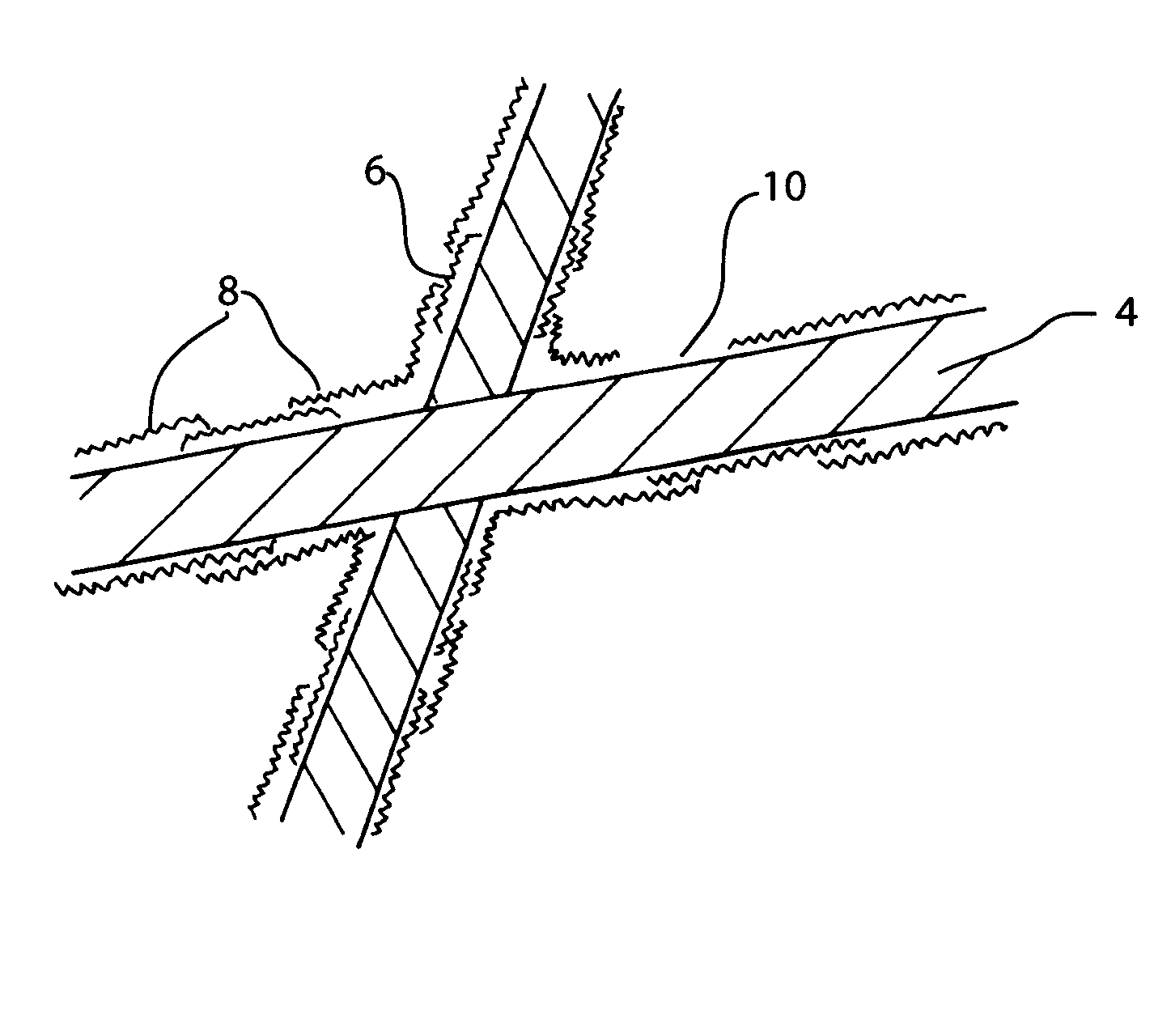
The present invention facilitates the thermal conductivity of fabrics by surface coating of the fabrics with high thermal conductivity materials 6. The fabrics may be surface coated when they are individual fibers or strands 4, bundles of strands, formed fabric or combinations therefore. A particular type of fibrous matrix used with the present invention is glass. Some fabrics may be a combination of more than one type of material, or may have different materials in alternating layers. HTC coatings of the present invention include diamond like coatings (DLC) and metal oxides, nitrides, carbides and mixed stoichiometric and non-stoichiometric combinations that can be applied to the host matrix.
Owner:SIEMENS ENERGY INC
Method and apparatus for maintenance and expansion of hemopoietic stem cells and/or progenitor cells
InactiveUS20050181504A1PromotePromote growthMicroorganism based processesMammal material medical ingredientsFiberProgenitor
A method of preparing a stromal cell conditioned medium useful in expanding undifferentiated hemopoietic stem cells to increase the number of the hemopoietic stem cells is provided. The method comprising: (a) establishing a stromal cell culture in a stationary phase plug-flow bioreactor under continuous flow on a substrate in the form of a sheet, the substrate including a non-woven fibrous matrix forming a physiologically acceptable three-dimensional network of fibers, thereby expanding undifferentiated hemopoietic stem cells; and (b) when a desired stromal cell density has been achieved, collecting medium from the stationary phase plug-flow bioreactor, thereby obtaining the stromal cell conditioned medium useful in expanding the undifferentiated hemopoietic stem cells.
