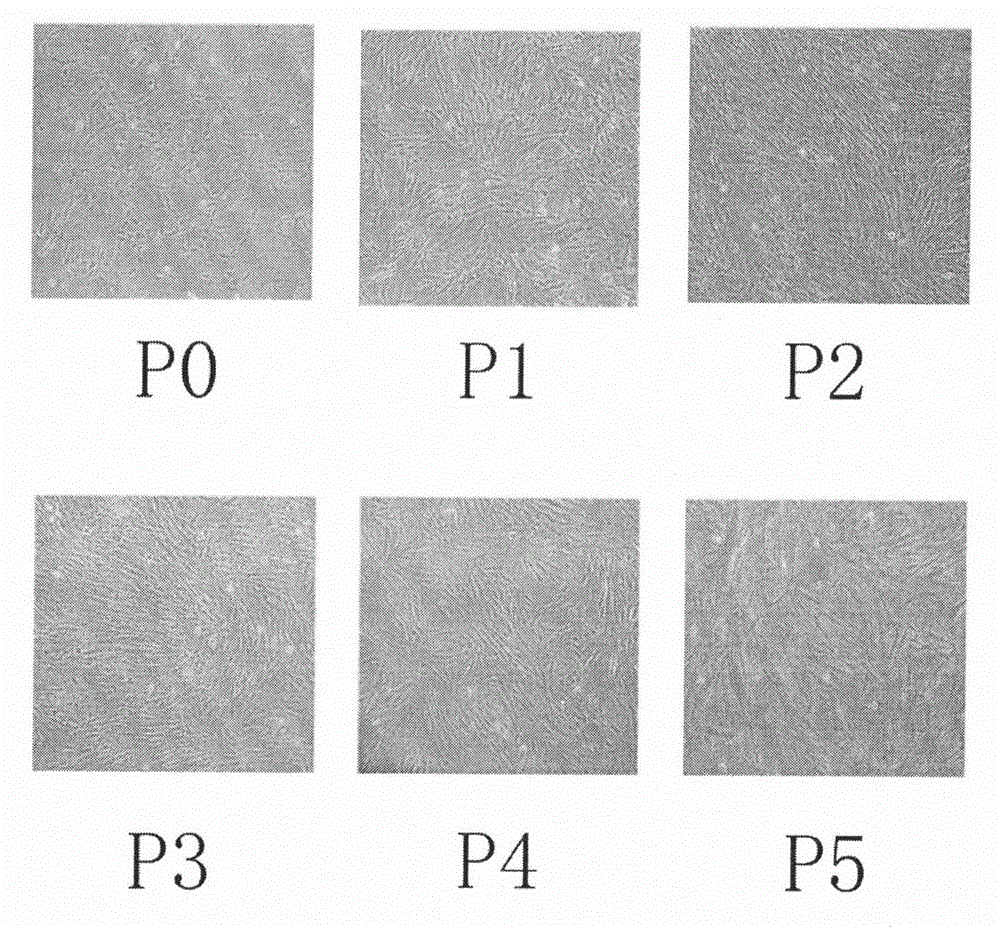
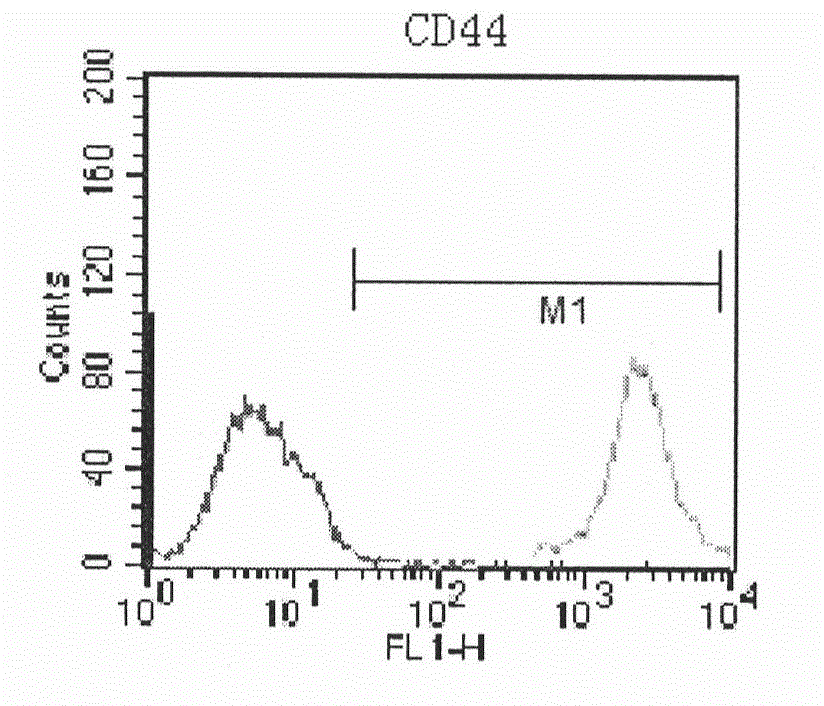
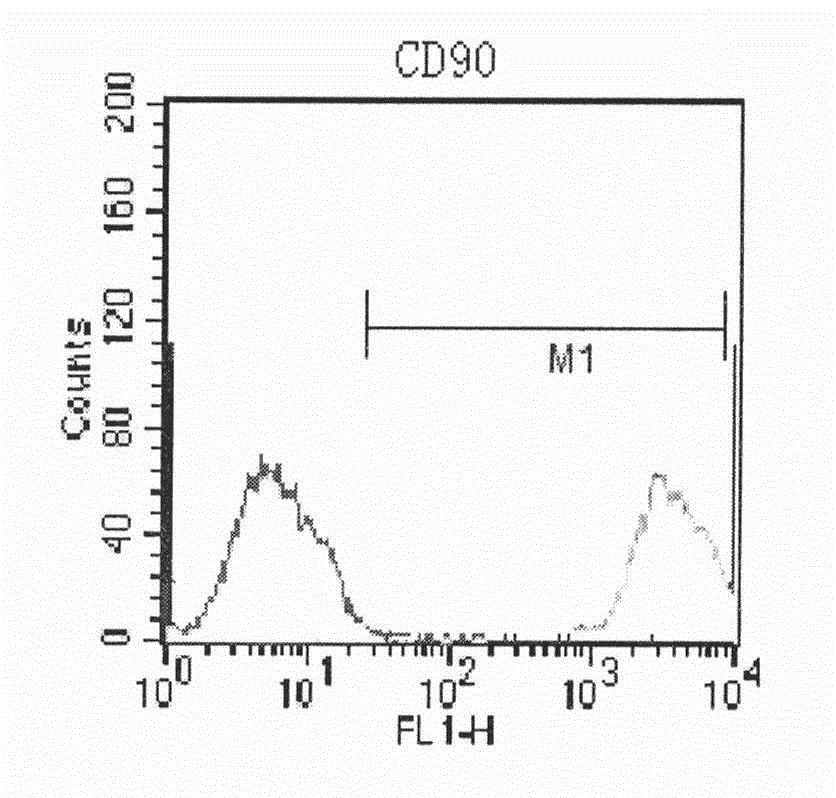
Human amnion mesenchymal stem cell serum-free culture medium and culture method thereof
A serum-free medium and serum-free culture technology, applied in the biological field, can solve the problems of limited quantity and achieve the effect of not being restricted by ethics, not being immune to heterogeneous immunogenicity, and having a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A serum-free medium for human amniotic mesenchymal stem cells of the present invention, comprising:
[0033] Basal medium DMEM / F12 15.6g / L
[0034] (According to the volume of 1:1, take 15.6g and dissolve in 1000ml water, supplier GIBCO)
[0035] Human serum albumin 8.0×10 -1 %
[0036] Human transferrin 5.0×10 -1 g / L
[0037] Human insulin 8.0×10 -1 g / L
[0038] Sodium selenite 6.0×10 -6 g / L
[0039] made into an aqueous solution.
Embodiment 2
[0041] A serum-free culture method for human amniotic mesenchymal stem cells of the present invention comprises the following steps:
[0042] 1. Isolation of human amniotic mesenchymal stem cells and preparation of single cell suspension
