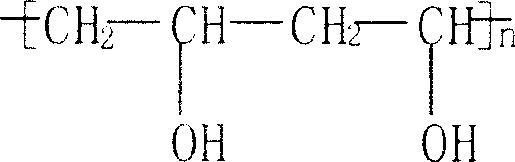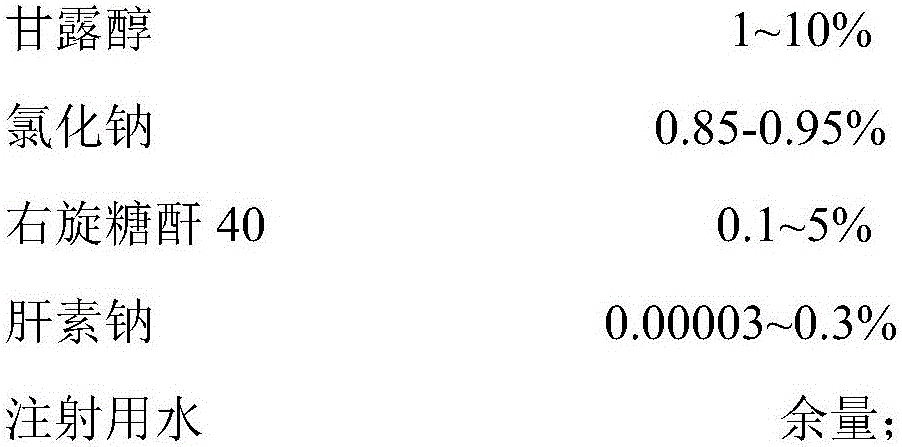Patents
Literature
105 results about "Dextran 40" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
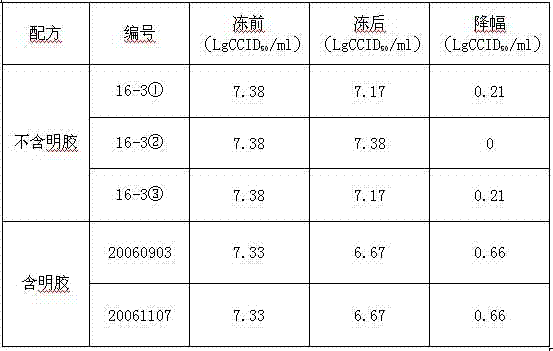
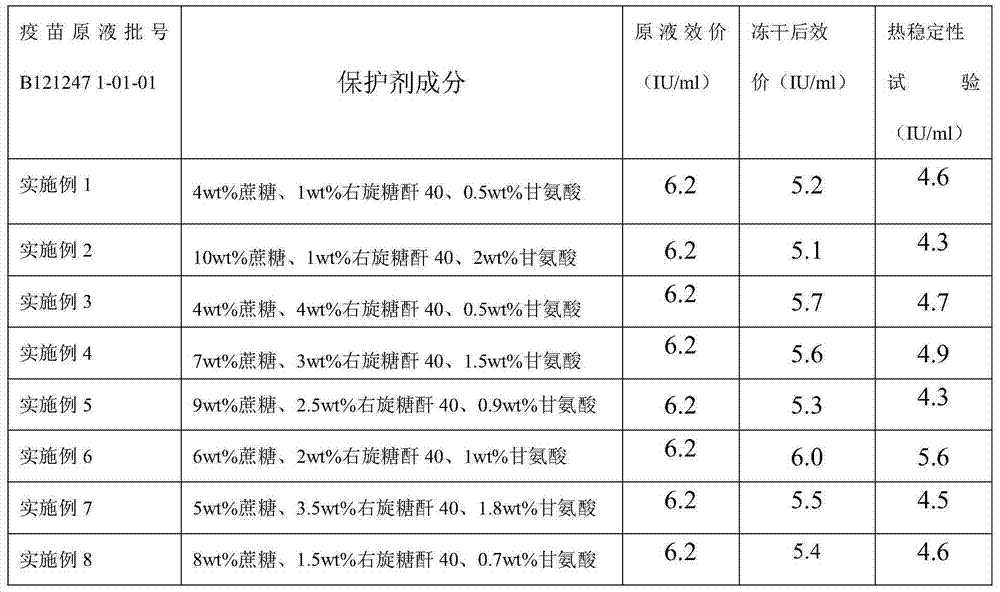
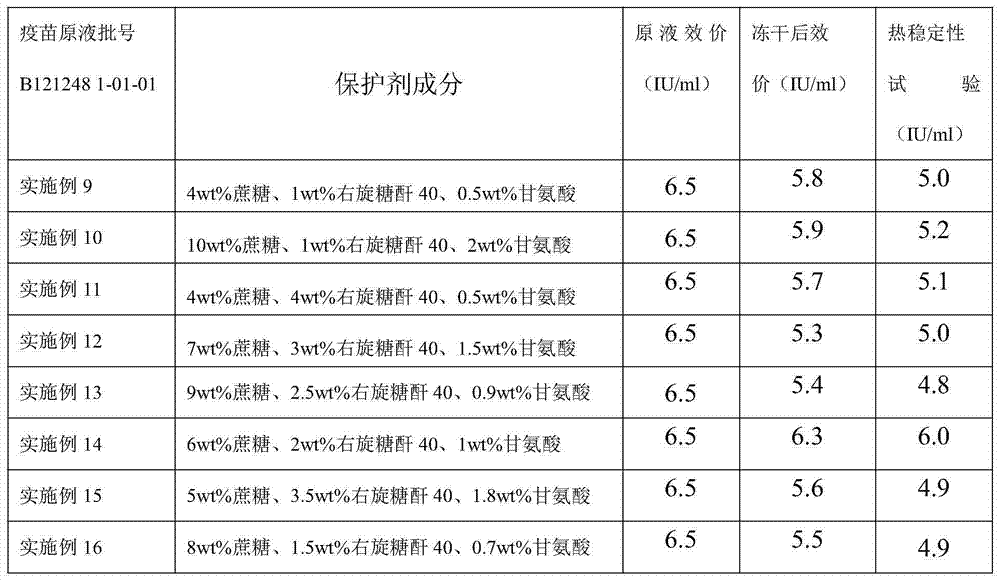
Dextran 40 is commonly used for use in the adjunctive treatment of shock or impending shock due to hemorrhage, burns, surgery or other trauma.
Plasma-like substance
InactiveUS6110504AEliminate circulationDelay EliminationBiocideSulfur/selenium/tellurium active ingredientsHydroxyethyl starchWater soluble polysaccharides
An artificial plasma-like substance having at least one water soluble polysaccharide oncotic agent selected from the group consisting of high molecular weight hydroxyethyl starch, low molecular weight hydroxyethyl starch, dextran 40 and dextran 70, and albumin which is buffered by lactate and has a pre-administration pH of between 5 and 6.5 is disclosed. Also disclosed is an artificial plasma-like solution having at least two water soluble polysaccharide oncotic agents one of which is eliminated from the circulation slowly and the other of which is eliminated from the circulation quickly. Supplimentation of the plasma-like solution with certain ions is described. A system for administration of the plasma-like solution to a subject wherein the system comprises a first and second solution each having particular buffers is described. The plasma-like solution including cryoprotective adducts is also disclosed. The use of the plasma-like solution in organ transplant, novel chemotherapy procedures, and tissue, organ and organism cryopreservation are also disclosed.
Owner:LINEAGE CELL THERAPEUTICS INC
Probiotic microcapsules as well as preparation method and application thereof
ActiveCN105310080AImprove the situation of low freeze-drying survival rateImprove stabilityFood freezingFood shapingFreeze-dryingK carrageenan