Owner:PLURISTEAM LTD
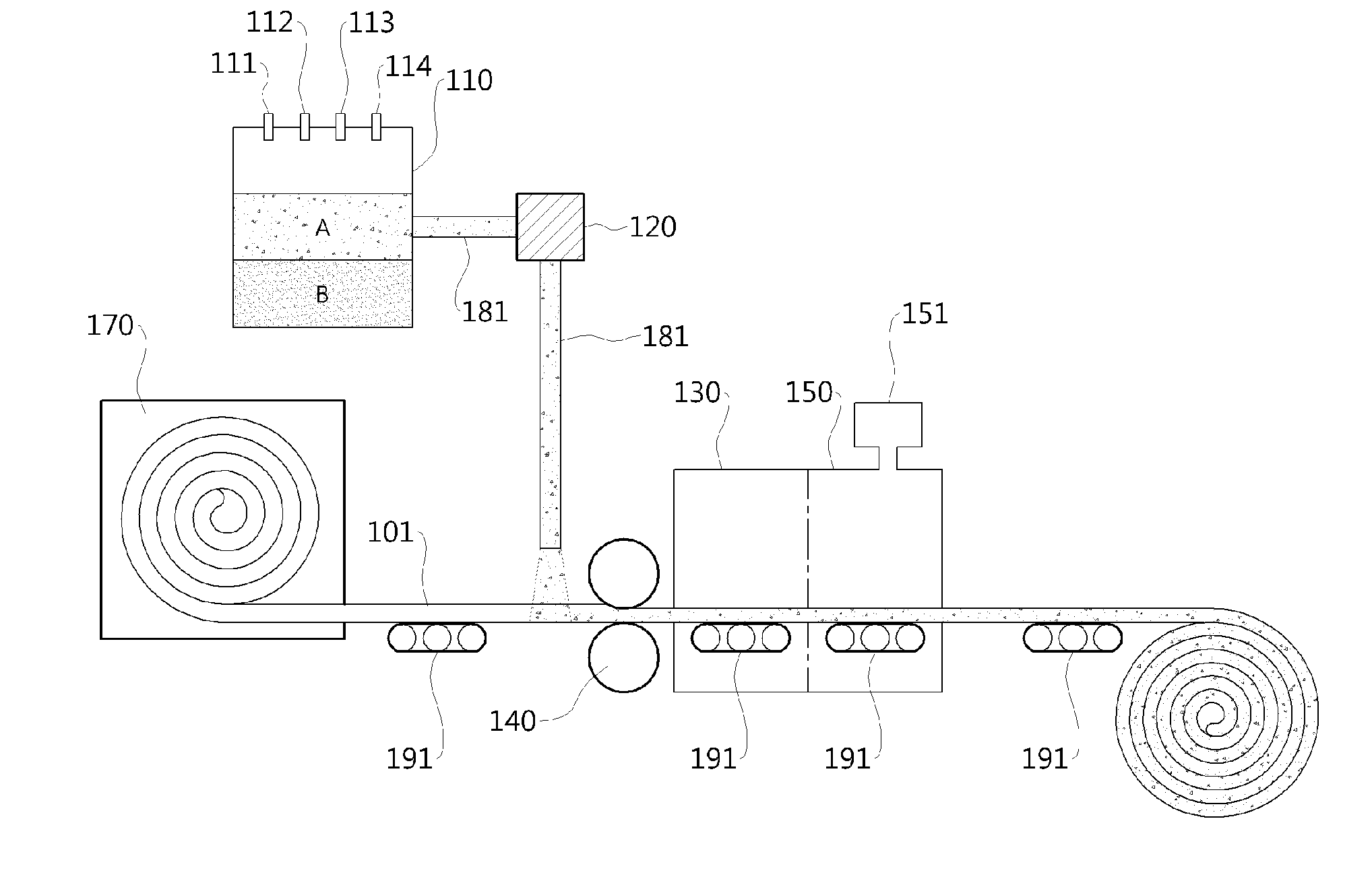
System and Mandrel for Creating Graft Devices
ActiveUS20140058194A1Enhanced AVGsImprove long-term patencySurgerySpraying apparatusFiberBiomedical engineering
Owner:XELTIS AG
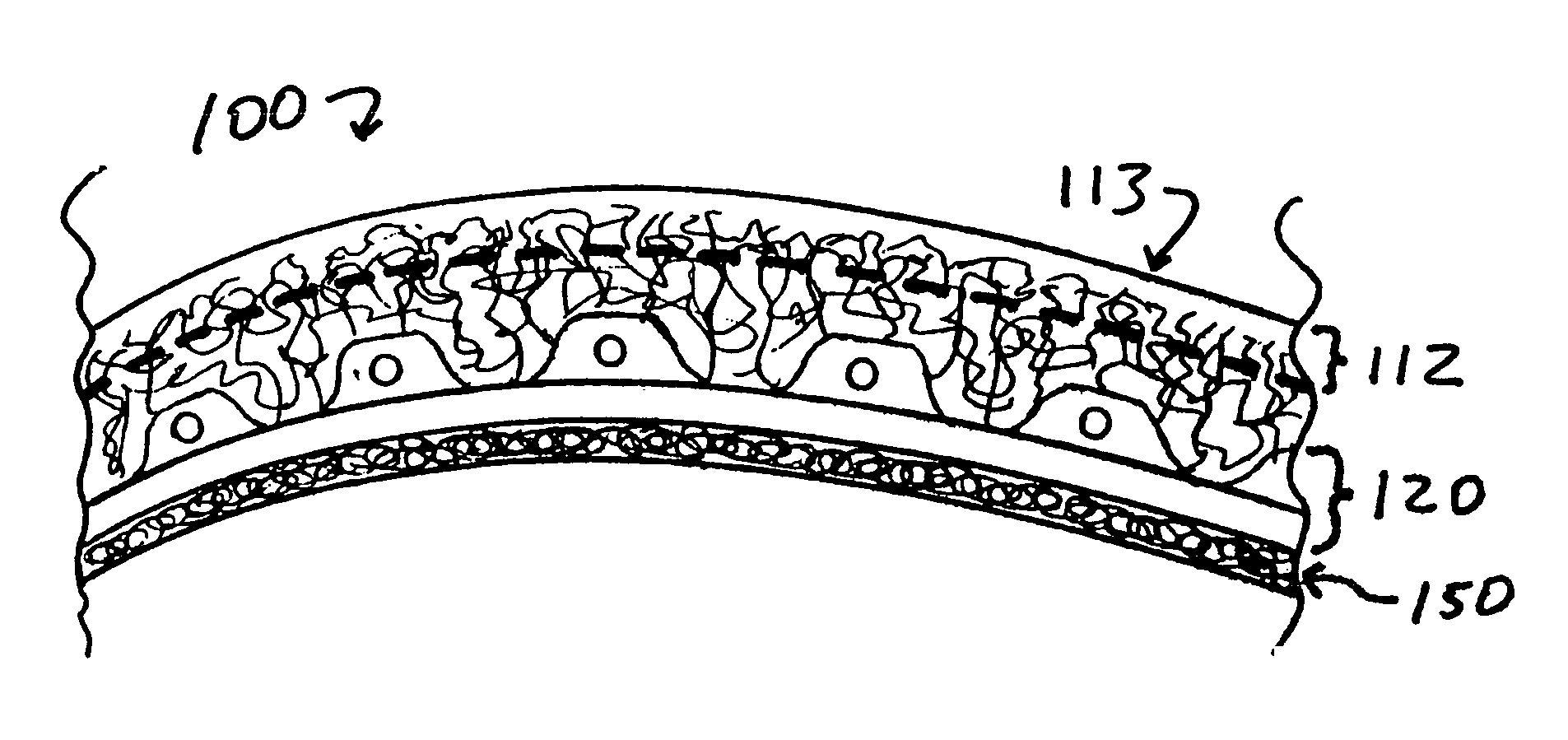
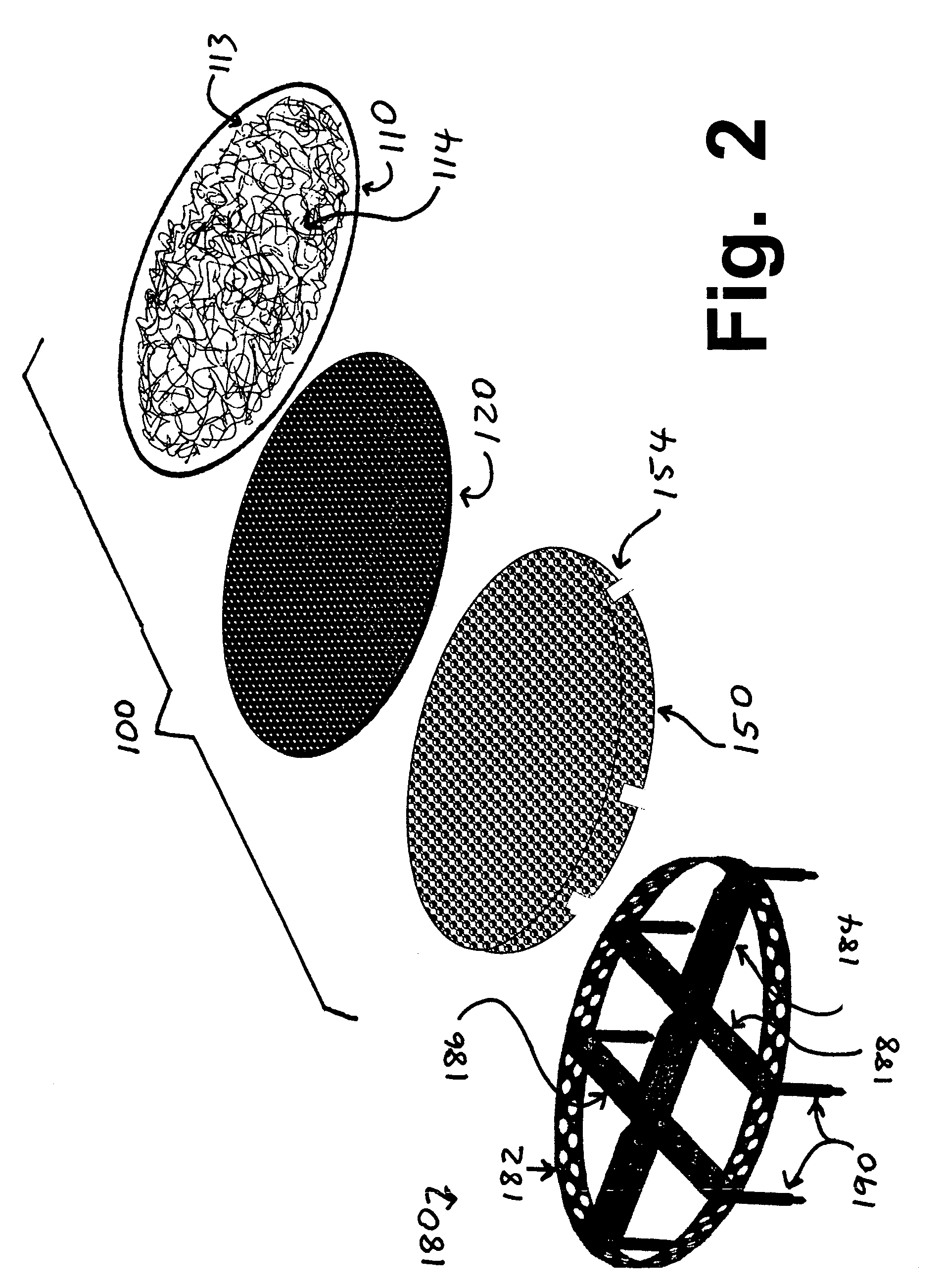
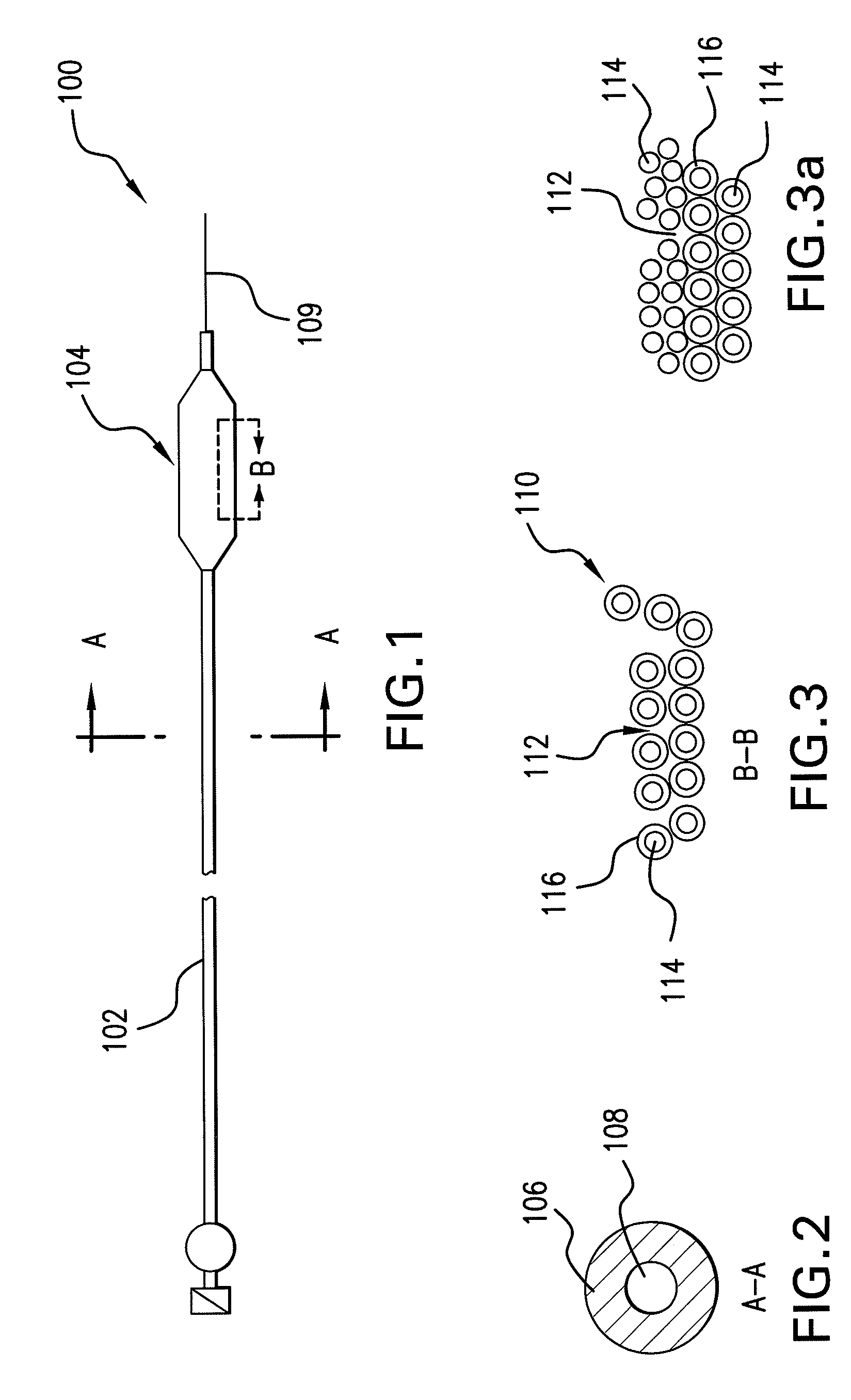
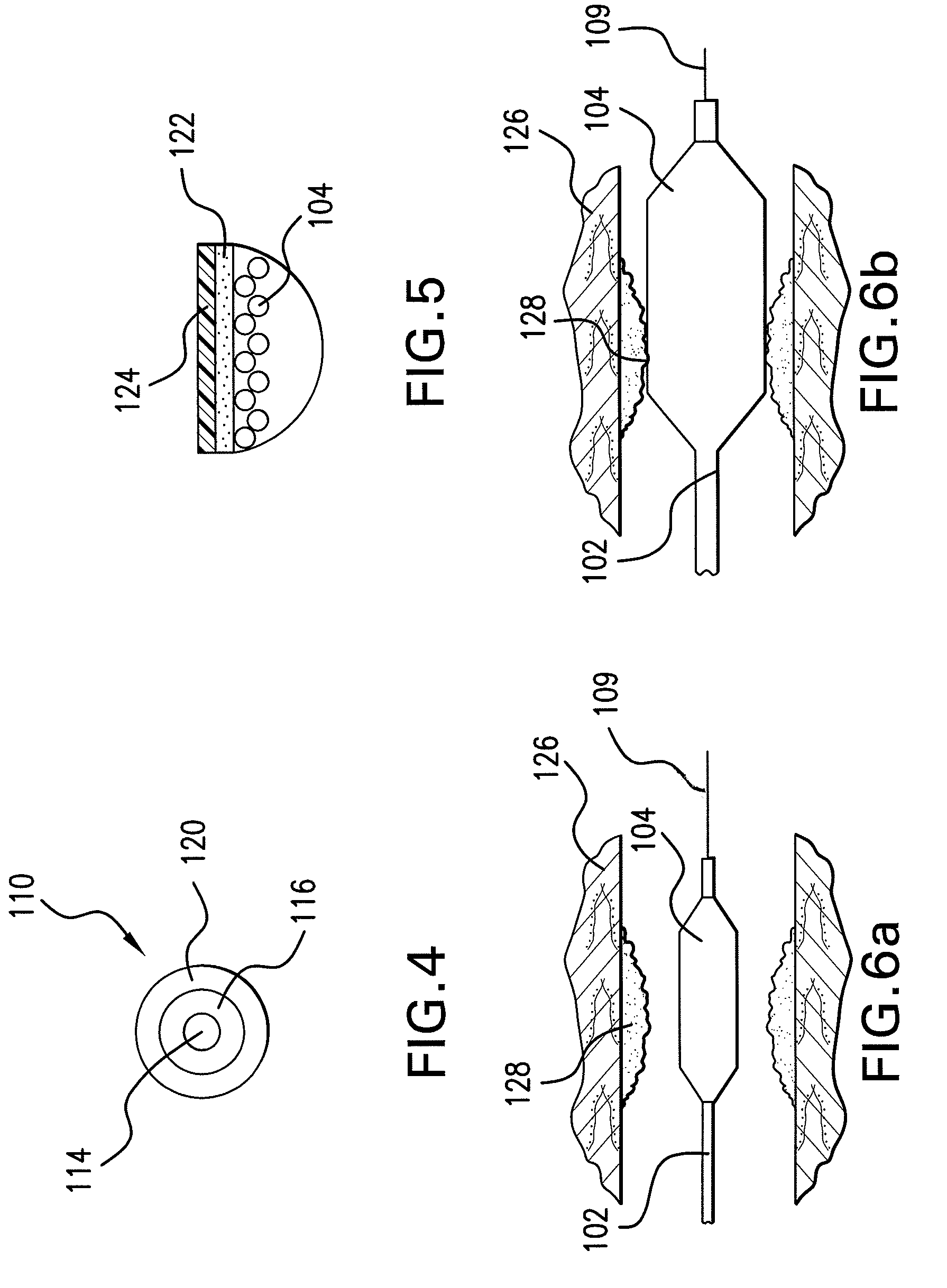
