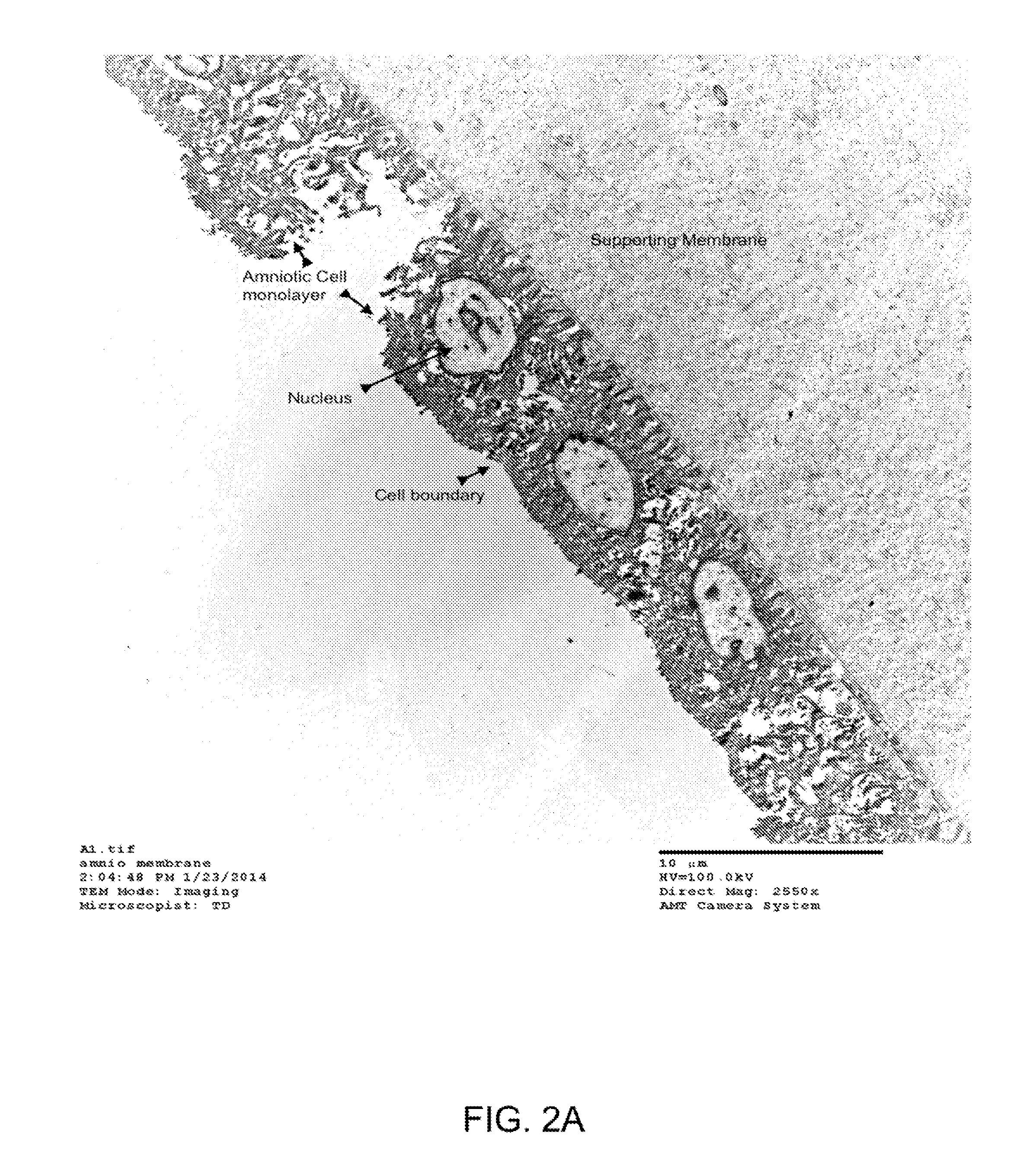
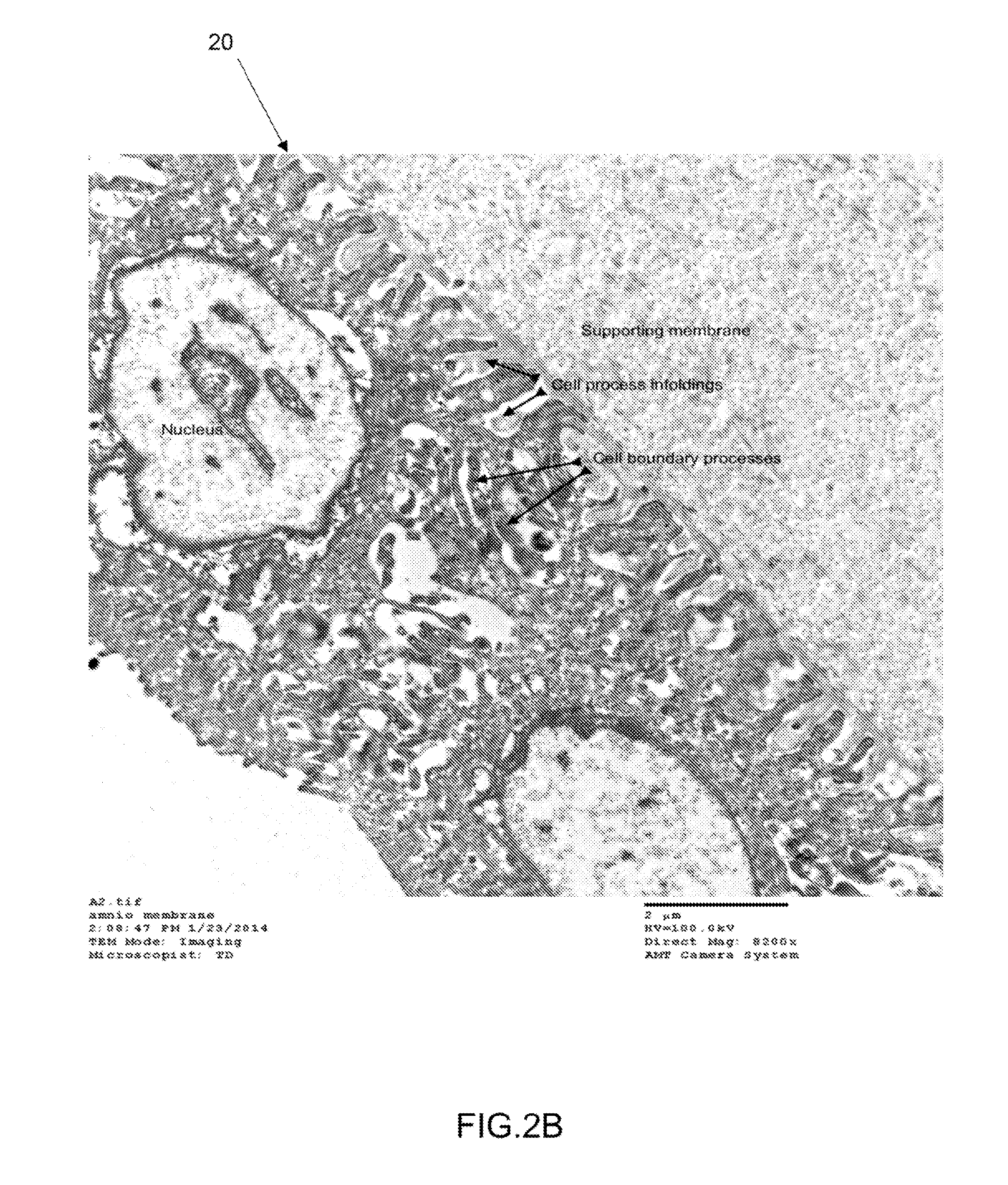
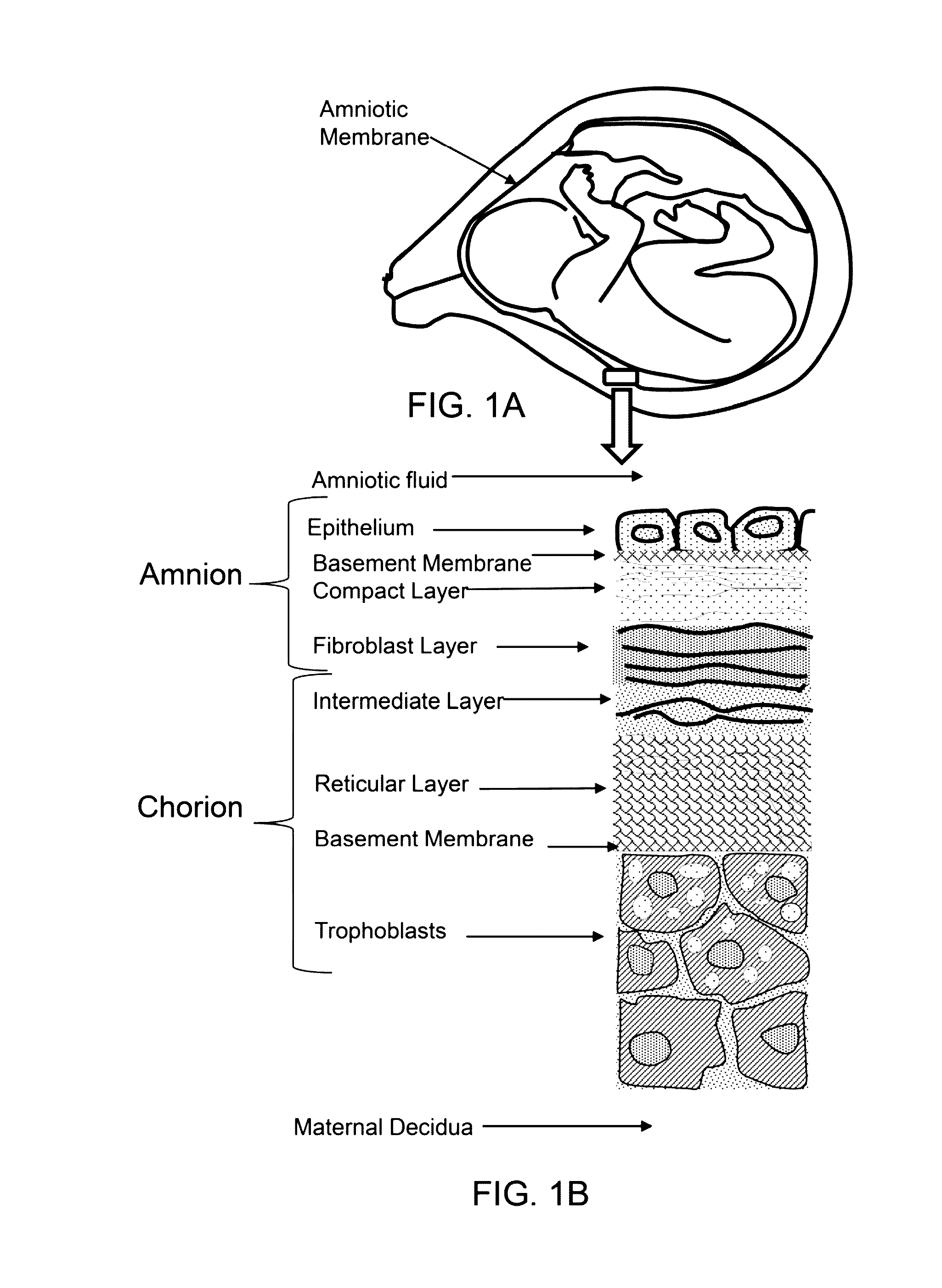
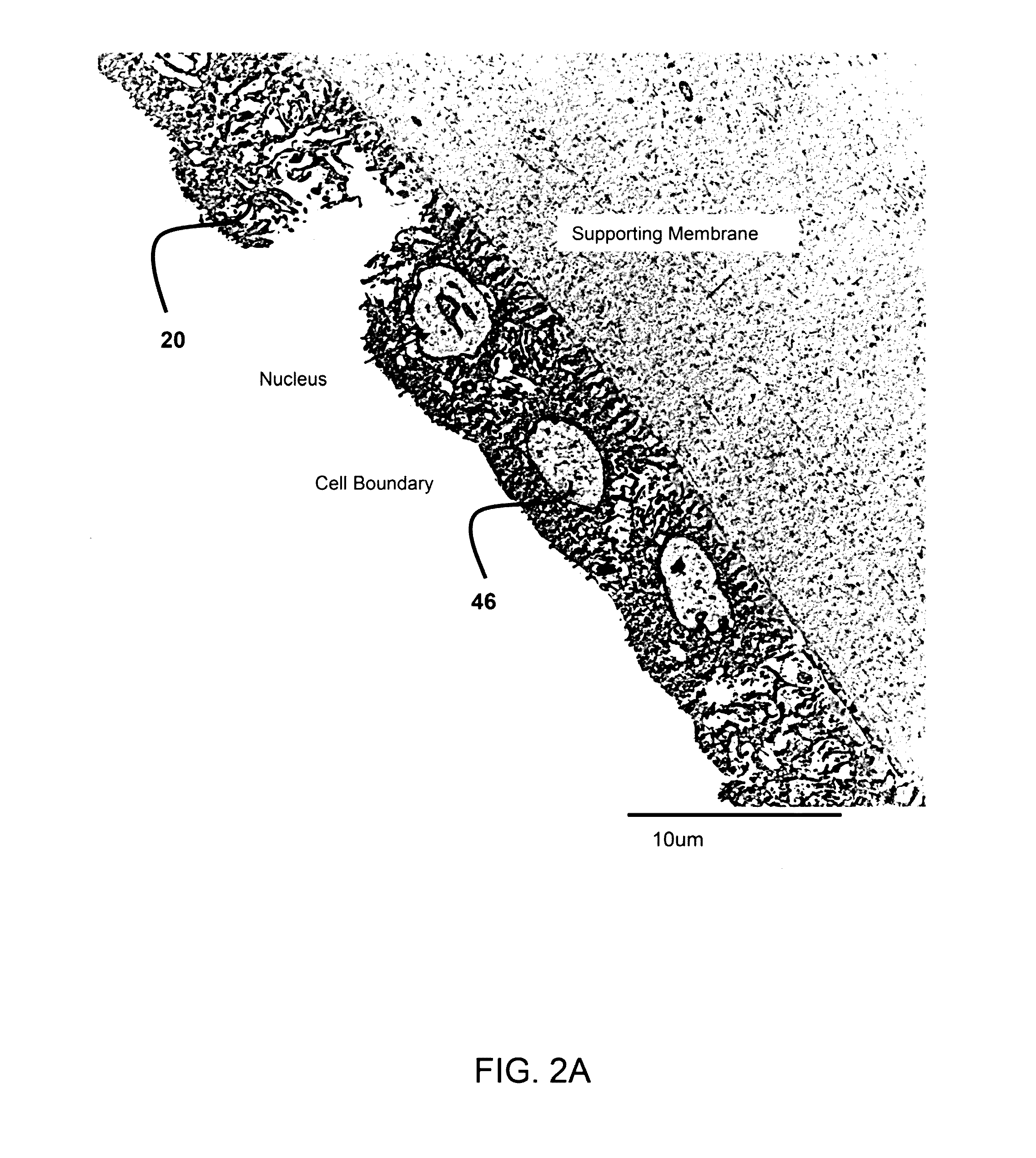
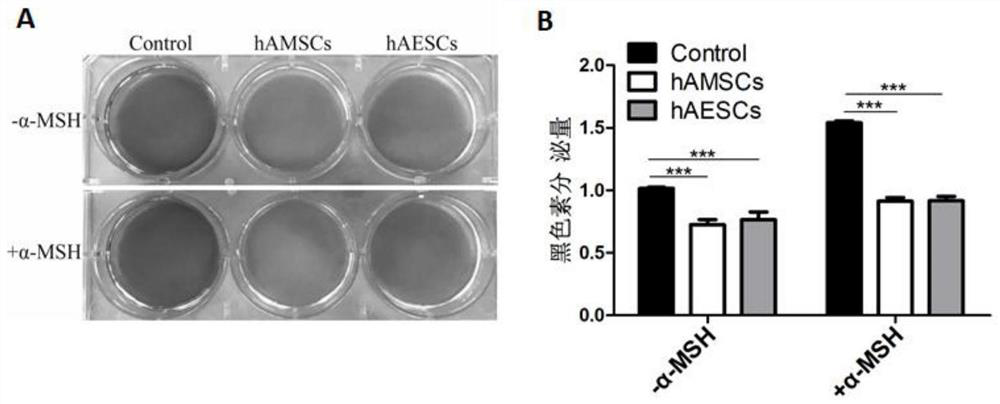
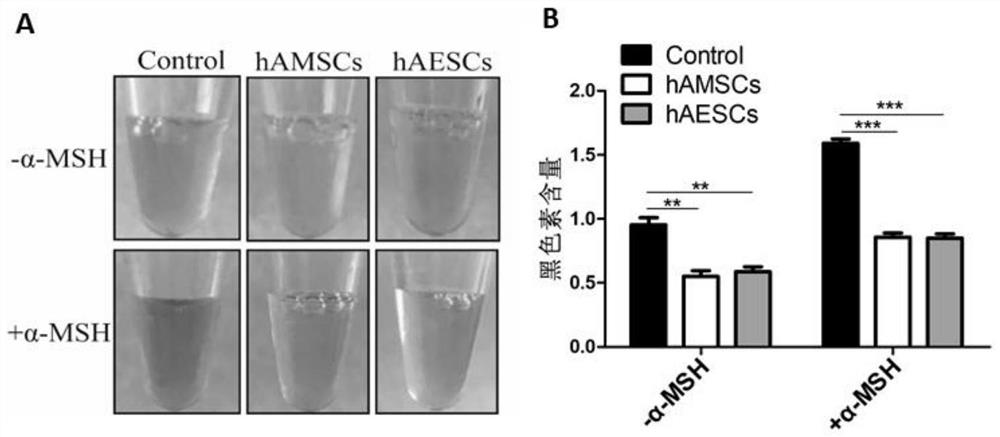
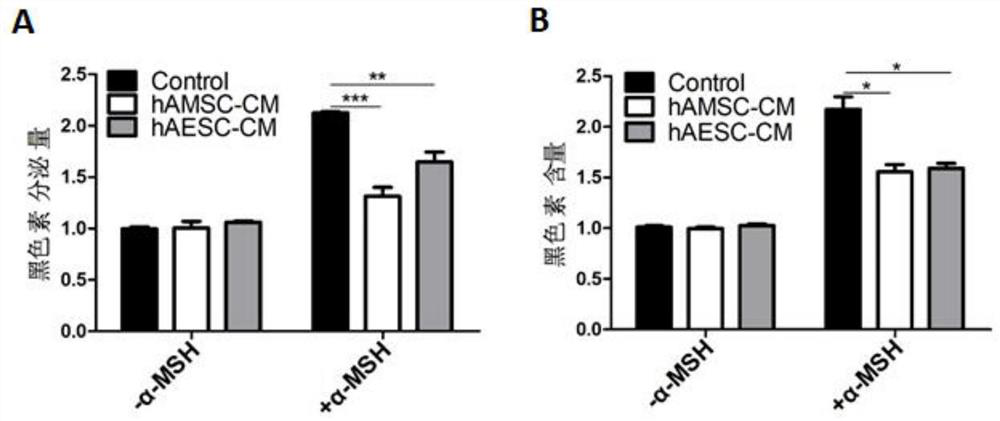
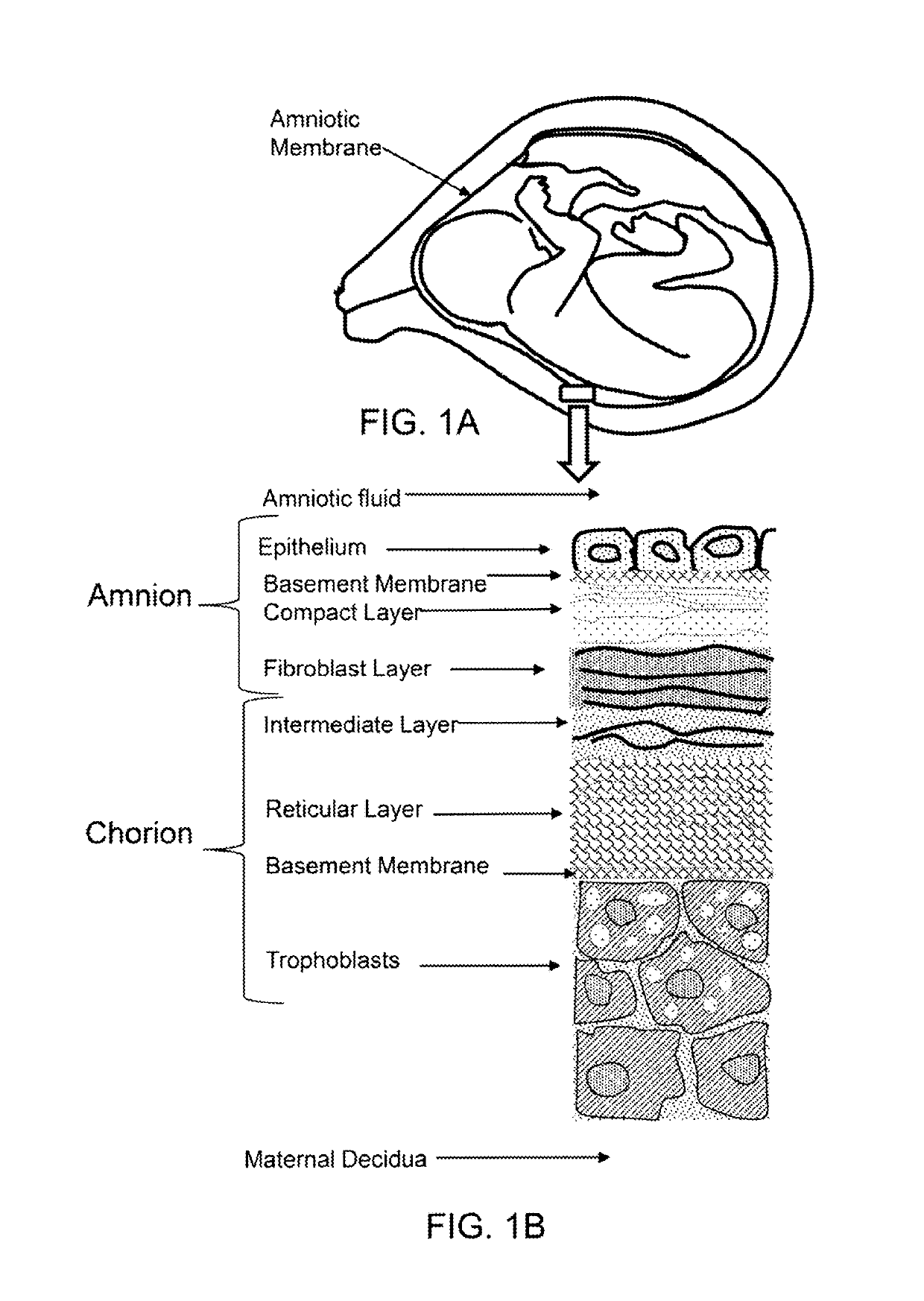