The invention relates to probiotic microcapsules as well as a preparation method and application thereof. The probiotic microcapsules comprise a core material and a wall material, wherein the core material is probiotics; the outer layer of the wall material is coated with chitosan; the wall material is prepared from an aqueous solution containing a natural polymer material and a freeze-drying protection agent; the freeze-drying protection agent comprises one or more of glucose, fructose, sucrose, lactose, trehalose, soluble starch, glycerin, mannitol, Arabic gum, dextran 40 and skim milk; the natural polymer material comprises one or more of gellan gum, xanthan gum, k-carrageenan, sodium alginate, cellulose acetate phthalate or gelatin; in the aqueous solution, the volume fraction of the freeze-drying protection agent is 4.0%-20.0% and the volume fraction of the polymer material is 0.5%-5.0%. The probiotic microcapsules can keep excellent acid resistance and storage stability before and after being freeze-dried.
Owner:SUN YAT SEN UNIV
Solutions for use as plasma expanders and substitutes
InactiveUS20030022147A1Eliminate circulationDelay EliminationPharmaceutical delivery mechanismDead animal preservationHydroxyethyl starchPlasma expander
An artificial plasma-like substance having at least one water soluble polysaccharide oncotic agent selected from the group consisting of high molecular weight hydroxyethyl starch, low molecular weight hydroxyethyl starch, dextran 40 and dextran 70, which is buffered by lactate and has a pre-administration pH of between 4 and 6.5 is disclosed. In one embodiment, the artificial plasma-like solution may have at least two water soluble polysaccharide oncotic agents one of which is eliminated from the circulation slowly and the other of which is eliminated from the circulation quickly. Supplementation of the plasma-like solution with certain ions is described. A system for administration of the plasma-like solution to a subject wherein the system comprises a first and second solution each having particular buffers is described. Methods for the administration of the plasma-like solution are also disclosed.