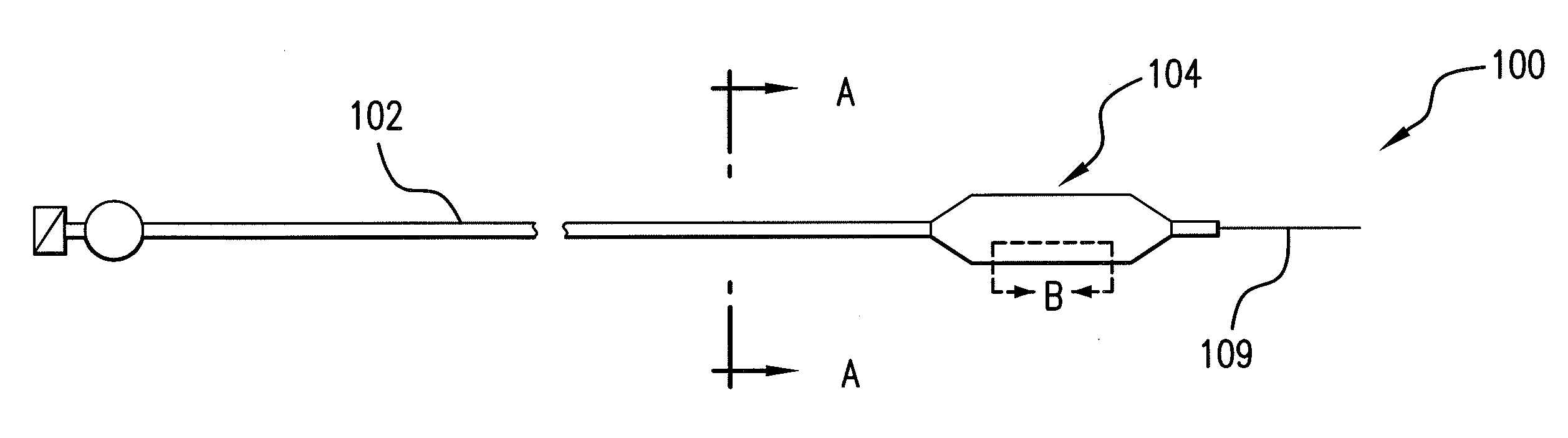
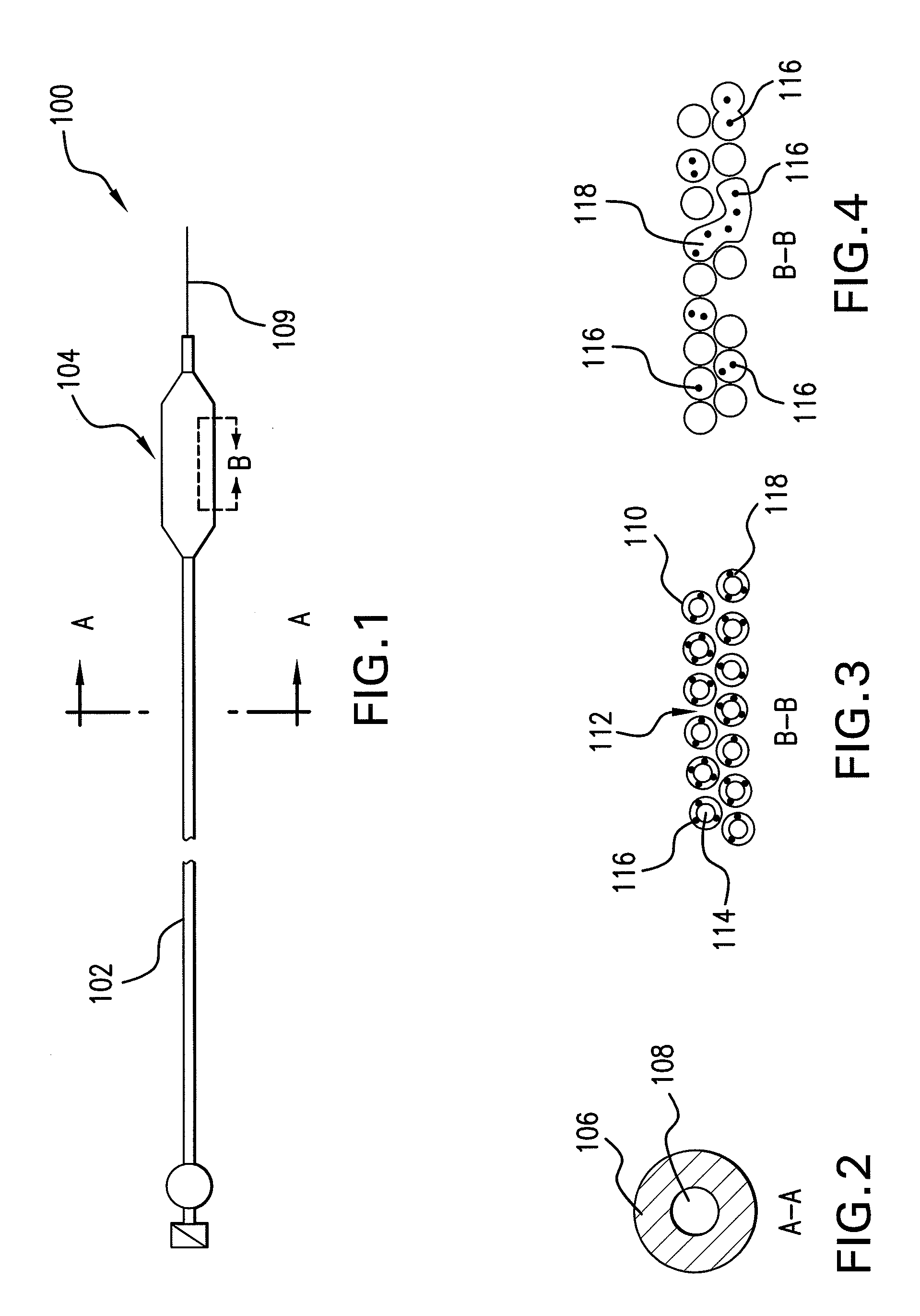
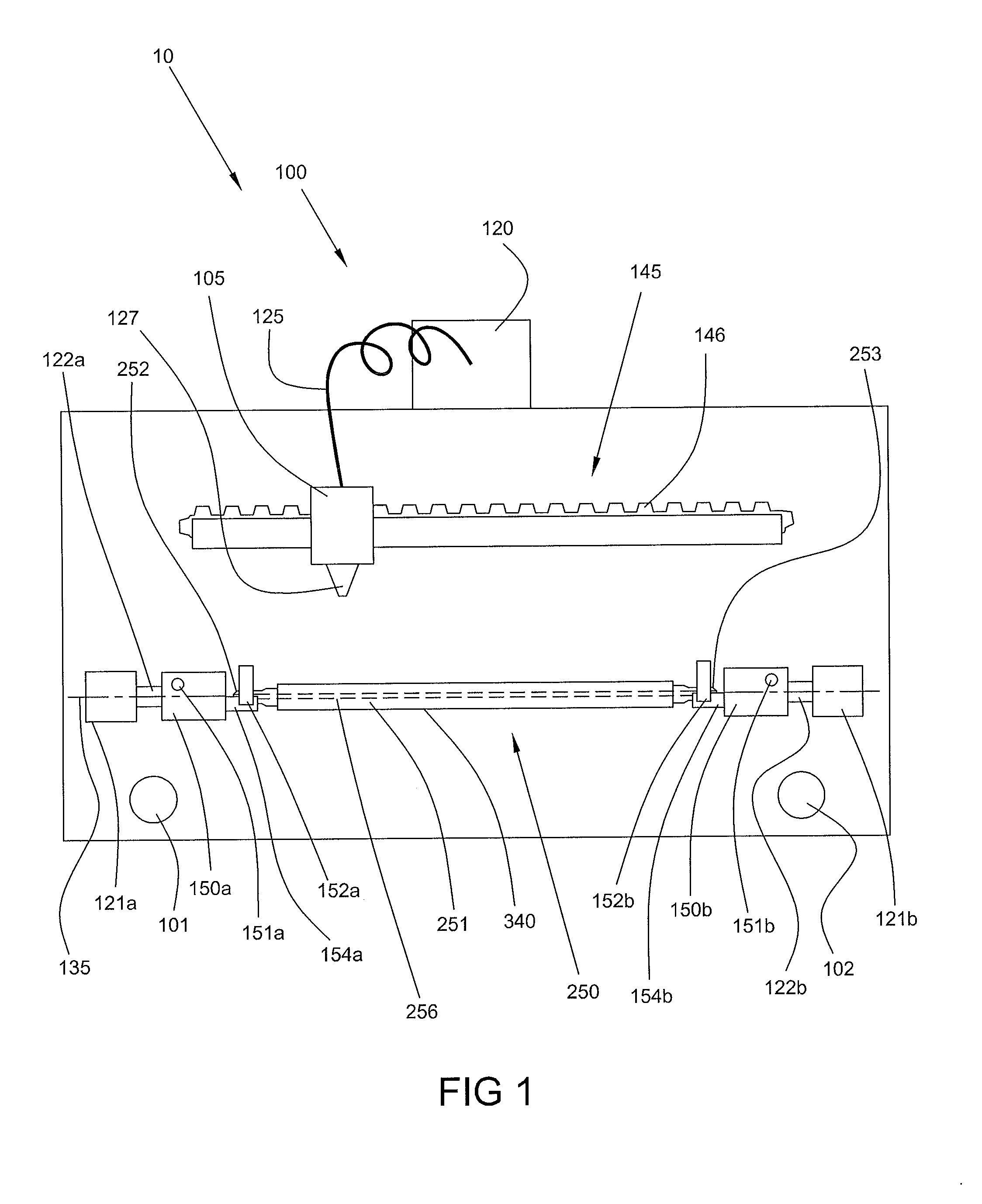
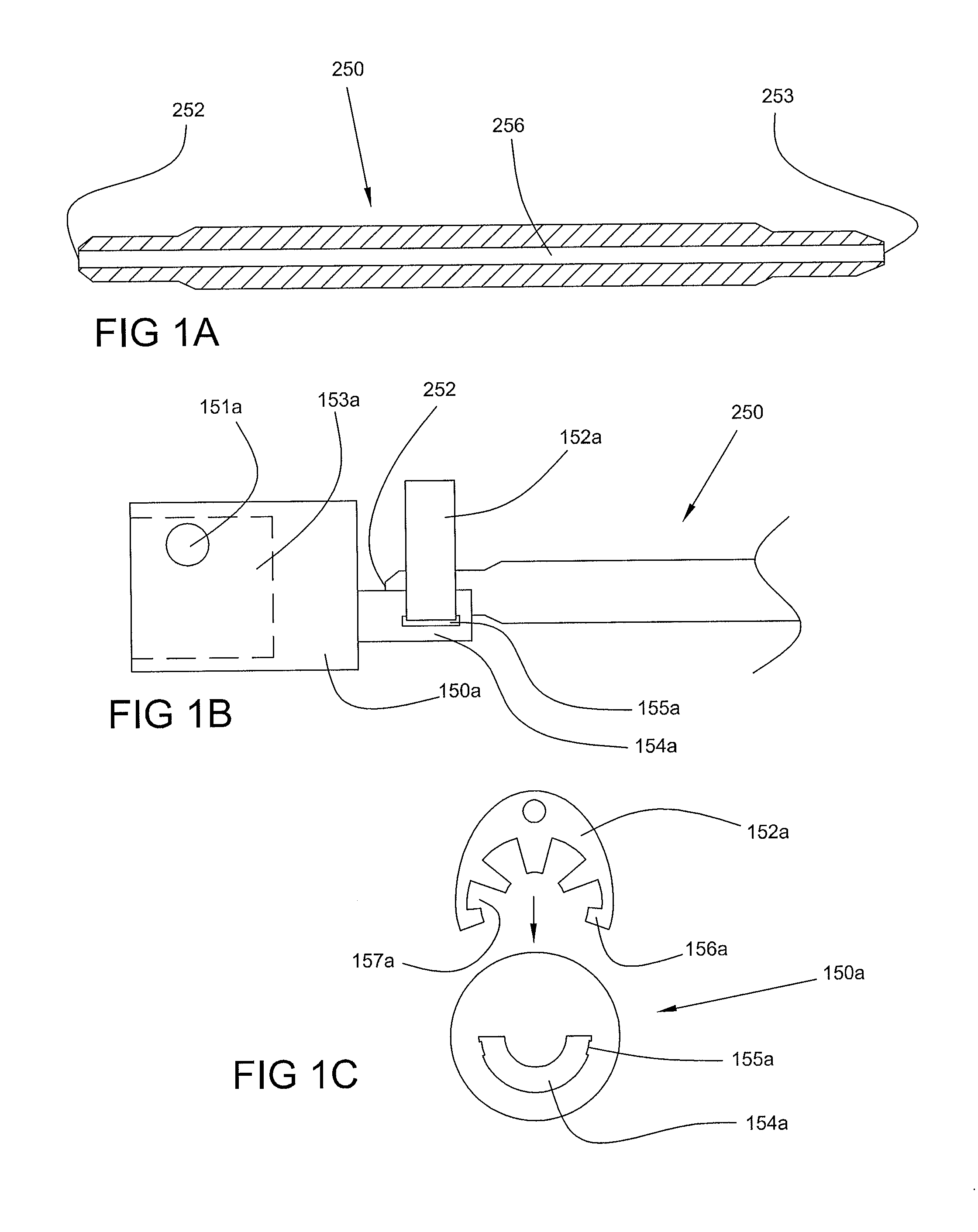
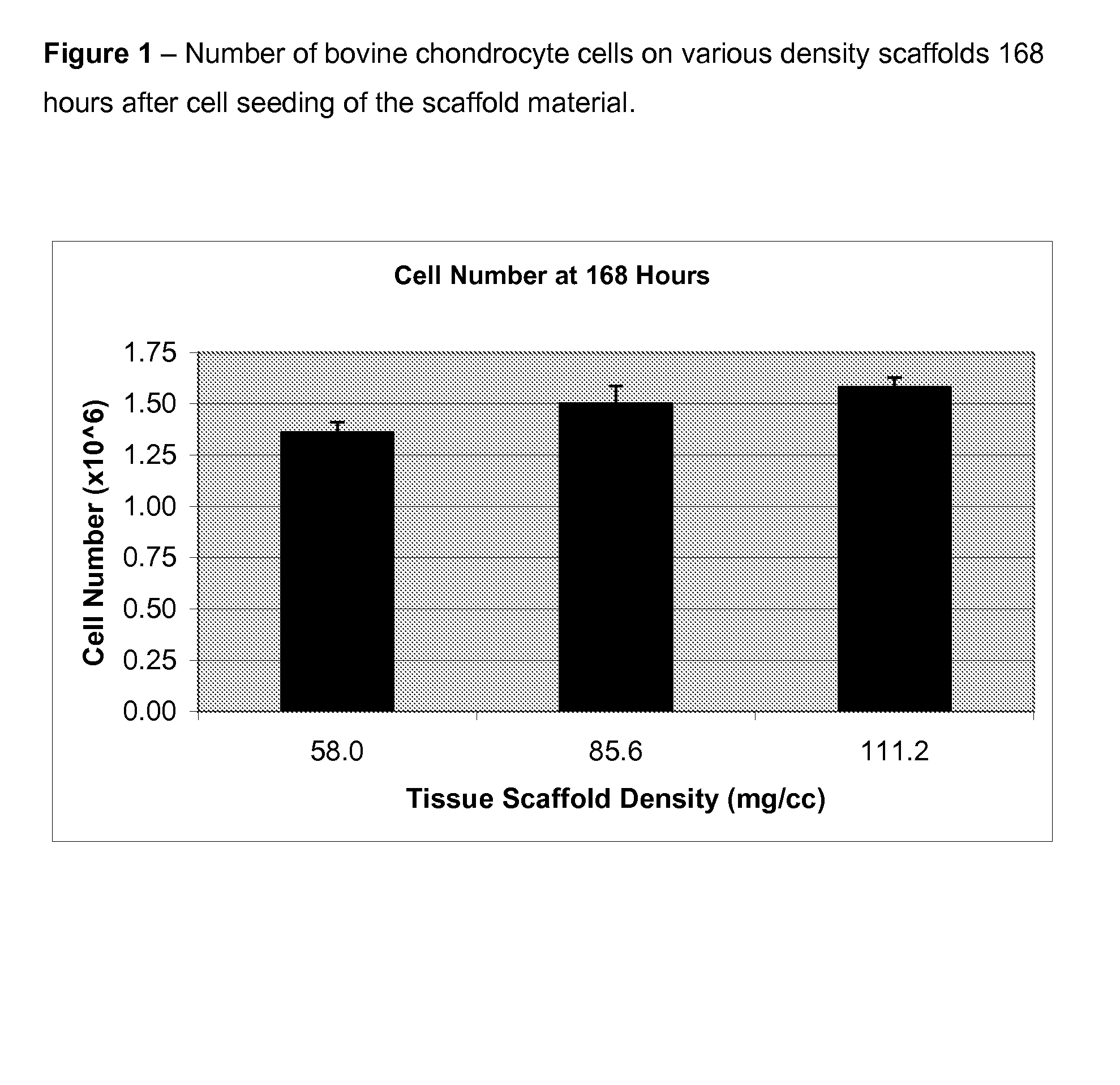
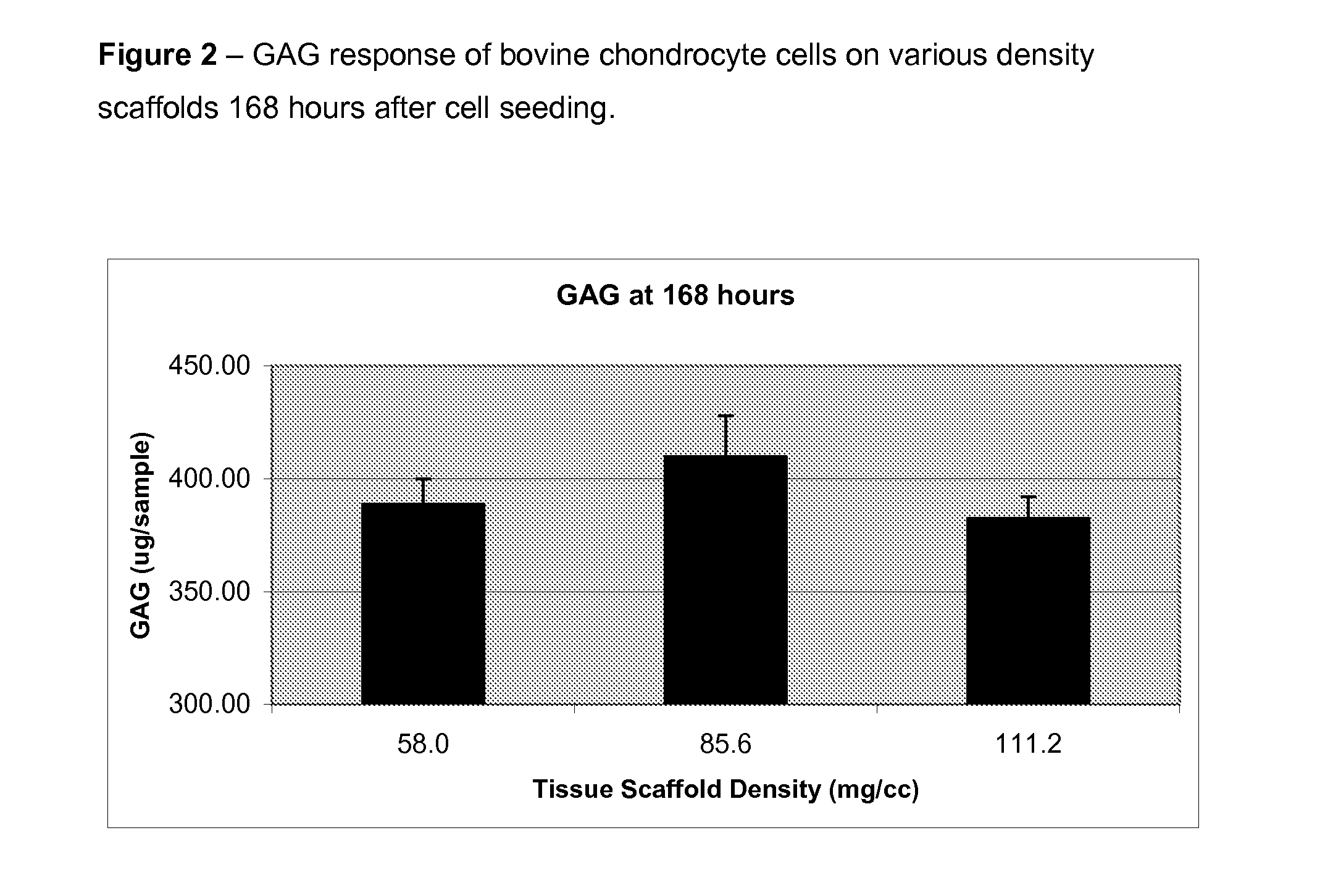
Optimum Density Fibrous Matrix
InactiveUS20090148495A1Faster cell infiltrationQuick migrationBiocidePeptide/protein ingredientsFiberMammalian tissue