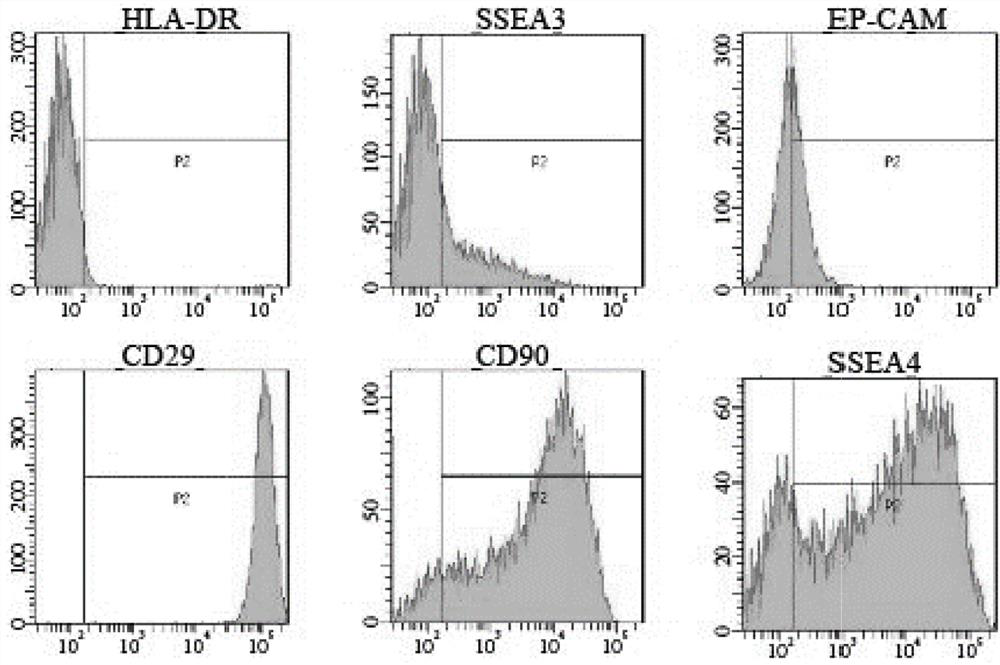
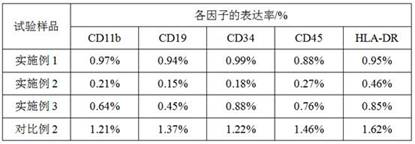
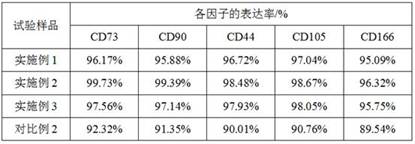
Patents
Literature
32 results about "Amniotic stem cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
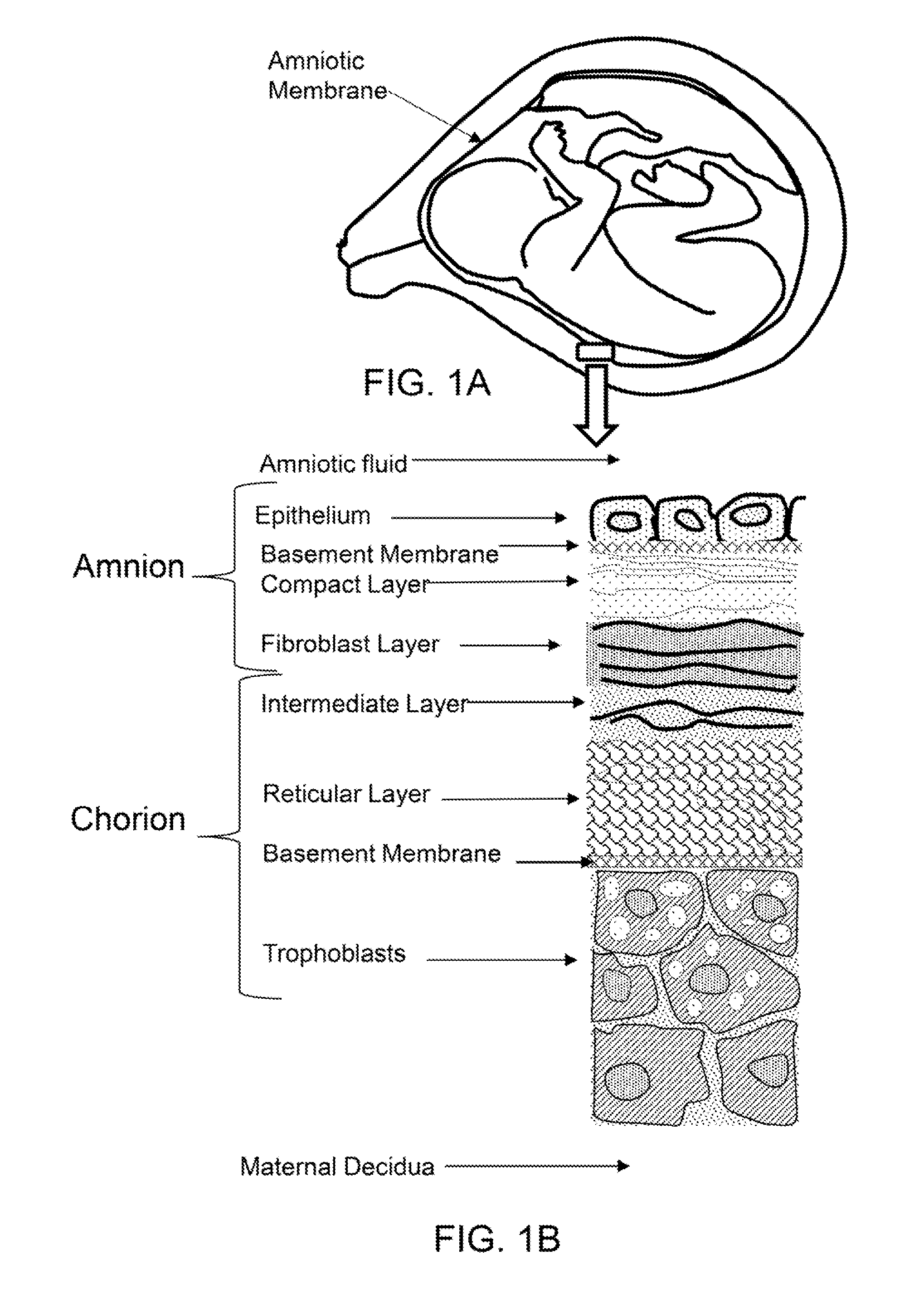
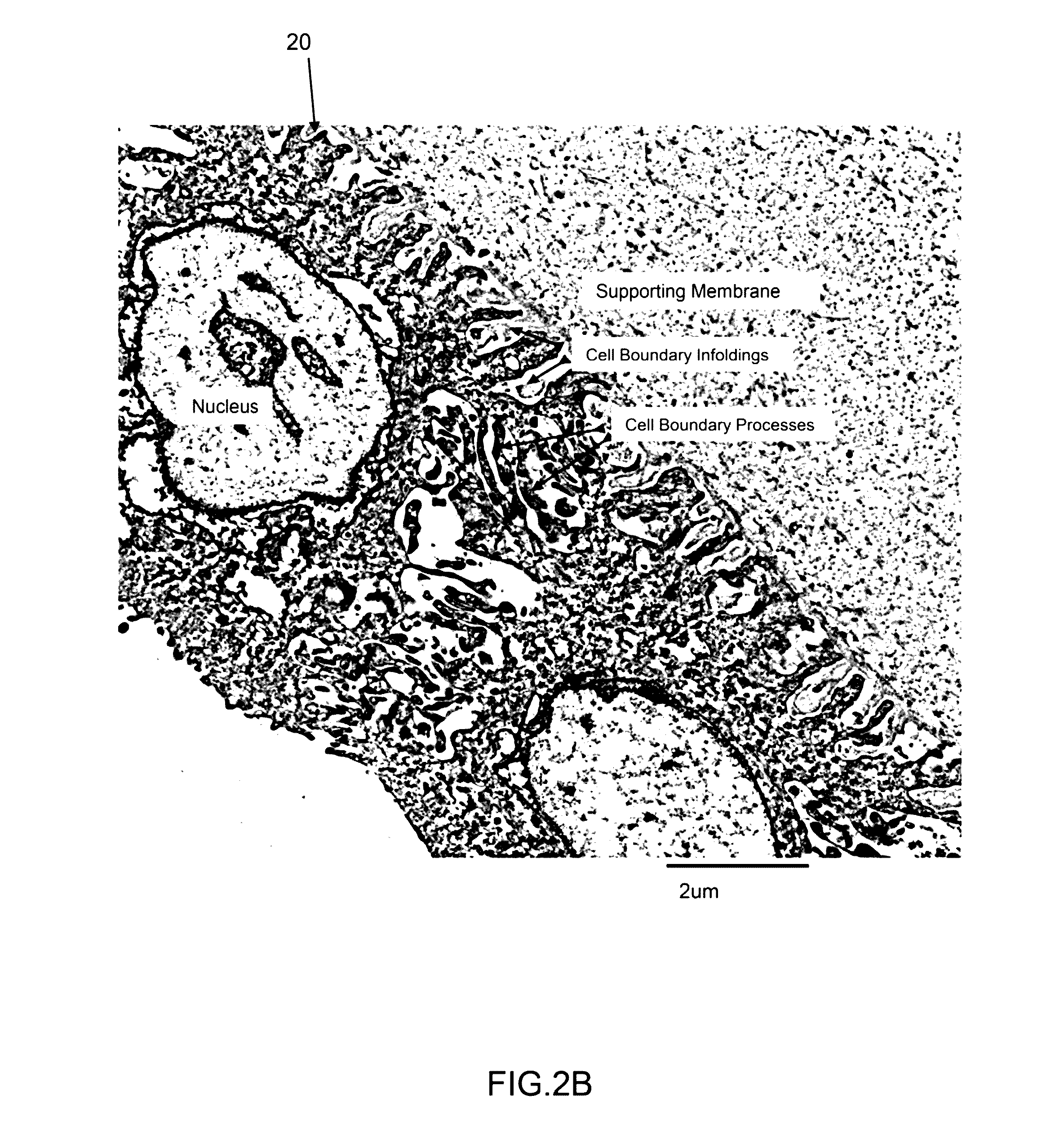
