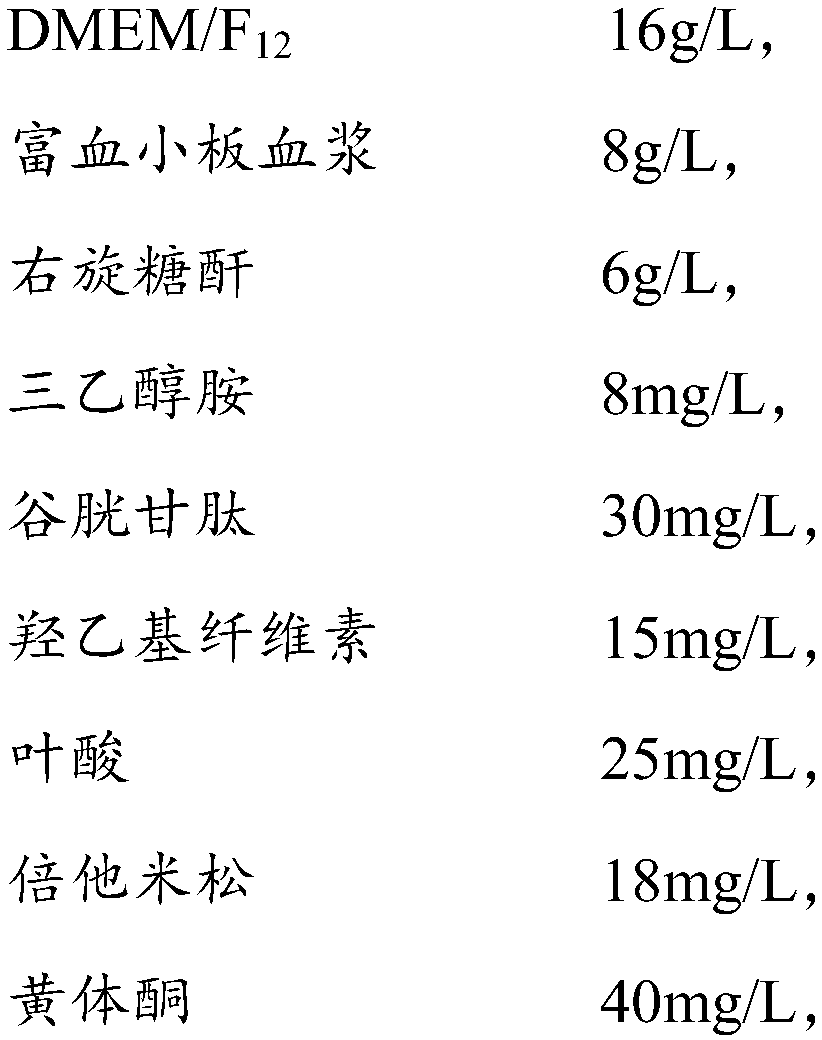
Amniotic stem cell culture medium and culture method thereof
A technology of stem cells and culture medium, applied in the field of amniotic membrane stem cell culture medium and its cultivation, can solve the problems of restricting the wide application in clinical practice, and achieve the effect of no heterogeneous immunogenicity, no ethical restrictions, and a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A method for culturing amniotic membrane stem cells, comprising the following steps:
[0024] (1) Isolation of human amniotic membrane stem cells and preparation of single cell suspension
[0025] Human amniotic membrane was first digested with trypsin at a concentration of 3g / L for 30-60 minutes at room temperature, 2-4 times in total, and then digested with V DMEM : VF 12 = 1:1 DMEM / F 12 Stop trypsin in the culture medium, use DMEM / F containing 1.0-2.0g / L collagenase IV and 0.1-0.2g / L deoxyribonuclease I 12 The culture medium was digested at 37°C for 30-60 minutes to obtain a cell suspension, which was filtered to make a single-cell suspension;
[0026] (2) Culture, purification and expansion of human amniotic stem cells
[0027] The cells obtained in step (1) were inoculated in the culture medium, and placed at 37°C, saturated humidity, and CO with a volume fraction of 5%. 2 Cultured in an incubator, and by changing the medium and passage, the human amniotic ste...
Embodiment 2
[0032] A method for culturing amniotic membrane stem cells, comprising the following steps:
[0033] (1) Isolation of human amniotic membrane stem cells and preparation of single cell suspension
[0034] Human amniotic membrane was first digested with trypsin at a concentration of 3g / L for 30-60 minutes at room temperature, 2-4 times in total, and then digested with V DMEM : VF 12 = 1:1 DMEM / F 12 Stop trypsin in the culture medium, use DMEM / F12 culture medium containing 1.0-2.0g / L collagenase IV and 0.1-0.2g / L deoxyribonuclease I, digest at 37°C for 30-60 minutes, and obtain cell suspension solution, filtered to make a single cell suspension;
[0035] (2) Culture, purification and expansion of human amniotic stem cells
[0036] The cells obtained in step (1) were inoculated in the culture medium, and placed at 37°C, saturated humidity, and CO with a volume fraction of 5%. 2 Cultured in an incubator, and by changing the medium and passage, the human amniotic stem cells wer...
Embodiment 3
[0041] A method for culturing amniotic membrane stem cells, comprising the following steps:
[0042] (1) Isolation of human amniotic membrane stem cells and preparation of single cell suspension
[0043] Human amniotic membrane was first digested with trypsin at a concentration of 3g / L for 30-60 minutes at room temperature, 2-4 times in total, and then digested with V DMEM : VF 12 = 1:1 DMEM / F 12 Stop trypsin in the culture medium, use DMEM / F containing 1.0-2.0g / L collagenase IV and 0.1-0.2g / L deoxyribonuclease I 12The culture medium was digested at 37°C for 30-60 minutes to obtain a cell suspension, which was filtered to make a single-cell suspension;
[0044] (2) Culture, purification and expansion of human amniotic stem cells
[0045] The cells obtained in step (1) were inoculated in the culture medium, and placed at 37°C, saturated humidity, and CO with a volume fraction of 5%. 2 Cultured in an incubator, and by changing the medium and passage, the human amniotic stem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com