An implantable biodegradable porous fibrous matrix is disclosed, the fibrous matrix being constructed of fibers arranged in a nonwoven array. The density of the nonwoven array is adjusted in the manufacturing process to obtain an optimum density of the array for tissue ingrowth. When implanted, the optimum density fibrous matrix provides for a superior biological response of the host in terms of tissue growth, especially for tissues containing glycosaminoglycans (GAGs). The optimum density fibrous matrix is therefore provided with properties useful in repair and / or regeneration of mammalian tissue.
Owner:DEPUY SPINE INC (US) +1
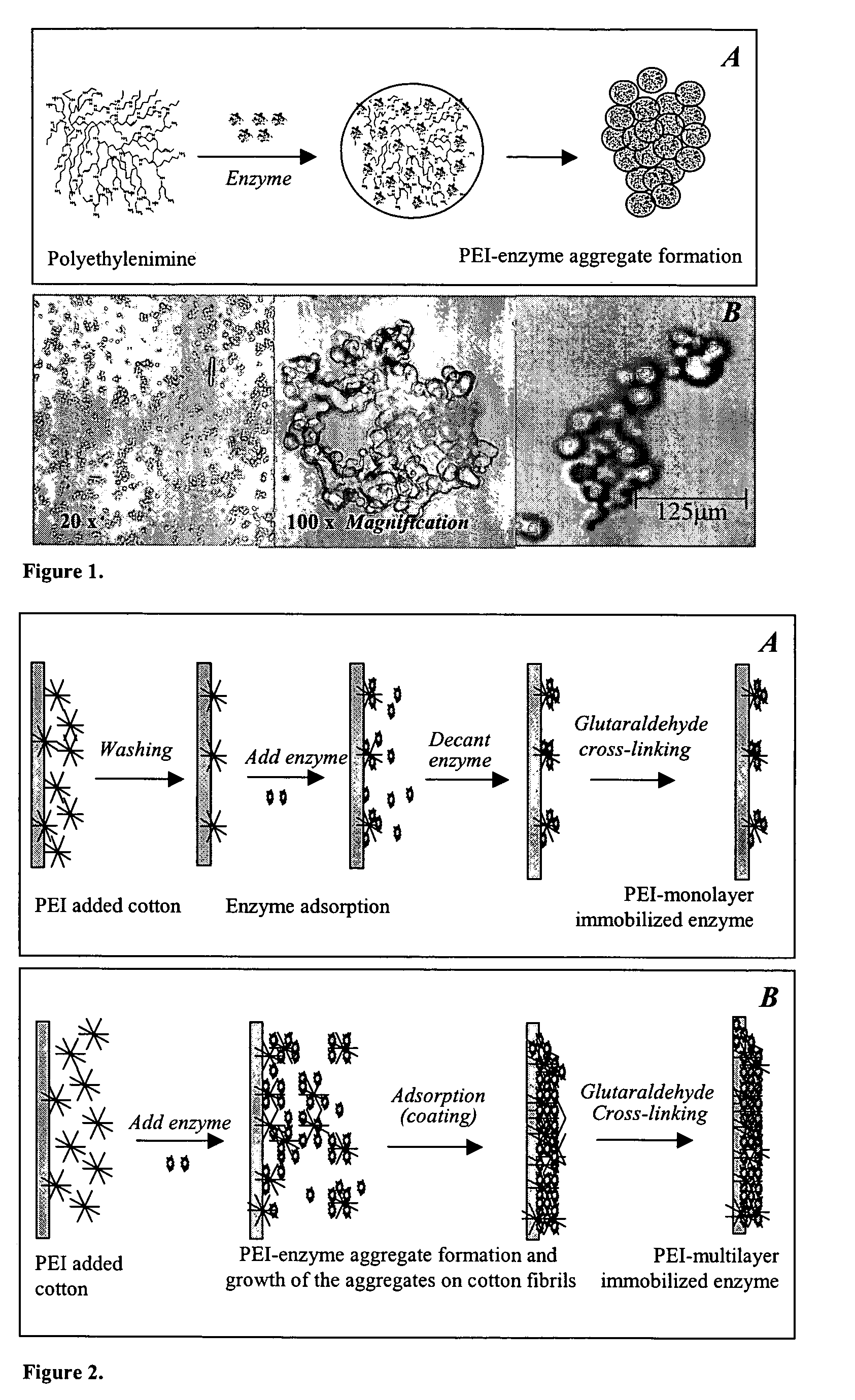
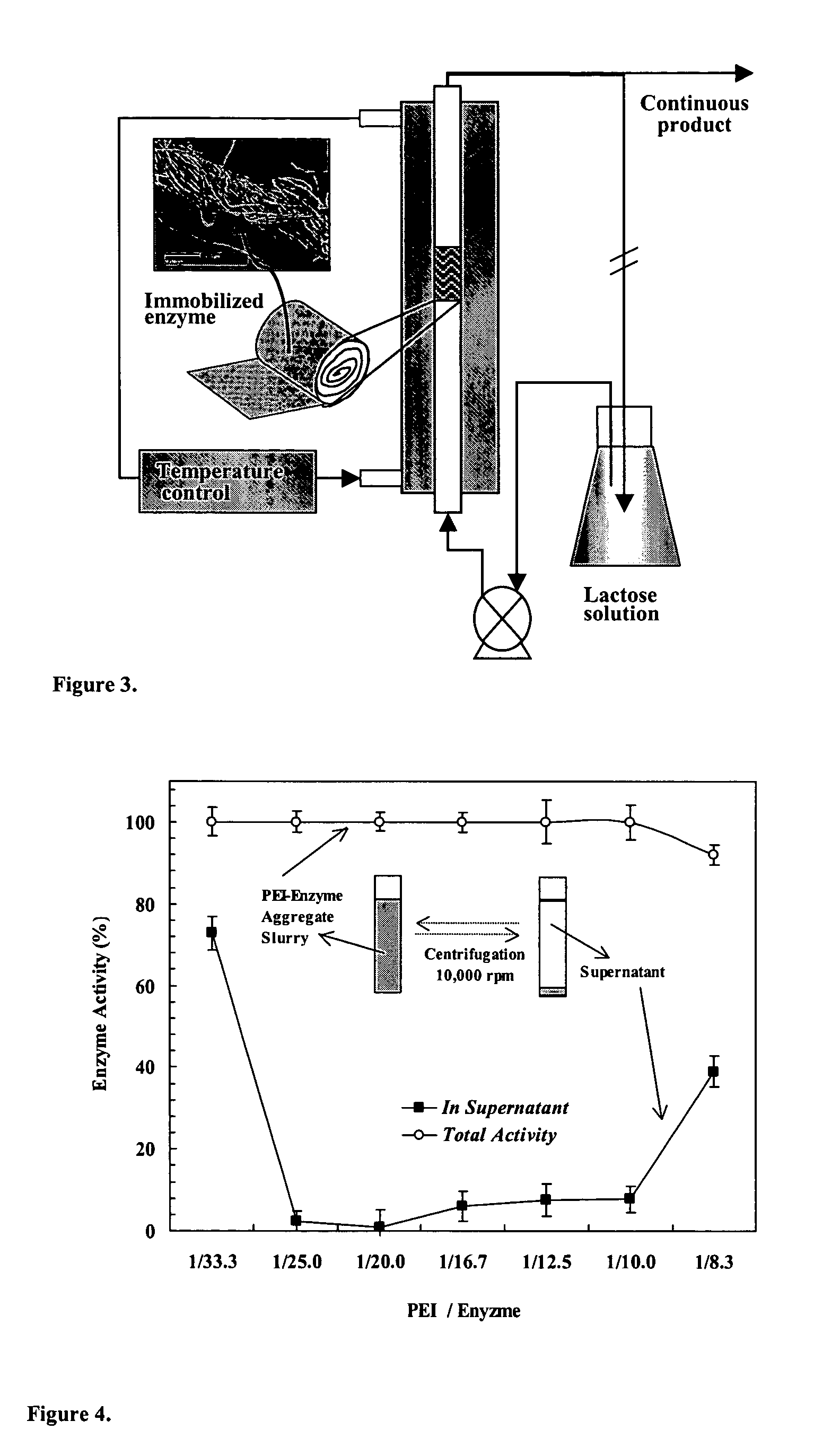
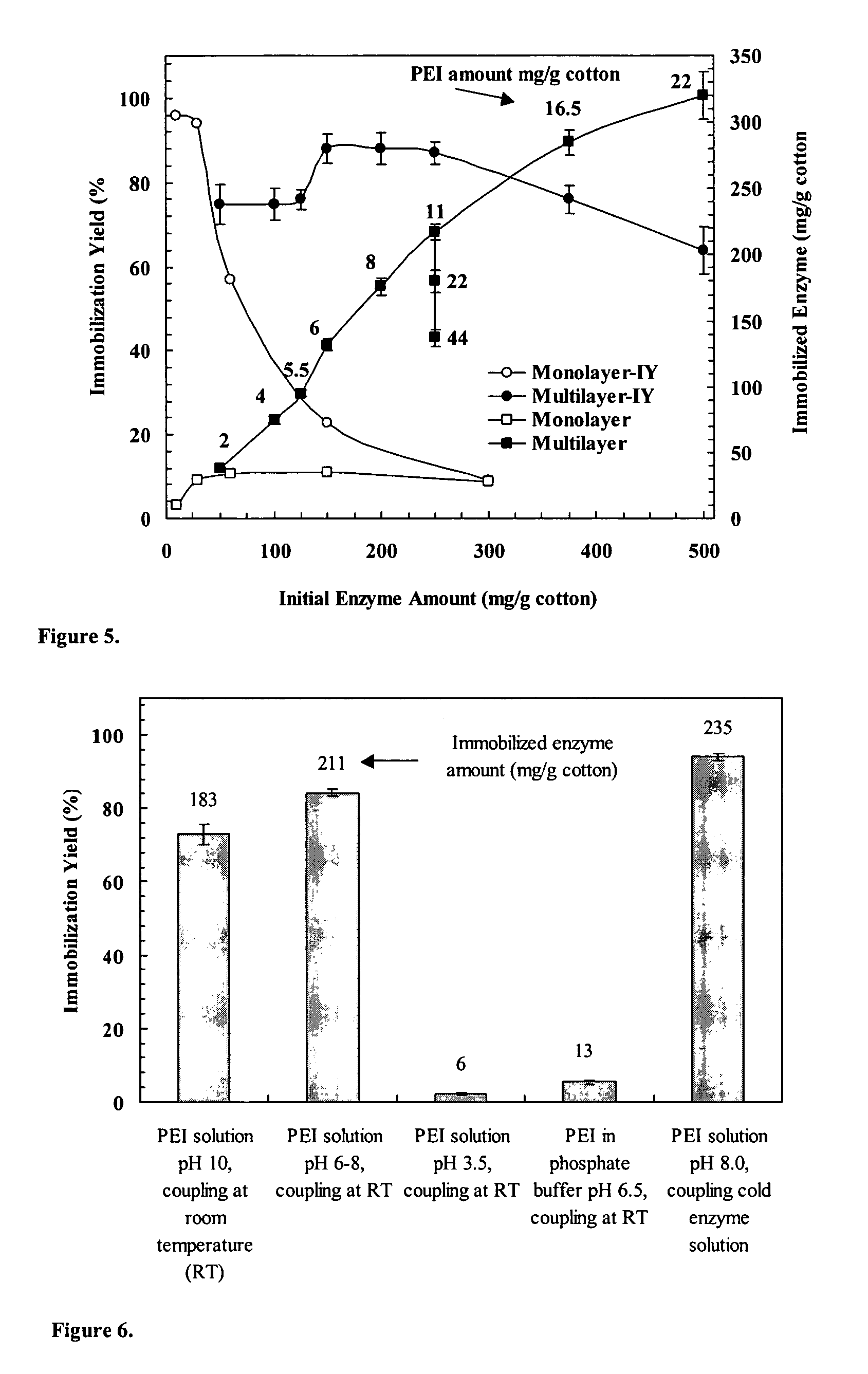
Immobilization of enzyme on a fibrous matrix
ActiveUS7166451B1The process is simple and clearHigh enzyme loadingOn/in organic carrierFermentationFiberCross-link
A multilayer enzyme immobilization process is provided comprising adsorbing a polyethyleneimine (PEI) solution in a fibrous matrix, and adding an enzyme to the fibrous matrix, which comprises a plurality of fibrils. The process further comprises forming at least two layers of PEI-enzyme aggregates on the fibrils, and cross-linking the multilayer PEI-enzyme aggregates. The process can further comprise washing the fibrils containing the cross-linked PEI-enzyme aggregates with distilled water and acetic acid buffer subsequent to cross-linking. However, the PEI-containing matrix is not washed prior to the addition of enzyme. The enzyme can be β-galactosidase and the fibrous matrix can be cotton cloth. The multilayer immobilized enzyme can be employed in a biocatalyst reactor for production of galacto-oligosaccharides from lactose and the hydrolysis of lactose to glucose and galactose.
Owner:THE OHIO STATE UNIV RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com