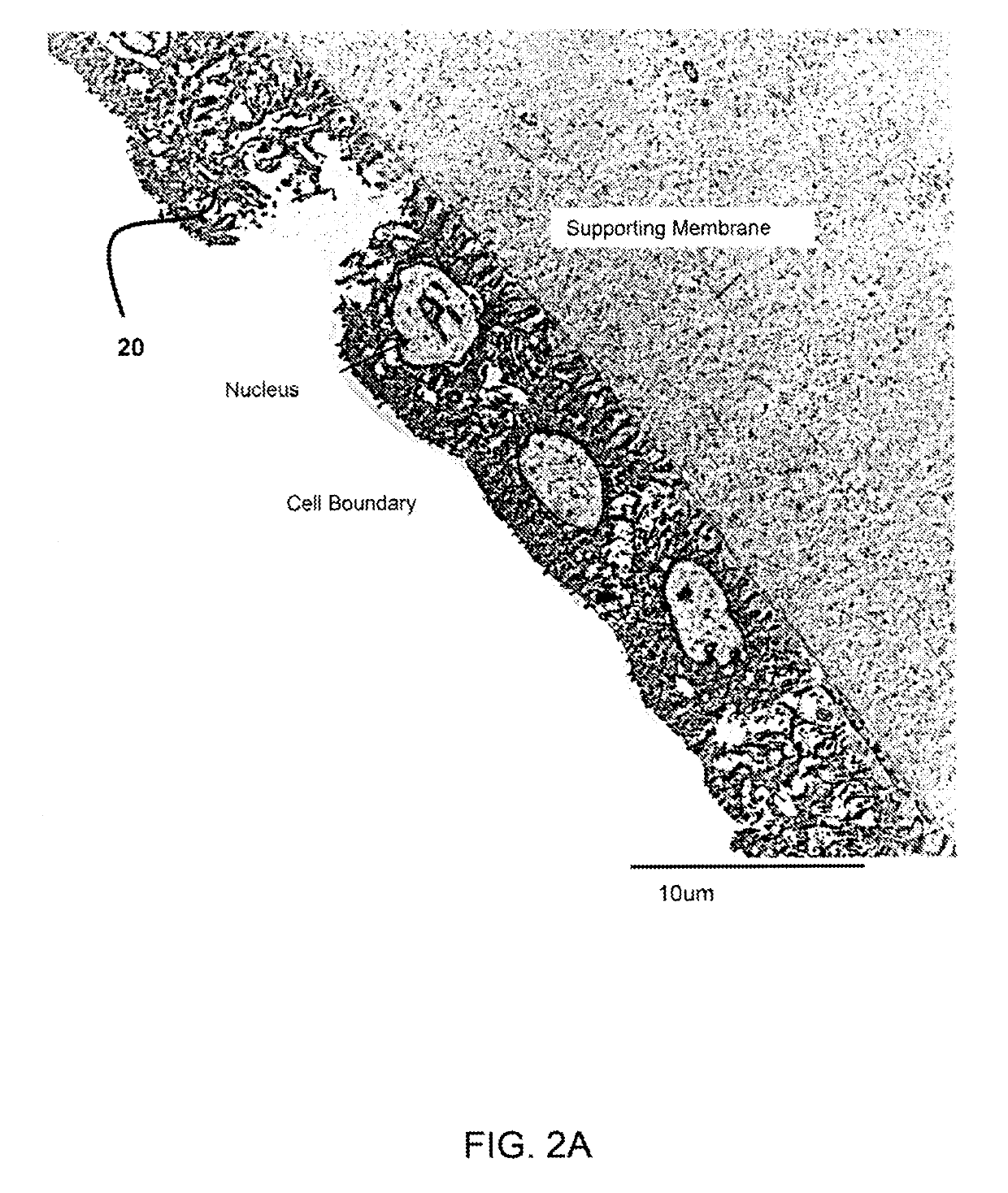
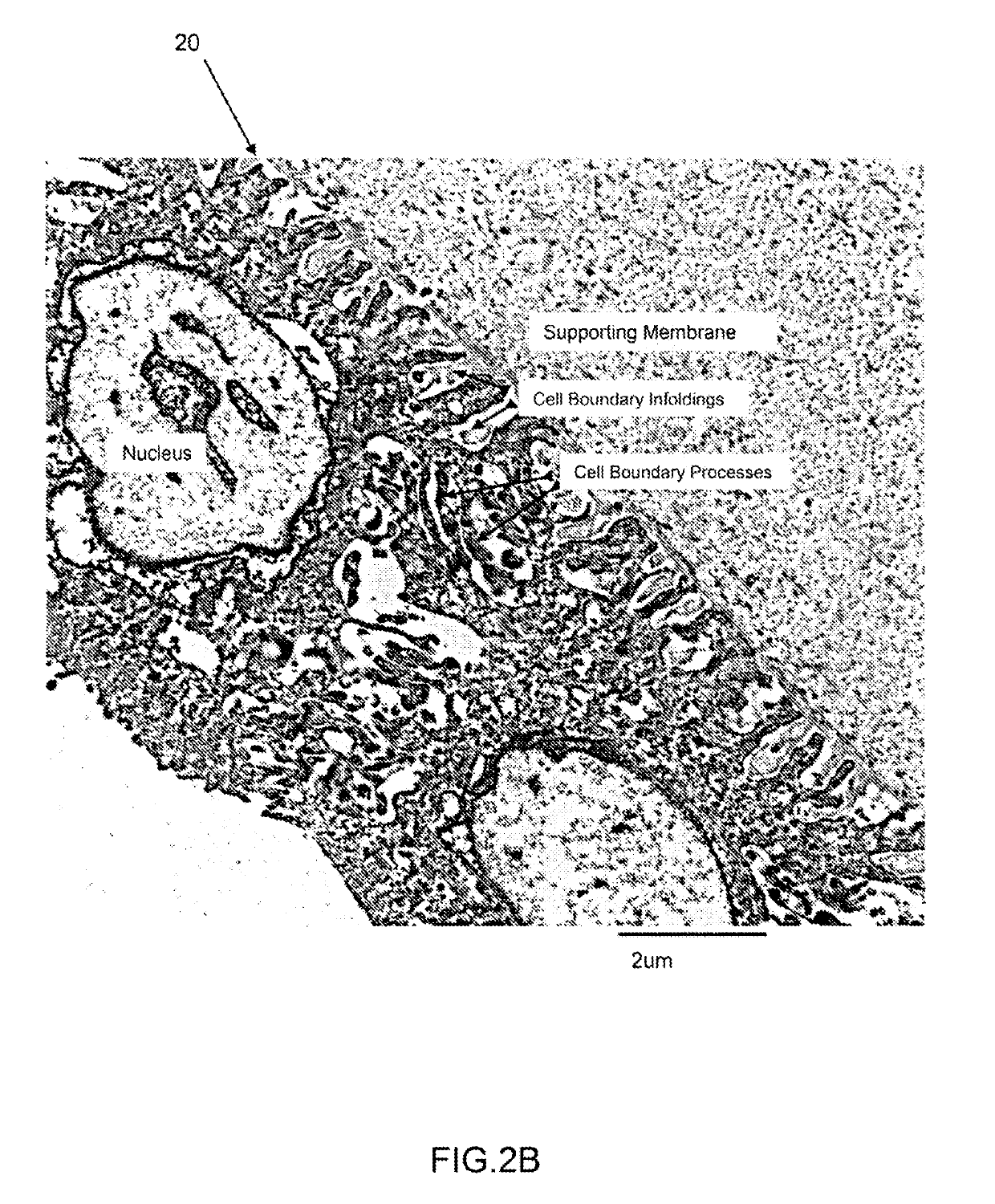
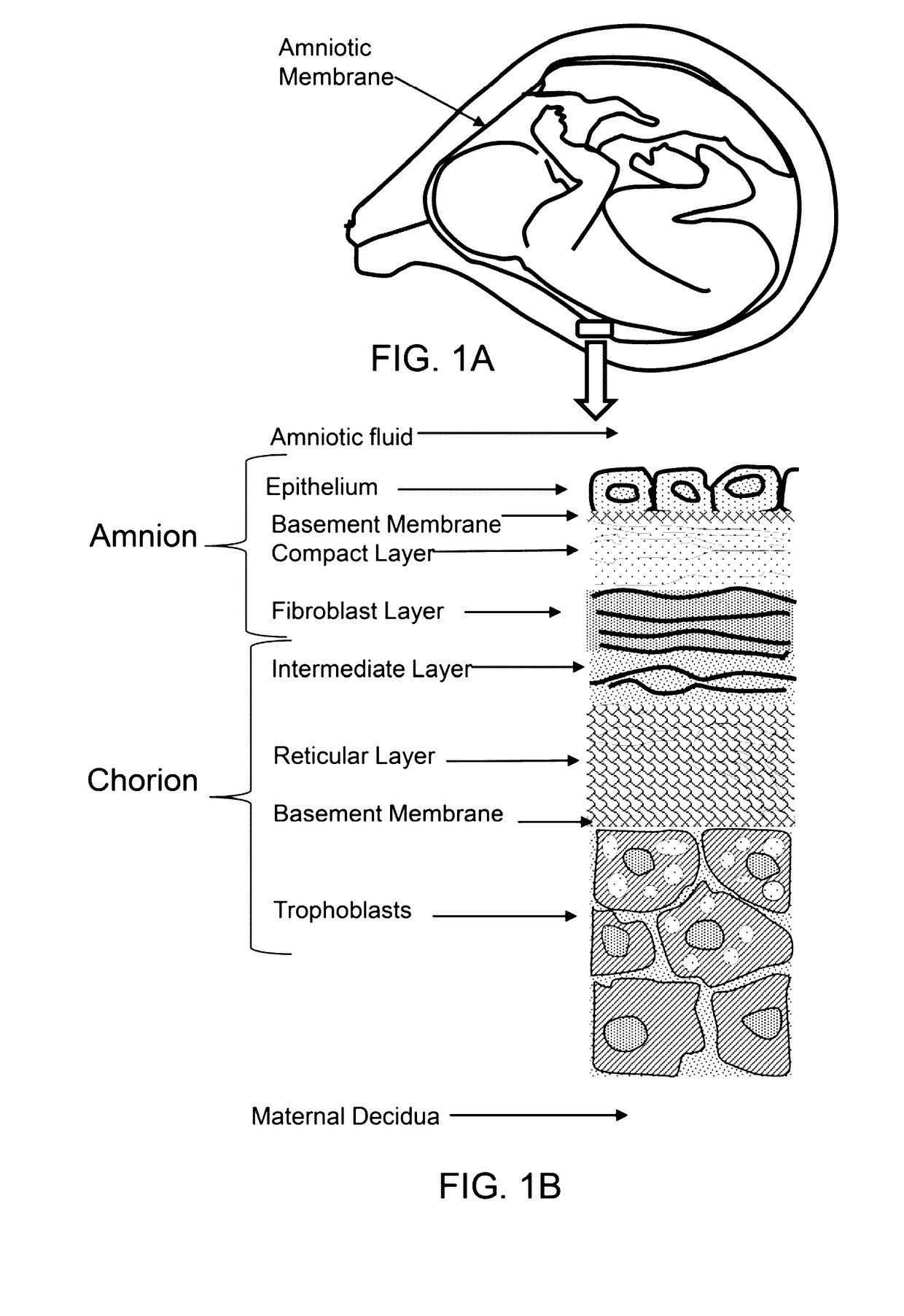
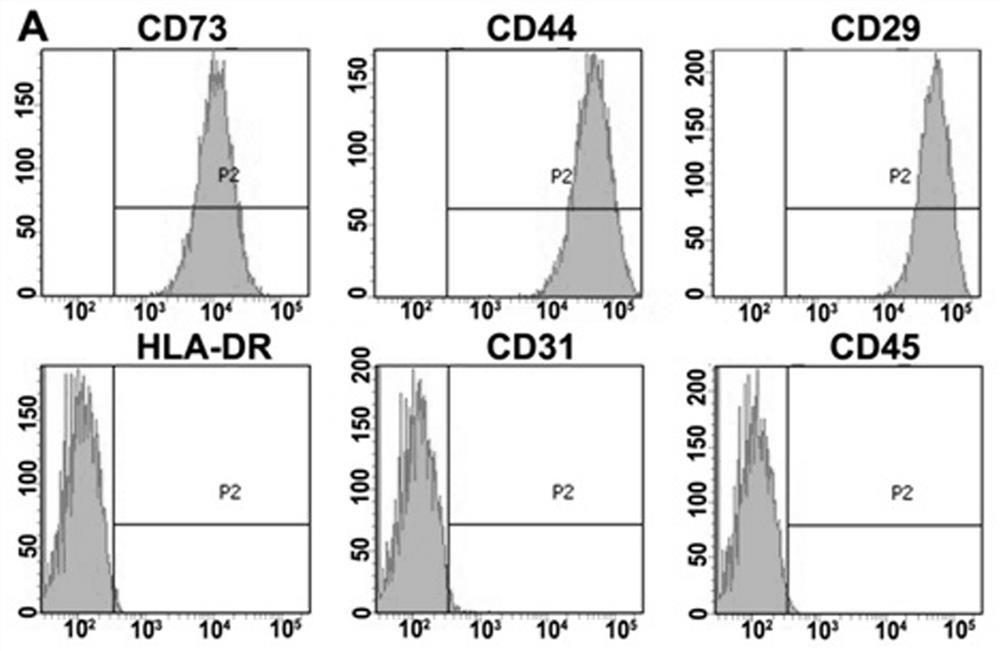
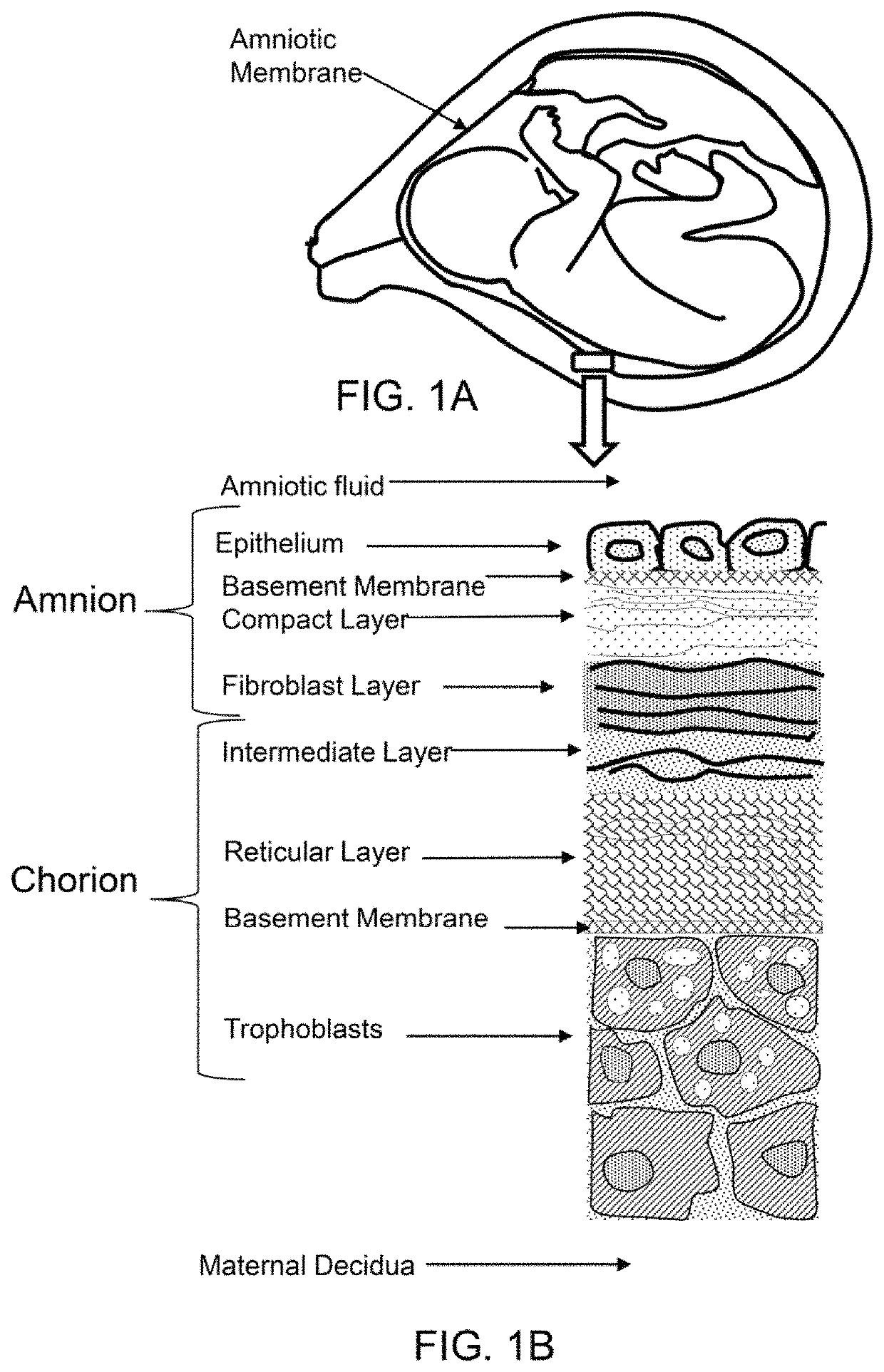
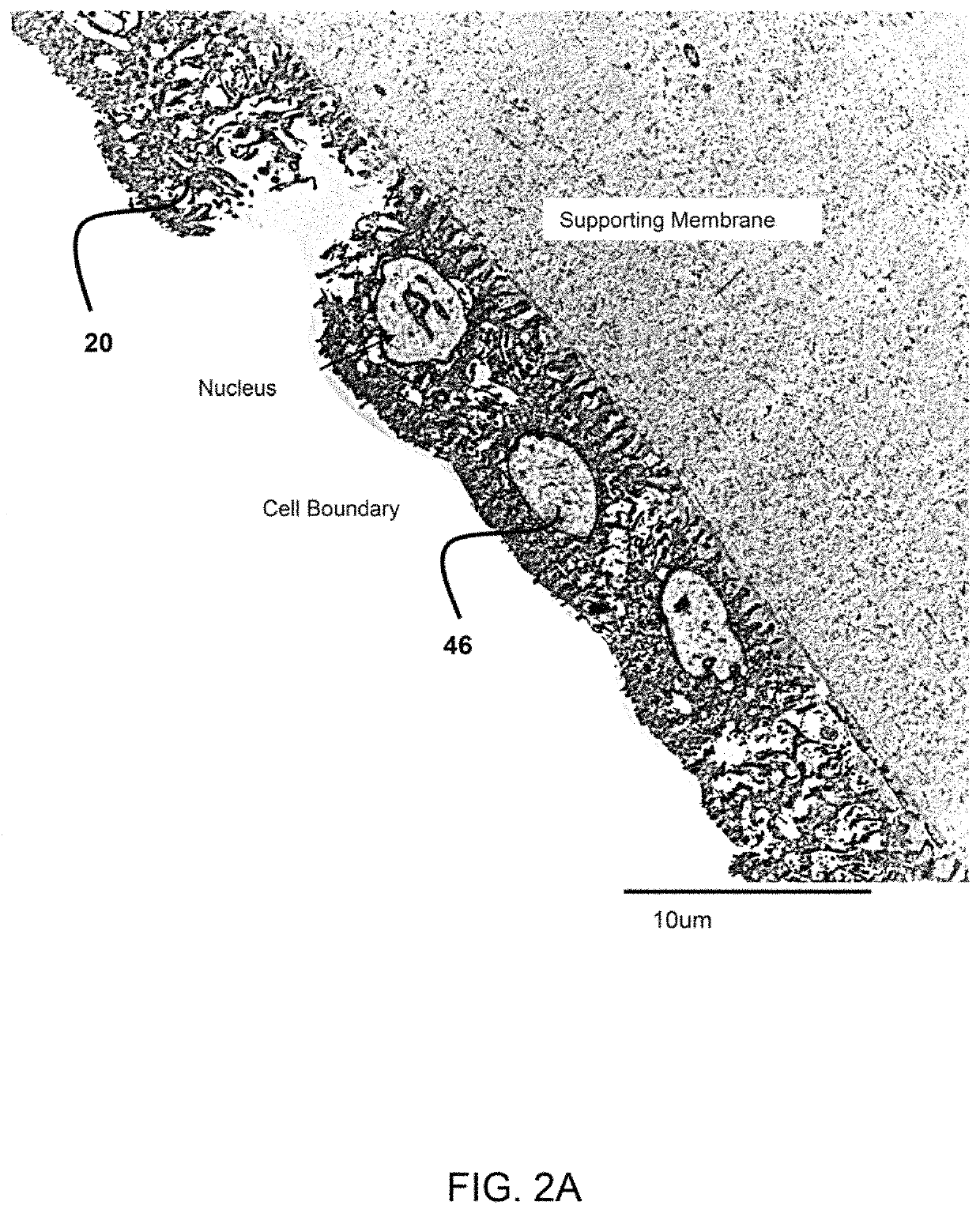
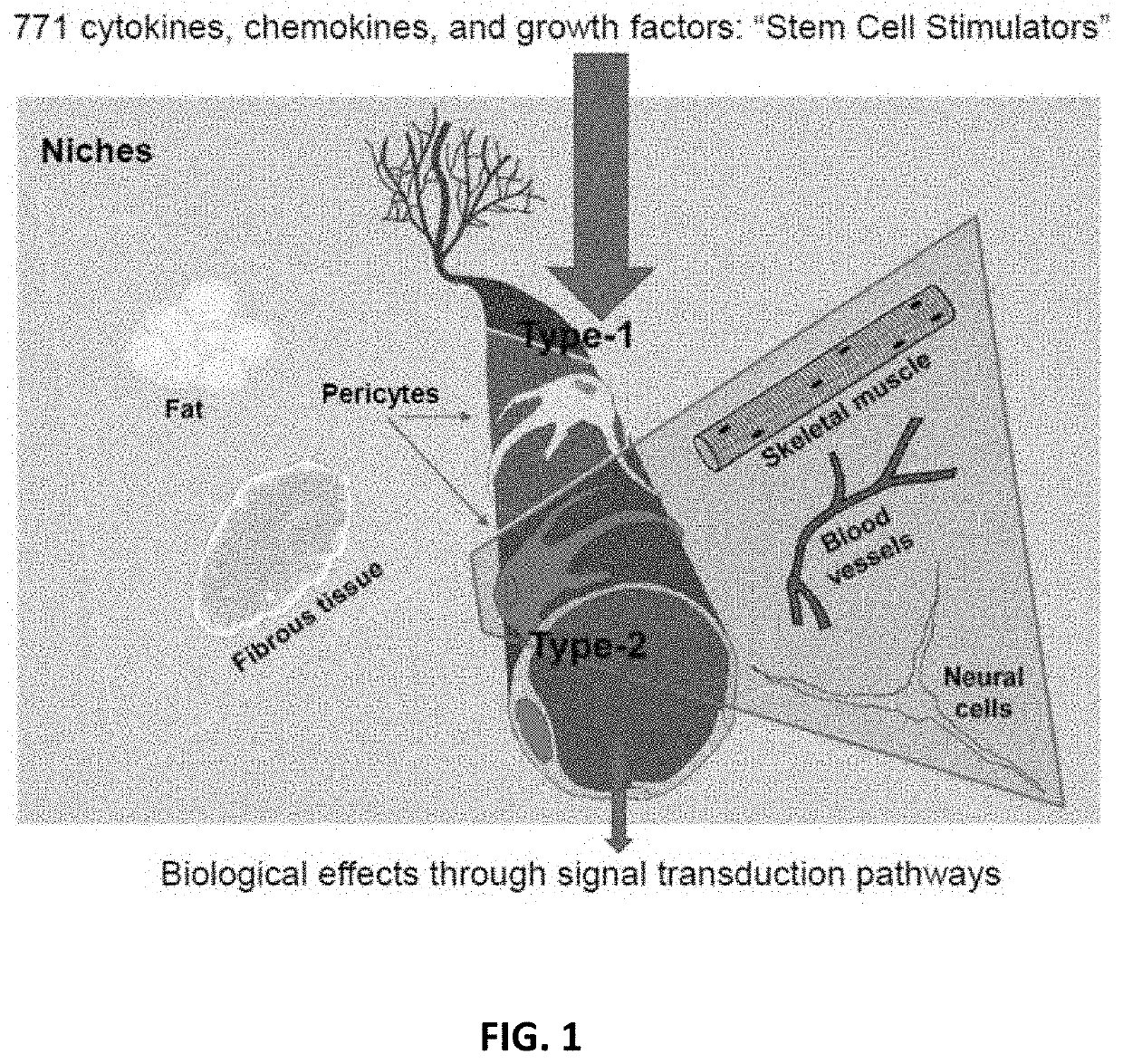
Amniotic stem cells are the mixture of stem cells that can be obtained from the amniotic fluid as well as the amniotic membrane. They can develop into various tissue types including skin, cartilage, cardiac tissue, nerves, muscle, and bone. The cells also have potential medical applications, especially in organ regeneration.