[0043] Take the human placenta of normal full-term caesarean section fetus (male) under sterile conditions; detect hepatitis A antibody, hepatitis B virus surface antigen, hepatitis B virus surface antibody, hepatitis B virus e antigen, hepatitis B virus e Antibody, hepatitis B core antibody IgM, hepatitis C antibody, hepatitis E antibody, HIV antibody, Treponema pallidum antibody and other related infectious indicators were all negative; Amniotic membrane 5×5cm 2 , fully rinsed with PH7.2 phosphate buffer solution (PBS), put the amniotic membrane in physiological saline containing 0.1 million U / ml gentamicin and 2.5ug / ml amphotericin B, and soak for 20 minutes; Cut into pieces, add 5ml of trypsin at a final concentration of 2.5g / L per gra...
Embodiment 3
[0054] A method for the separation and serum-free culture of human amniotic mesenchymal stem cells of the present invention, comprising the following steps:
[0055] The 1st step of the present invention can also be: trypsin final concentration is 2.5g / L, and digestion time is 30-60 minutes, and number of times is 2-4 times; Score can be 5.0×10 -1 %; When digested with collagenase IV and deoxyribonuclease I, V DMEM :V F12 =The concentration of collagenase IV in the DMEM / F12 culture fluid of 1:1 adopts 0.5-1.5g / L, deoxyribonuclease 0.05-0.15g / L, and digestion time is 1-2 hour; All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com