Acellular amnion derived therapeutic compositions
ActiveUS9132156B1Reduce in quantityInduce and responsePowder deliveryCosmetic preparationsAmniotic fluidViable cell
Acellular amnion derived therapeutic compositions are described having a number of various compositional embodiments. An acellular amnion derived therapeutic composition has essentially no live or active amniotic stems cells. The amniotic stem cells may be destroyed, and the cells and cell debris may be removed from the acellular amnion derived therapeutic composition. An acellular amnion derived therapeutic composition may comprise micronized amniotic membrane particles, and / or amniotic fluid. An acellular amnion derived therapeutic composition may be a dispersion of micronized amniotic membrane combined with a fluid, such as plasma, saline, amniotic fluid, combinations thereof and the like. An acellular amnion derived therapeutic composition may be combined with a matrix component to form a composite. An acellular amnion derived therapeutic composition may be used in conjunction with a composition comprising viable cells, such as stem cells.
Owner:AMNIO TECH +1
Cryoprotectant and method for cryopreserving placenta amnion and chorion
The invention relates to the technical field of tissue engineering and discloses a cryoprotectant and a method for cryopreserving placenta amnion and chorion. The cryoprotectant is composed of fetal calf serum, dimethyl sulfoxide and dextran 40, and the volume ratio of the fetal calf serum, the dimethyl sulfoxide and the dextran 40 is 7-9:1:1. On the basis of deep research of placenta amnion and chorion structures, the dimethyl sulfoxide, the dextran 40 and the fetal calf serum suitable for protecting placenta amnion stem cells and chorion stem cells are selected to form the cryoprotectant. The fetal calf serum, the dimethyl sulfoxide and the dextran 40 coordinate to protect activity of cells, the cells are prevented from forming ice crystal and being damaged, and the activity of recovered stem cells and the activity of newly prepared amnion chorion stem cells have no difference.
Owner:BOYALIFE
Cryoprotectant and method for cryopreserving placenta amnion and chorion
ActiveCN102763642BHigh activityNo freeze damageArtificial cell constructsVertebrate cellsBiologyCattle calf
The invention relates to the technical field of tissue engineering and discloses a cryoprotectant and a method for cryopreserving placenta amnion and chorion. The cryoprotectant is composed of fetal calf serum, dimethyl sulfoxide and dextran 40, and the volume ratio of the fetal calf serum, the dimethyl sulfoxide and the dextran 40 is 7-9:1:1. On the basis of deep research of placenta amnion and chorion structures, the dimethyl sulfoxide, the dextran 40 and the fetal calf serum suitable for protecting placenta amnion stem cells and chorion stem cells are selected to form the cryoprotectant. The fetal calf serum, the dimethyl sulfoxide and the dextran 40 coordinate to protect activity of cells, the cells are prevented from forming ice crystal and being damaged, and the activity of recovered stem cells and the activity of newly prepared amnion chorion stem cells have no difference.
Owner:BOYALIFE
Amnion derived therapeutic compositions and methods of use
ActiveUS20160375064A1Reduce scarsReduce blockingPowder deliveryCosmetic preparationsMedicineAmniotic fluid
Therapeutic compositions are described for the treatment of a variety of conditions including heart, eye, lungs, organs, joints, dermal, nerve, and the like. s, A therapeutic composition may be a fluid comprising amniotic fluid or micronized amniotic particles. A therapeutic composite may be a dispersion of micronized amniotic membrane combined with a fluid, such as plasma, saline, amniotic fluid, combinations thereof and the like. In another embodiment, the therapeutic composite is a mixture of micronized amniotic membrane particles combined with an amniotic rich stem cell fluid. An amniotic rich or concentrated stem cell fluid comprises at least 0.5×106 amniotic stem cells per milliliter of fluid or composition. A therapeutic composite may be used to treat any number of conditions through topical application, surgical introduction, and / or injection.
Owner:AMNIO TECH
Hyaluronic acid method for promoting proliferation of human amniotic membrane stem cells and application thereof
ActiveCN105861425ADoes not affect potentialWide variety of sourcesArtificially induced pluripotent cellsNon-embryonic pluripotent stem cellsMedicinePharmaceutical drug
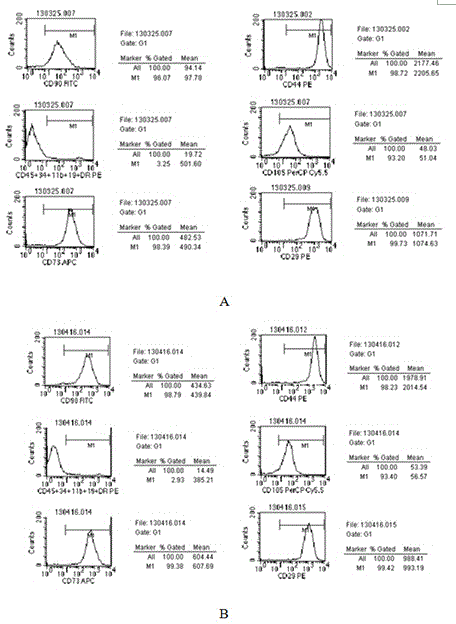
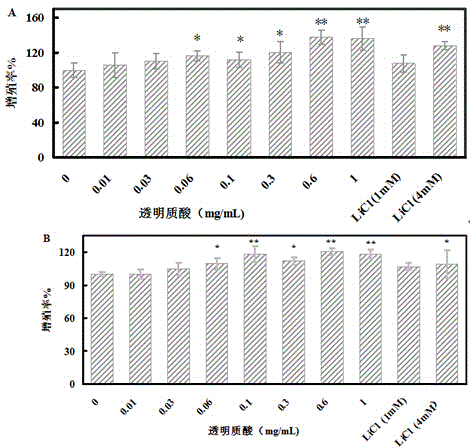
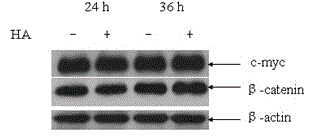
The invention discloses a hyaluronic acid method for promoting proliferation of human amniotic membrane stem cells and application thereof. The present invention applies hyaluronic acid in drugs for promoting proliferation of human amniotic stem cells. Through a series of tests, it is fully proved that hyaluronic acid can significantly reduce the doubling time of human amniotic stem cells (hASCs) and promote hASCs proliferation, has safety, and does not affect hASCs multi-differentiation potential of stem cells. Hyaluronic acid can be used alone, or a composition containing hyaluronic acid can be used for promoting the hASCs proliferation or manufacturing pharmaceuticals for the same purpose. The present invention has the advantages of wide range of sources, low cost, and side effects.
Owner:AFFILIATED HOSPITAL OF ZUNYI UNIV
Method for extracting exosome from amniotic mesenchymal stem cells and application of exosome
PendingCN110904037AWide variety of sourcesHigh purityCell dissociation methodsSkeletal disorderVascular endotheliumThelial cell
The invention discloses a method for extracting exosome from amniotic mesenchymal stem cells and application of the exosome. The exosome is obtained through the steps of amnion separation, washing, epithelial cell removal, shearing, digestion, centrifugation, second-layer suspension taking, resuspension, inoculated culture, subsequent extraction preparation and the like. When the exosome is applied to a medicine for promoting angiogenesis and osteogenesis, vascular endothelial cell aggregation and angiogenesis can be achieved, a microcirculation is reconstructed, blood perfusion is increased,lost of other functions is not caused, and the application prospect is promising. According to the method, the cell extraction process is optimized, the amniotic stem cell extraction efficiency is higher, the purity is higher, and on the premise that more cells are obtained, it can be guaranteed that more exosomes are obtained.
Owner:AFFILIATED STOMATOLOGICAL HOSPITAL OF NANJING MEDICAL UNIV
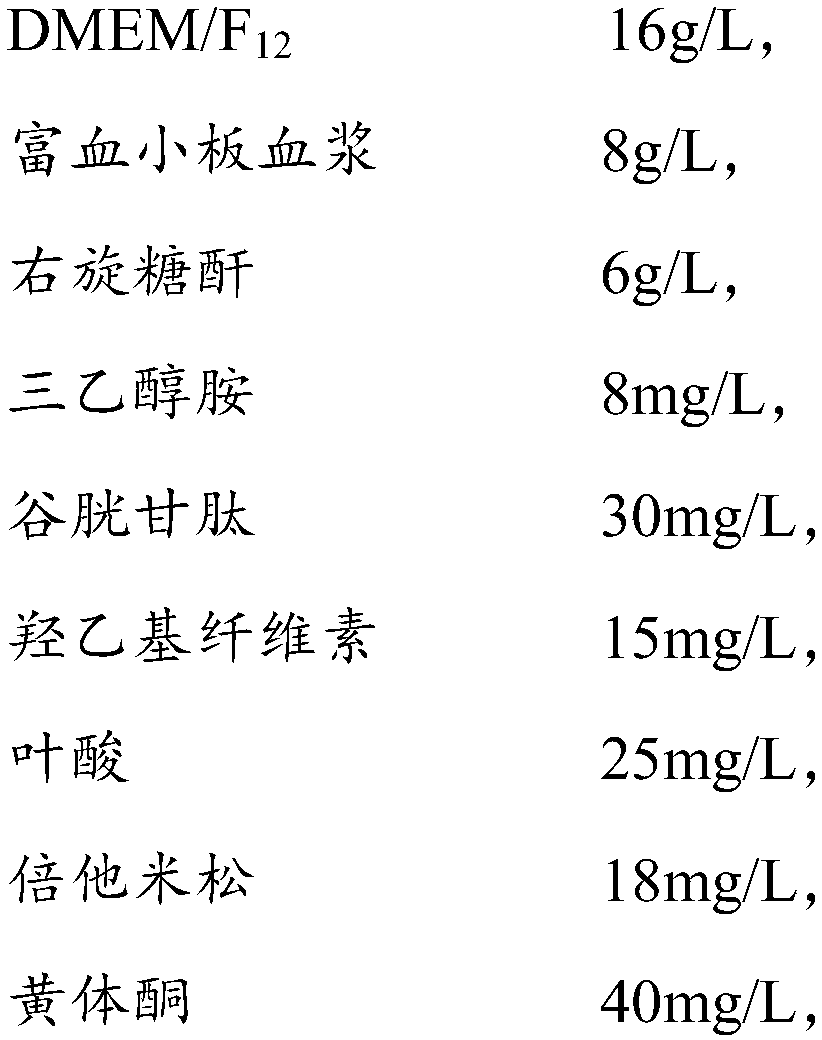
Amniotic stem cell culture medium and culture method thereof
InactiveCN107653223ANo other animal originNon-xenoimmunogenicCulture processCell culture active agentsCulture fluidStem cell culture
The invention provides an amniotic stem cell culture medium which is prepared from the following ingredients of DMEM / F12, platelet rich plasma, dextranum, triethanolamine, glutathione, hydroxyethyl cellulose, folic acid, betamethasone, progesterone, dulcite and sodium citrate. According to the amniotic stem cell culture medium disclosed by the invention, a human amniotic membrane is taken in vitroto extract and culture amniotic stem cells; compared with a method of utilizing a culture solution containing fetal calf serum to culture human amniotic mes-enchymal stromal cells, the amniotic stemcell culture medium has the advantages of no other animal source, wide source, no ethic limit, no heterogeneous immunogenicity and the like.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Method for extracting, culturing and purifying amniotic stem cells
InactiveCN108103010AMeet the needs of clinical applicationKeep aliveCell dissociation methodsEmbryonic cellsSyphilisDigestion
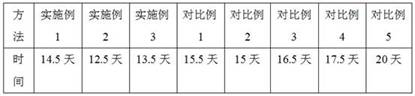
The invention discloses a method for extracting, culturing and purifying amniotic stem cells. The method comprises S1: material preparation: taking a normal placenta which is acquired in 4h after cesarean section in 38+ / -1 weeks and has no hepatitis, syphilis, HIV and other infectious diseases, mechanically separating an amniotic membrane from the placenta, simultaneously, taking off a large areaof the amniotic membrane around the umbilical region, putting the amniotic membrane into a double-antibody-containing PBS container and repeatedly washing the amniotic membrane to remove blood and fragments, and S2: amniotic stem cell extraction: (1) directly adding the amniotic membrane pieces into a container containing an uniform mixture of 0.25% of trypsin and 0.02% of an EDTA digestion solution without cutting, putting the container into an incubator at 37 DEG C and carrying out treatment for 0.5h. The method separates, extracts, cultures and purifies the amniotic membrane pieces in the placenta tissue and carries out in-vitro expansion multiplication culture so that the amniotic stem cells can be simply and conveniently acquired in large scale.
Owner:宁波东钱湖旅游度假区靖芮医疗美容诊所有限公司
Preparation method and application of human amniotic stem cell culture
PendingCN112891293AStay healthy and naturalCompensation for high allergenicityCosmetic preparationsToilet preparationsSkin colourMedicine
The invention relates to the technical field of biomedicine, and discloses a preparation method of a human amniotic stem cell culture. The preparation method comprises the following steps of: firstly, culturing separated and extracted human amniotic stem cells by using a human amniotic stem cell culture medium; and when the human amniotic stem cells are amplified to a cell confluence degree of 90%, replacing a serum-free culture medium to continuously culture the human amniotic stem cells for 48 hours; then collecting supernatant (namely the culture), and concentrating the supernatant by 10 times to obtain the human amniotic stem cell culture. The invention also discloses application. The invention discovers and proves that the human amniotic stem cell culture has the effects of whitening skin, brightening skin color and keeping the skin healthy and natural for the first time, has good prevention and treatment effects on melanin deposition caused by endocrine dyscrasia or chemical substance stimulation or physical method stimulation, is safe in components, does not have immunological rejection or tumorigenic effect, and does not have obvious toxic or side effect on a human body; the preparation method is simple, the operation process is controllable, and the preparation method has important industrialization value.
Owner:NANCHANG UNIV
Frozen therapeutic dose and package
ActiveUS10363278B2Preserver viabilityImprove efficiencyCosmetic preparationsPowder deliveryBiomedical engineeringMulti-pack
A frozen therapeutic dose includes an amniotic material and is configured into a pack for easy administering of the dose to a treatment location. A frozen therapeutic dose may contain a concentration of live amniotic stem cells. A frozen therapeutic dose may be provided in a form, such as a multi-pack form, to enable a person to administer a dose to a treatment location without the need of traveling to a doctor's office or clinic. A frozen therapeutic dose package may be kept in a conventional freezer at −20° C. for example, for extended periods of time and a person may remove the package as needed for treatment. A frozen dose package or pack may contain a secondary material configured to mix with the frozen therapeutic dose. A secondary material may be configured within a single dose compartment with the frozen dose or within a separate compartment.
Owner:AMNIO TECH
Stem cell treatment matter microneedle for skin antioxidation
InactiveCN107661569ARelieve painNo pollutionCosmetic preparationsToilet preparationsVeinIntramuscular injection
The invention discloses a stem cell treatment matter microneedle for skin antioxidation. The stem cell treatment matter microneedle is composed of a microneedle made of a metal material and stem celltreatment matter, wherein the stem cell treatment matter is a stem cell medium, and stem cells are hematopoietic stem cells, bone marrow stem cells, neural stem cells, amnion stem cells, dental pulp stem cells or uterine membrane stem cells. Compared with intravenous or intramuscular injection, the stem cell treatment matter microneedle for the skin antioxidation reduces the pain of users, is moresimple and convenient in operation, and will not cause environmental pollution; meanwhile, compared with stem cells or stem cell extractive used in the prior art, the stem cell treatment matter microneedle is low in cost and simple in operation, the medium in which the stem cells are cultured is utilized directly, and thus resources are prevented from being wasted.
Owner:上海坤爱生物科技股份有限公司 +1
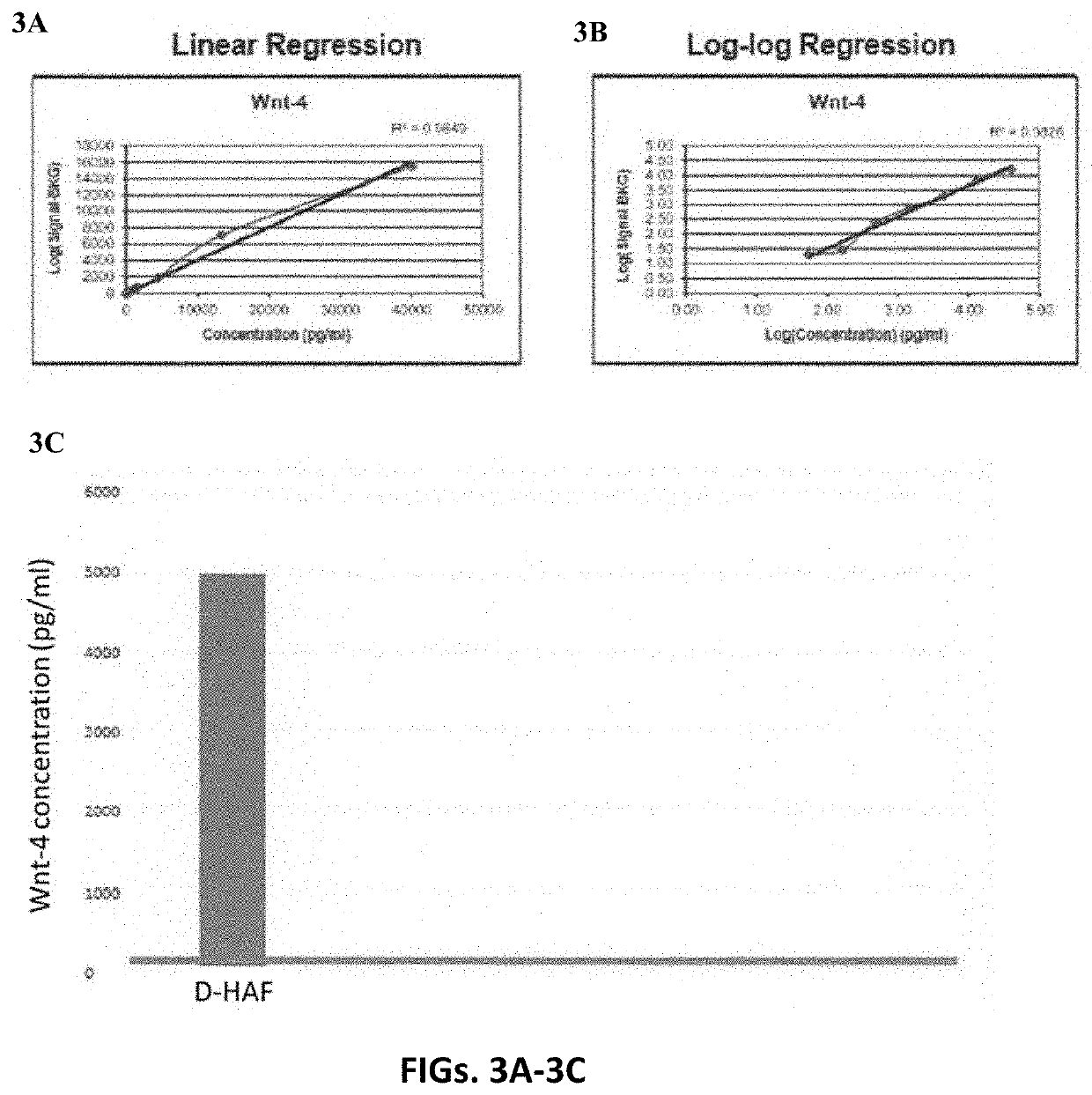
Amniotic fluid topical formulation
ActiveUS20180311284A1Preventing and alleviatingImprove rednessPowder deliverySenses disorderDiseaseDiagnostic agent
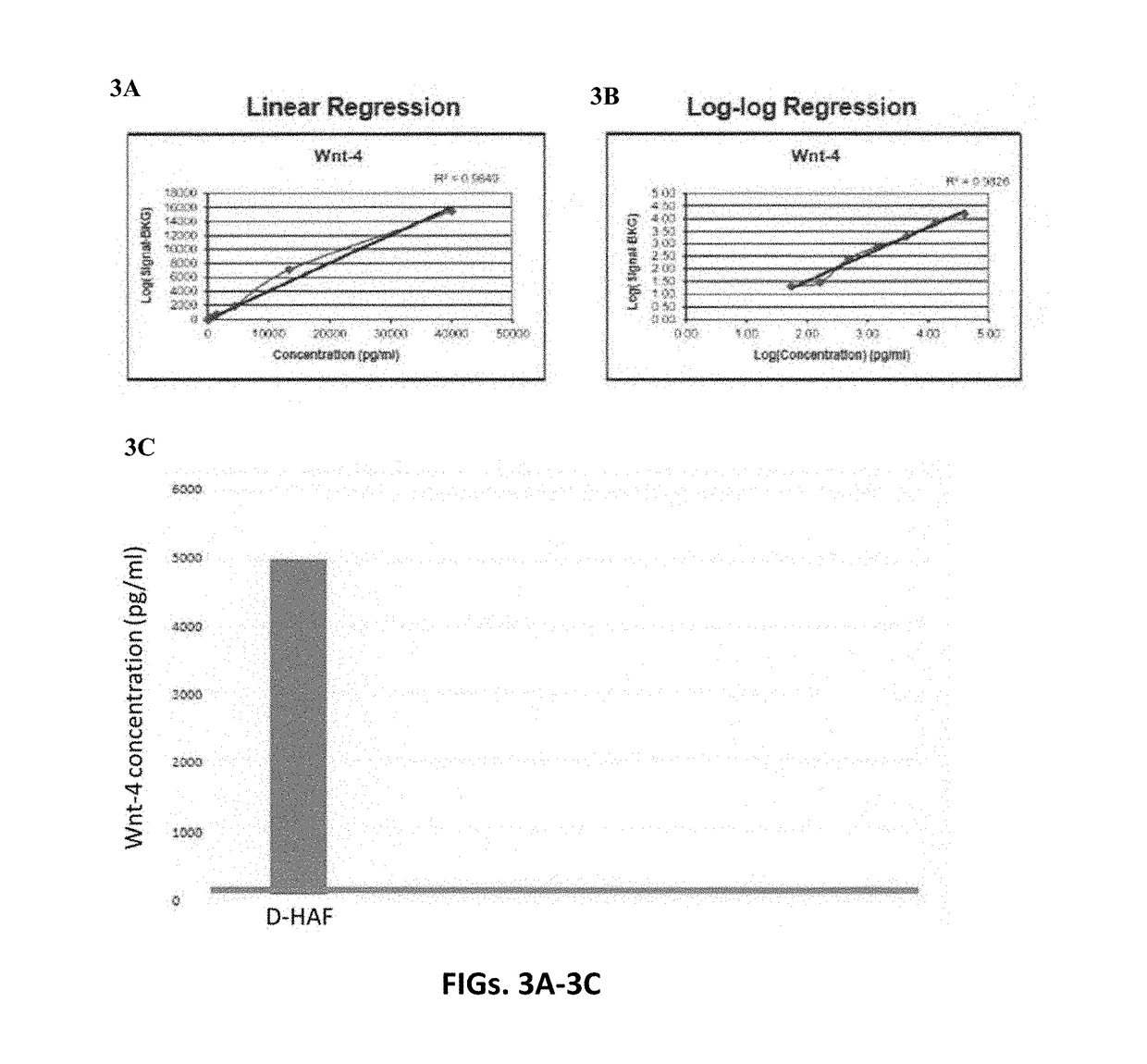
A human amniotic fluid formulation has been developed for topical application to the eye, which is useful for the treatment of ocular diseases and injuries including dry eyes, Sjogren's Syndrome, cataracts, burns and injuries to the eye tissues. The formulation is a sterile de-cellularized human amniotic fluid (D-HAF), devoid of amniotic stem cells and elements of micronized membrane or chorion particles. Methods for treating, or preventing various ocular diseases, injuries and disorders using the formulation, optionally in combination with one or more therapeutic, prophylactic or diagnostic agents are described.
Owner:MAM HLDG OF WEST FLORIDA L L C
Frozen therapeutic dose and package
ActiveUS20170095515A1Preserver viabilityImprove effectivenessCosmetic preparationsPowder deliveryBiomedical engineeringMulti-pack
A frozen therapeutic dose includes an amniotic material and is configured into a pack for easy administering of the dose to a treatment location. A frozen therapeutic dose may contain a concentration of live amniotic stem cells. A frozen therapeutic dose may be provided in a form, such as a multi-pack form, to enable a person to administer a dose to a treatment location without the need of traveling to a doctor's office or clinic. A frozen therapeutic dose package may be kept in a conventional freezer at −20° C. for example, for extended periods of time and a person may remove the package as needed for treatment. A frozen dose package or pack may contain a secondary material configured to mix with the frozen therapeutic dose. A secondary material may be configured within a single dose compartment with the frozen dose or within a separate compartment.
Owner:AMNIO TECH
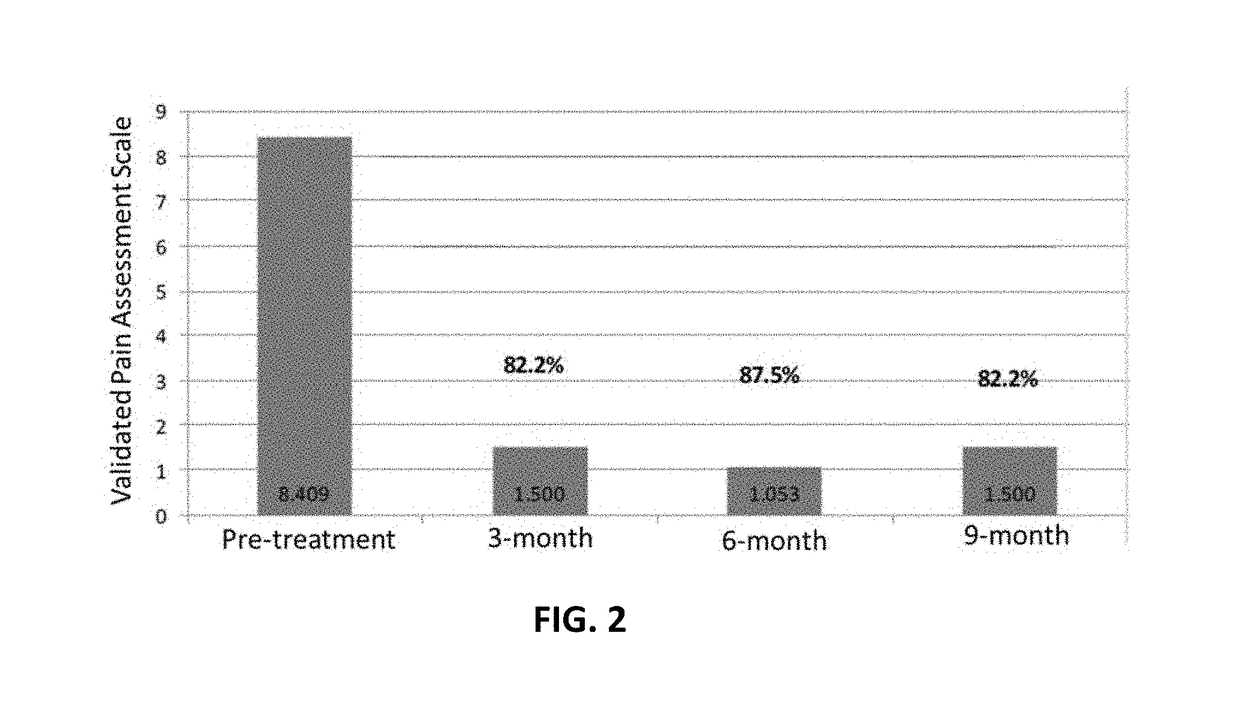
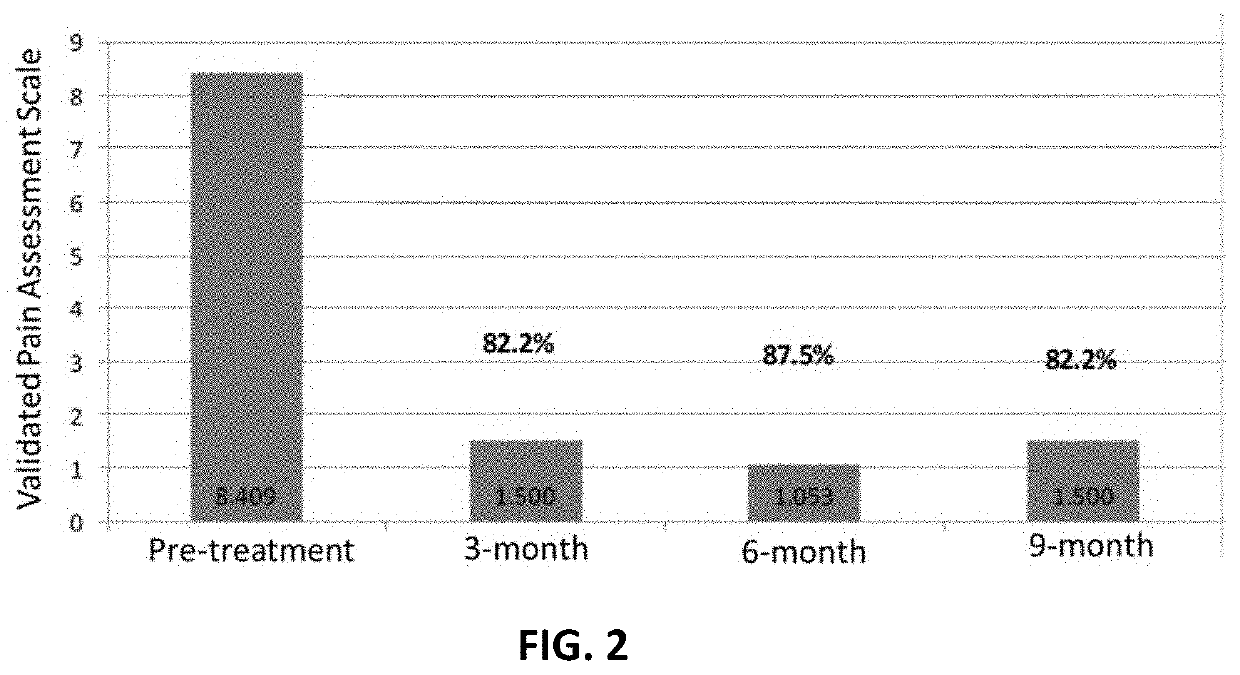
Amniotic fluid formulation for treatment of joint pain or disorders
A human amniotic fluid formulation has been developed for administration into a joint or associated soft tissue such as a tendon or ligament for treatment of pain, degeneration or injury. The formulation is a sterile de-cellularized human amniotic fluid (D-HAF), devoid of amniotic stemcells and elements of micronized membrane or chorion particles, which has not been heat treated or treated with ethidium bromide. The formulation is optionally diluted, or concentrated, depending on the severity of the disorder or injury. Examples demonstrate efficacy in treatment of pain, disease, disorder, degeneration or injury of a joint or associated soft.
Owner:MAM HLDG OF WEST FLORIDA L L C
Treatment of stroke by amniotic fluid derived stem cell conditioned media and products derived thereof
InactiveUS20180085405A1Easy maintenanceEasy to produceMammal material medical ingredientsNon-embryonic pluripotent stem cellsStress conditionsAmniotic fluid
Disclosed are compositions of matter useful for treatment of stroke derived from amniotic fluid stem cell produced factors. In one embodiment the invention teaches the use of products derived from amniotic fluid stem cells cultured under basal conditions. In another embodiment the invention teaches the utilization of amniotic stem cell derived products from said amniotic stem cells cultured under conditions of stress. Said amniotic stem cell derived products include small molecules, proteins, peptides, conditioned media, microvesicles, including exosomes and apoptotic bodies. In one embodiment, the invention teaches administration of amniotic fluid stem cells that have been exposed to a stress condition.
Owner:CREATIVE MEDICAL TECH INC
Method for extracting and culturing amniotic stem cells
ActiveCN110218697AImprove extraction efficiencyHigh purityCell dissociation methodsSkeletal/connective tissue cellsMicrobiologyDigestion
The invention discloses a method for extracting and culturing amniotic stem cells. The method comprises the steps of soaking a cleaned healthy amnion mechanically separated from a placenta into a first mixed solution for 0.5-1 hour, cleaning the healthy amnion with a trimethylolaminomethane buffer solution, scraping the surface of the amnion slightly with a cell scraper to remove epithelial cells,flushing the amnion with PBS 3-5 times, and mechanically shearing the amnion; adding amnion fragments into a second mixed solution, and digesting the amnion fragments until the amnion fragments basically disappear; superposing 1 / 2X ml of an amnion digestion suspension on a separated solution, performing centrifuging for 30 minutes at the speed of 1,500-2,000 rpm, and sucking a second suspension layer; finally, putting the second suspension layer into a culture medium for resuspension, and inoculating an obtained suspension into a cell culture dish for culturing at the constant temperature. According to the method, the efficiency of extracting the amniotic stem cells is higher, and the purity is higher.
Owner:AFFILIATED STOMATOLOGICAL HOSPITAL OF NANJING MEDICAL UNIV
Method for extracting amniotic stem cells
PendingCN112280733AClimb out in favor ofUniform dryingCell dissociation methodsSkeletal/connective tissue cellsEnzymatic digestionAnimal science
The invention discloses a method for extracting amniotic stem cells. The method comprises the following steps: A1, mechanically separating amniotic epidermis from a healthy placenta, and performing washing; a2, placing a first culture dish in a second culture dish, wherein a plurality of holes are formed in the bottom surface of the first culture dish; a3, cutting the cleaned amnion into pieces, putting the amnion pieces into a first culture dish, adding a buffer solution, then taking out the first culture dish, and paving cut amnion tissue blocks at the bottom of the first culture dish; and A4, putting the first culture dish with the amnion tissue block into another second culture dish again, adding the culture solution for culture, allowing standing for 15-18 days, adding pancreatin fordigestion, then adding the culture solution to stop digestion, taking out the first culture dish, and then extracting stem cells from the culture solution mixed with the cells. The method can effectively solve the problems of high cost and low efficiency in the prior art and the operation complexity caused by a manual inoculation mode, can quickly achieve extraction operation, reduces the cost andimproves the working efficiency.
Owner:上海坤爱生物科技股份有限公司
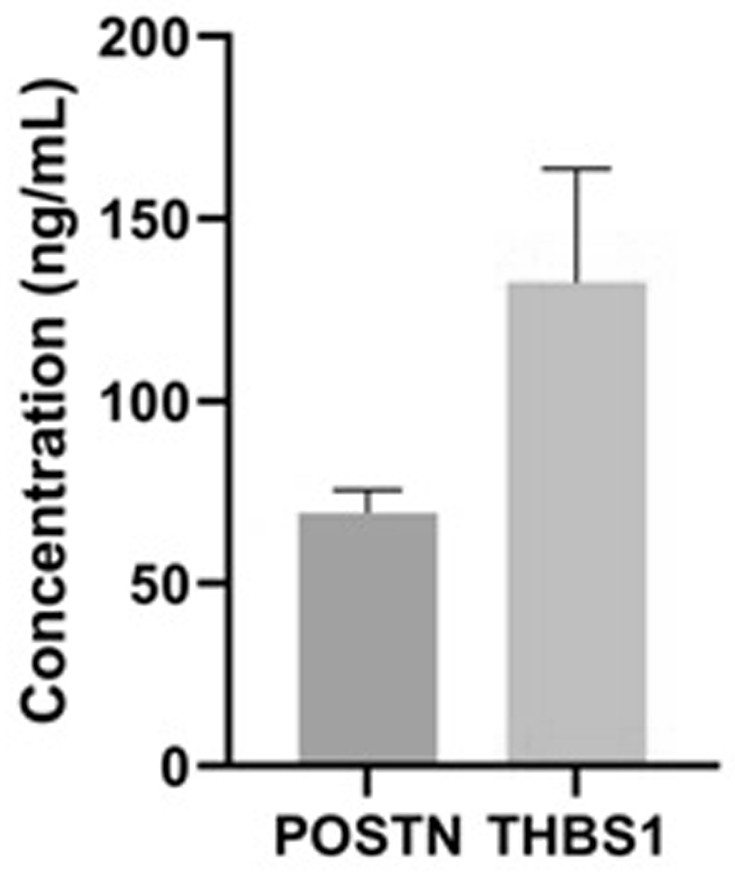
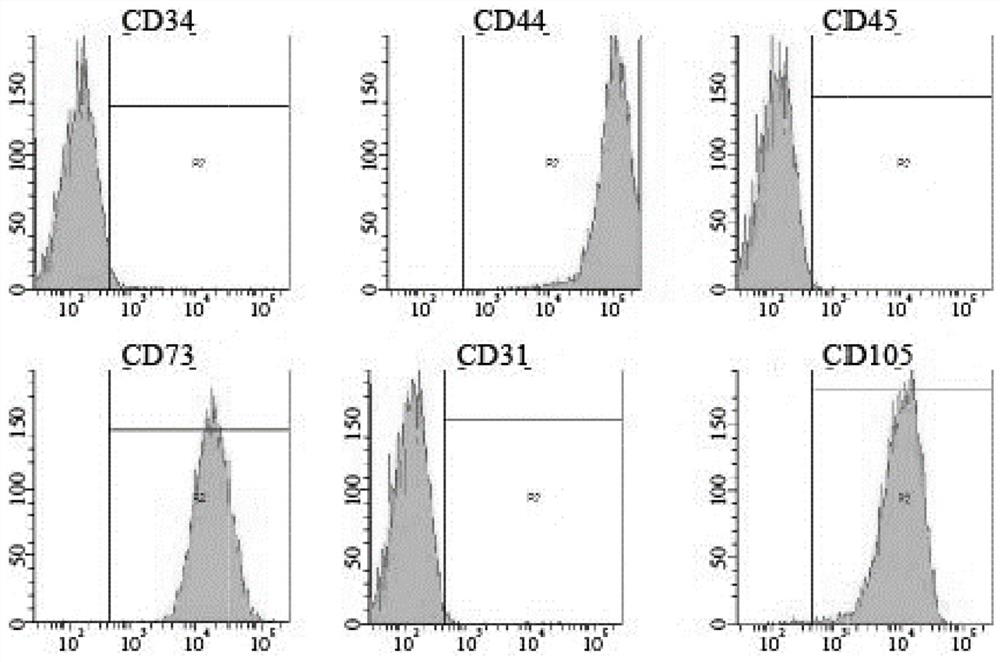
Identification and application of human amniotic mesenchymal stem cell exocrine protein POSTN
ActiveCN113262295AEasy to digPeptide/protein ingredientsMaterial analysis by electric/magnetic meansMesenchymal stem cellMass Spectrometry-Mass Spectrometry
The invention relates to application of human amniotic mesenchymal stem cell exocrine protein POSTN in preparation of a product for promoting spermatogonial stem cell proliferation and an identification method of the exocrine protein POSTN. The identification method comprises the following steps: collecting a human amniotic mesenchymal stem cell conditioned medium; and carrying out mass spectrometry on the exocrine protein of the amniotic mesenchymal stem cells in the conditioned culture medium, and screening out the high-expression protein POSTN. The exocrine proteome of the amniotic mesenchymal stem cells is identified, the results are analyzed and integrated, the spermatogonial stem cell proliferation positive factor POSTN is screened out, and a detailed foundation and a new thought are provided for subsequent research on amniotic stem cell paracrine effects. The POSTN provides a new cell factor for in-vitro proliferation of spermatogonial stem cells, and provides a direction for better excavating the characteristics of exocrine proteins of amniotic stem cells.
Owner:中国医科大学
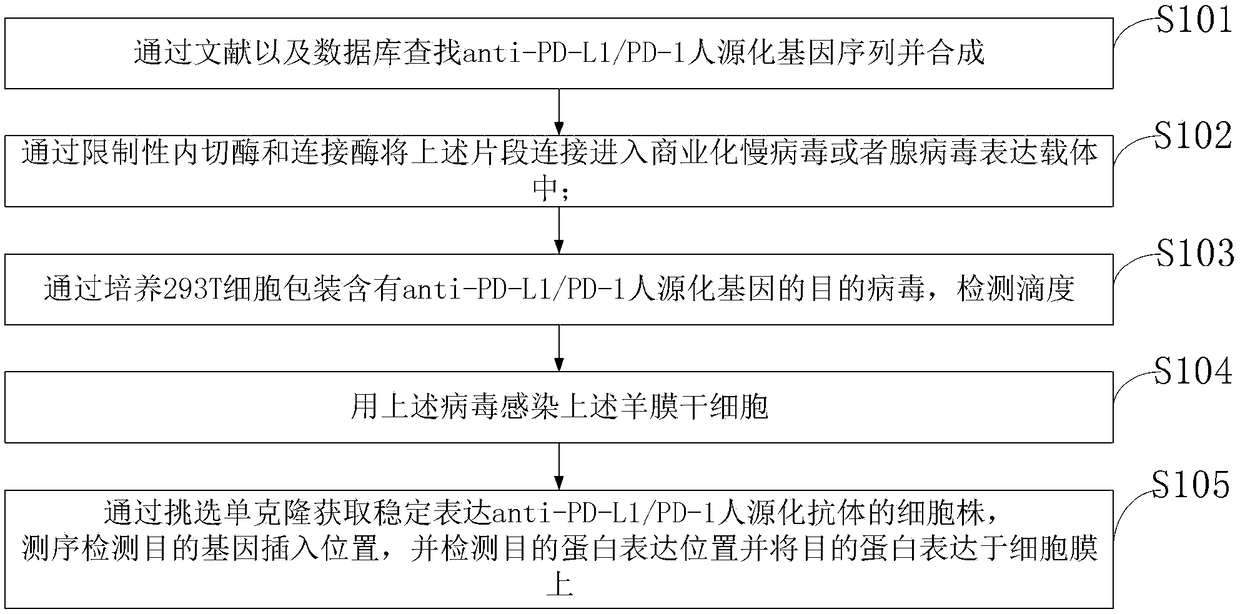
Anti-tumor preparation and preparation method thereof
InactiveCN108904799ATargetedImprove the quality of lifeMammal material medical ingredientsNucleic acid vectorProtein targetSide effect
The invention belongs to the technical field of medicine, and discloses an anti-tumor preparation and a preparation method thereof, wherein the anti-tumor preparation is stem cells loading an anti-PD-L1 / PD-1 humanized antibody. The preparation method comprises: linking the fragment into a commercial lentivirus or adenovirus expression vector through restriction endonuclease and ligase; packaging target viruses containing an anti-PD-L1 / PD-1 humanized gene by culturing 293T cells, and detecting the titer; infecting amnion stem cells with the viruses; and obtaining a cell line stably expressing the anti-PD-L1 / PD-1 humanized antibody by selecting a monoclone, detecting the insertion position of the target gene through sequencing, detecting the expression position of the target protein, and expressing the target protein on the cell membrane. According to the present invention, the anti-PD-L1 / PD-1 humanized antibody can be targeted to tumor tissues by stem cells so as to reduce the side effects caused by systemic administration.
Owner:夏荣木
Efficient preparation device and preparation method for exosome
PendingCN114797173AIncrease productivityRealize assembly line productionBioreactor/fermenter combinationsBiological substance pretreatmentsReal-time dataBiochemical engineering
The invention discloses an efficient preparation device and a preparation method for exosomes, and belongs to the technical field of exosome preparation.The efficient preparation device comprises a chromatography mechanism for performing chromatography on substances needing to be removed in extracellular fluid through a carbon nano tube composite filter layer to obtain an external fluid semi-finished product; the purification unit is connected with the chromatography mechanism and is used for purifying the external liquid semi-finished product to obtain the exosome; a detection unit; according to the efficient preparation device and method for the exosome, flow line production is achieved, the production efficiency of the exosome is greatly improved, consumption of manpower and material resources is reduced, and the occupied area is small; the preparation time is 1 / 4 of the traditional preparation time, the efficiency is improved by 8 times, the obtaining rate is improved by two times, and 8ml of CB solution can be prepared when the exosome is collected by amniotic stem cell engineering and reaches 100ml. The chromatography effect is improved, and the influence of incomplete chromatography on the purification process is reduced; and real-time data in the chromatography process is acquired through the detection unit and is processed by the controller, so that the problem of long production period in the preparation process is solved.
Owner:南通星中细胞工程有限公司
Amnion derived therapeutic compositions and methods of use
ActiveUS10894066B2Improve efficiencyReduce Shrinkage ProblemsCosmetic preparationsToilet preparationsBlood plasmaRat heart
Therapeutic compositions are described for the treatment of a variety of conditions including heart, eye, lungs, organs, joints, dermal, nerve, and the like. A therapeutic composition may be a fluid comprising amniotic fluid or micronized amniotic particles. A therapeutic composite may be a dispersion of micronized amniotic membrane combined with a fluid, such as plasma, saline, amniotic fluid, combinations thereof and the like. In another embodiment, the therapeutic composite is a mixture of micronized amniotic membrane particles combined with an amniotic rich stem cell fluid. An amniotic rich or concentrated stem cell fluid comprises at least 0.5×106 amniotic stem cells per milliliter of fluid or composition. A therapeutic composite may be used to treat any number of conditions through topical application, surgical introduction, and / or injection.
Owner:AMNIO TECH
A kind of amniotic membrane stem cell gel and its preparation method and application
ActiveCN107349468BGood repair functionImprove moisturizing functionAerosol deliveryOintment deliveryTissue repairSODIUM ACRYLOYLDIMETHYLTAURATE
Owner:JIANGXI RUIJI BIOTECH CO LTD
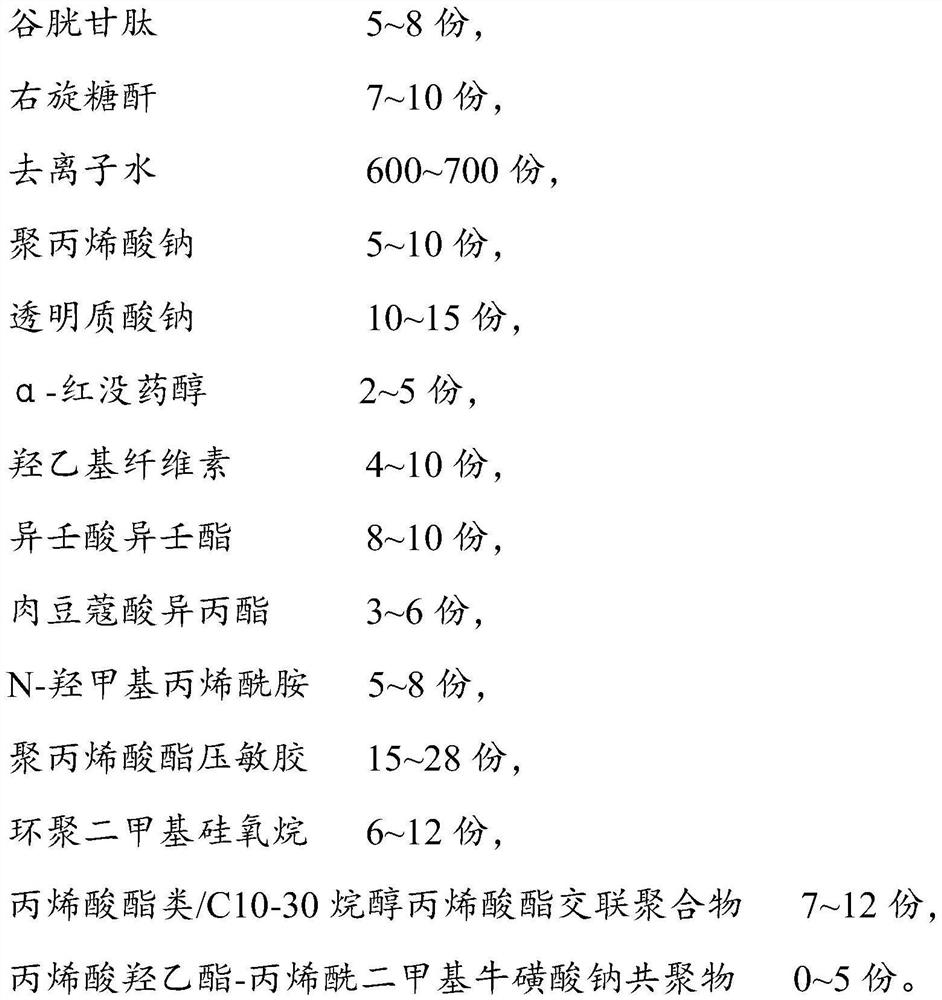
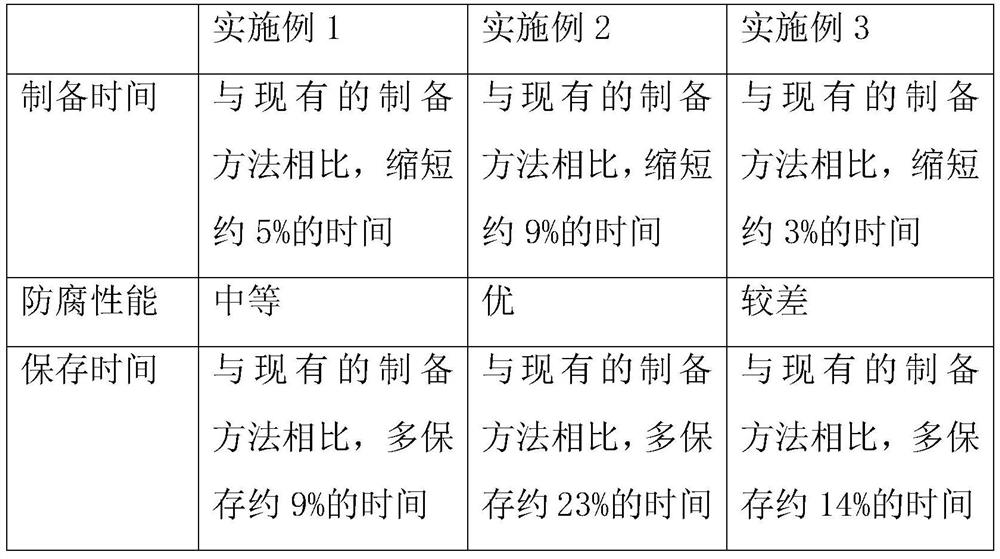
Preparation method of freeze-dried powder containing placenta amniotic stem cell cytokine extracting solution
InactiveCN114796281AImprove anti-corrosion performanceExtended storage timePowder deliveryMammal material medical ingredientsWhey proteinPreservative
The invention discloses a preparation method of freeze-dried powder containing a placenta amniotic stem cell cytokine extracting solution, and relates to the technical field of freeze-dried powder, the technical scheme is that the preparation method comprises the following specific steps: S1, putting a first material into a stirrer for stirring and heating, and filtering to obtain a mixture A, the first material is prepared from an extracting solution A, whey protein concentrate powder and stachyose, and the second material is prepared from a second material, a third material and a fourth material; s2, putting the second material into a stirrer for stirring and heating, filtering to obtain a mixture B, and storing the mixture B for later use in an environment of 4-6 DEG C. The preparation method of the freeze-dried powder containing the placenta amniotic membrane stem cell cytokine extracting solution has the beneficial effects that the preservative has the effects of improving the corrosion resistance of the freeze-dried powder; therefore, the preservation time of the freeze-dried powder is prolonged, and the cost investment is reduced.
Owner:上海南滨江细胞生物科技有限公司
Screening and identifying method and application of amniotic stem cell exocrine protein
ActiveCN112462062AEasy to digPeptide/protein ingredientsEpidermal cells/skin cellsMesenchymal stem cellSecretory protein
The invention relates to a method for screening and identifying exocrine proteins of amniotic stem cells and application, and the method identifies exocrine proteomes of amniotic epithelial stem cellsand amniotic mesenchymal stem cells, analyzes and integrates results, and further analyzes differential expression proteins of the amniotic epithelial stem cells and the amniotic mesenchymal stem cells. The positive factor LOXL2 for epithelial development and epidermal regeneration is screened out, and a detailed basis and a new thought are provided for subsequent research on the paracrine effectof the amniotic stem cells. LOXL2 provides a new idea for epithelialization of clinical large-area difficult-to-heal wounds, and provides a direction for better mining the characteristics of different amnion stem cell exocrine proteins. And a basis is provided for development and clinical application of specific cell-free dressings.
Owner:沈阳艾米奥生物工程技术研发中心有限公司
Culture method and application of primary amniotic stem cells
PendingCN114686426AAdvantages of cultivation methodExcellent adhesionCell dissociation methodsEpidermal cells/skin cellsVitamin CStem cell culture
The invention belongs to the technical field of stem cell culture, and particularly relates to a culture method and application of primary amniotic stem cells. The culture method of the primary amniotic stem cells mainly comprises the following steps: stripping a placenta under a sterile condition, washing with normal saline, washing with a mixed solution containing a bactericide, treating with digestive enzyme, culturing with a culture medium and the like. The mixed solution containing the bactericide is prepared from the following components in percentage by weight: 1 to 2 percent of herba taraxaci extract, 0.5 to 0.8 percent of rosemary hydrolat, 0.3 to 0.8 percent of vitamin C and 96.4 to 98.2 percent of purified water; the culture medium is composed of an LB liquid culture medium, tween-20, type IV collagen and BI recombinant vitreous adhesion protein. By adopting the culture method provided by the invention, cell adherence can be accelerated, tissues in the cells can be cleaned up, and the cell purity is improved on the premise of shortening the culture time of the amniotic stem cells.
Owner:SHENZHEN WINGOR BIO TECH
Amniotic fluid formulation for treatment of joint pain or disorders
A human amniotic fluid formulation has been developed for administration into a joint or associated soft tissue such as a tendon or ligament for treatment of pain, degeneration or injury. The formulation is a sterile de-cellularized human amniotic fluid (D-HAF), devoid of amniotic stem cells and elements of micronized membrane or chorion particles, which has not been heat treated or treated with ethidium bromide. The formulation is optionally diluted, or concentrated, depending on he severity of the disorder or injury. Examples demonstrate efficacy in treatment of pain, disease, disorder, degeneration or injury of a joint or associated soft.
Owner:MAM HLDG OF WEST FLORIDA L L C
Amniotic fluid formulation for treatment of joint pain or disorders
A human amniotic fluid formulation has been developed for administration into a joint or associated soft tissue such as a tendon or ligament for treatment of pain, degeneration or injury. The formulation is a sterile de-cellularized human amniotic fluid (D-HAF), devoid of amniotic stem cells and elements of micronized membrane or chorion particles, which has not been heat treated or treated with ethidium bromide. The formulation is optionally diluted, or concentrated, depending on he severity of the disorder or injury. Examples demonstrate efficacy in treatment of pain, disease, disorder, degeneration or injury of a joint or associated soft.
Owner:MAM HLDG OF WEST FLORIDA L L C
Amniotic fluid formulation for treatment of joint pain or disorders
Owner:MAM HLDG OF WEST FLORIDA L L C
Method for separating and extracting amniotic membrane stem cells from placentas
PendingCN111518747AEasy to operateReduce manufacturing costCell dissociation methodsSkeletal/connective tissue cellsBiophysicsAmniotic stem cells
The invention discloses a method for separating and extracting amniotic membrane stem cells from placentas. The method is used for extracting the amniotic membrane stem cells by simply cleaning and sterilizing the waste placentas in hospitals, so that resources are fully utilized. The method comprises the following steps of firstly, cleaning, dicing, culturing and digesting amniotic membranes to obtain a small amount of the amniotic membrane stem cells from the amniotic membranes, and then continuously culturing, digesting and centrifuging the obtained amniotic membrane stem cells to obtain alarge amount of the amniotic membrane stem cells. The preparation method is simple, convenient and easy to operate; and when the amniotic membrane stem cells are extracted from the amniotic membranes,the amniotic membranes are sheared into meat paste of 2-3 mm<3>, and more amniotic membrane stem cells are promoted to be exposed, so that the contact area between the amniotic membrane stem cells and a culture medium is increased, in-vitro amplification of the amniotic membrane stem cells is promoted, and the amount of the amniotic membrane stem cells extracted from the amniotic membranes is increased.
Owner:成都容医汇生物技术研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com