Owner:LINEAGE CELL THERAPEUTICS INC
Peripheral blood mononuclear cell serum-free freezing medium and freezing method
ActiveCN104719282AImprove securityTo ensure the effect of freezingDead animal preservationSerum igePeripheral blood mononuclear cell
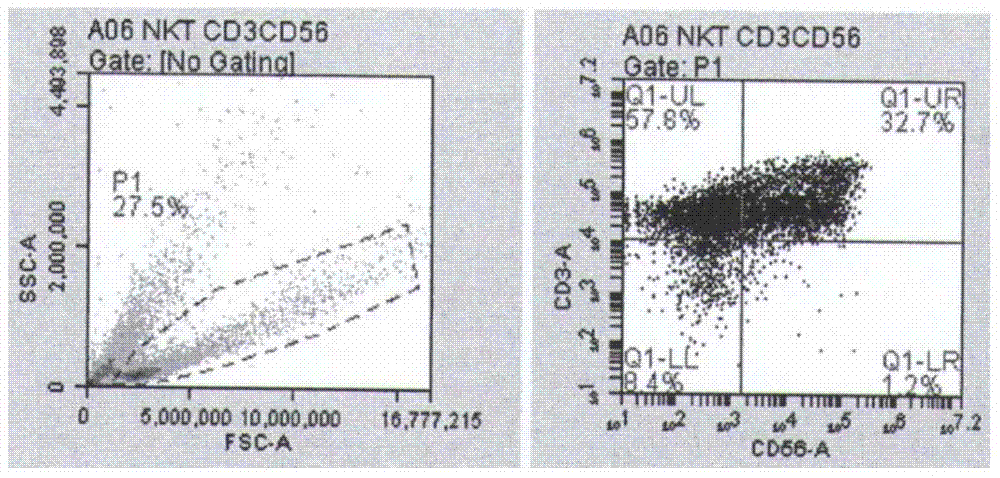
The invention discloses a peripheral blood mononuclear cell serum-free freezing medium. The peripheral blood mononuclear cell serum-free freezing medium is prepared from the following components in percentage by volume: 5 to 20% of dimethyl sulfoxide, 0.5 to 10% of dextran 40, and the balance of plasmalyte A. The invention further discloses a method for freezing peripheral blood mononuclear cells through the peripheral blood mononuclear cell serum-free freezing medium. Compared with the prior art, the freezing medium is free of animal serum, human serum and a cell culture medium, and has a good freezing effect; the cells can be directly applied to the clinic after resuscitation and can be also induced in vitro into immune cells NKT and CIK and have high application value in the clinic.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Recombined human alkaline fibroblast growth factor gelling agent and process for preparing the same
InactiveCN1733294AReduce formationEffective function recoveryPeptide/protein ingredientsPharmaceutical delivery mechanismMANNITOL/SORBITOLEffective treatment
The invention relates to a recombinant human basic fibroblast growth factor gelling agent and the process for preparation, which comprises recombinant human basic fibroblast growth factor (rh-bFGF) stock solution 0.1-100% (W / W), medicinal auxiliary material 0-99.9% (W / W), the stock solution comprises effective treatment dose of recombinant human basic fibroblast growth factor (rh-bFGF) 0.01-1% (ug / g), NaCl 0.01-20% (W / W), phosphates cushioning liquid 2m Mol - 200m Mol, and right amount of water for injection, the medicinal auxiliary materials can be selected from: carbomer 940NF, sodium hyaluronate, trehalose, mannitol, dextran 40 and sodium heparin.
Owner:朗肽生物制药股份有限公司
Stem cell freezing and storing medium and preparation method and freezing and storing method thereof
The invention discloses a stem cell freezing and storing medium and a preparation method and freezing and storing method thereof. The stem cell freezing and storing medium comprises, by weight, 3-10 parts of dimethyl sulfoxide, 2-7 parts of human serum albumin, 0.5-3 parts of mycose, 0.2-2 parts of dextran 40 and 2-6 parts of hetastarch. Cells can be frozen for a long time by the stem cell freezing and storing medium, freezing damage of the cells can be remarkably reduced, the resuscitated cells have a high degree of survival rate and adherent property, and freezing and storing effect of the cells is improved; meanwhile, the compositions of the stem cell freezing and storing medium are clear and are medical compendial injection-grade excipients without containing serum, the risk of contamination and allergen introduced by the use of heterogeneous serum is effectively prevented, the content of DMSO (dimethylsulfoxide) is low, the negative effect of the DMSO on the cells is lowered, highsecurity and good stability are achieved, requirements of CFDA (China food and drug administration) and FDA (food and drug administration) are met, and the stem cell freezing and storing medium can be directly used in human infusion and is suitable for clinical research and treatment.
Owner:广州赛隽生物科技有限公司
System and method for regeneration of a fluid
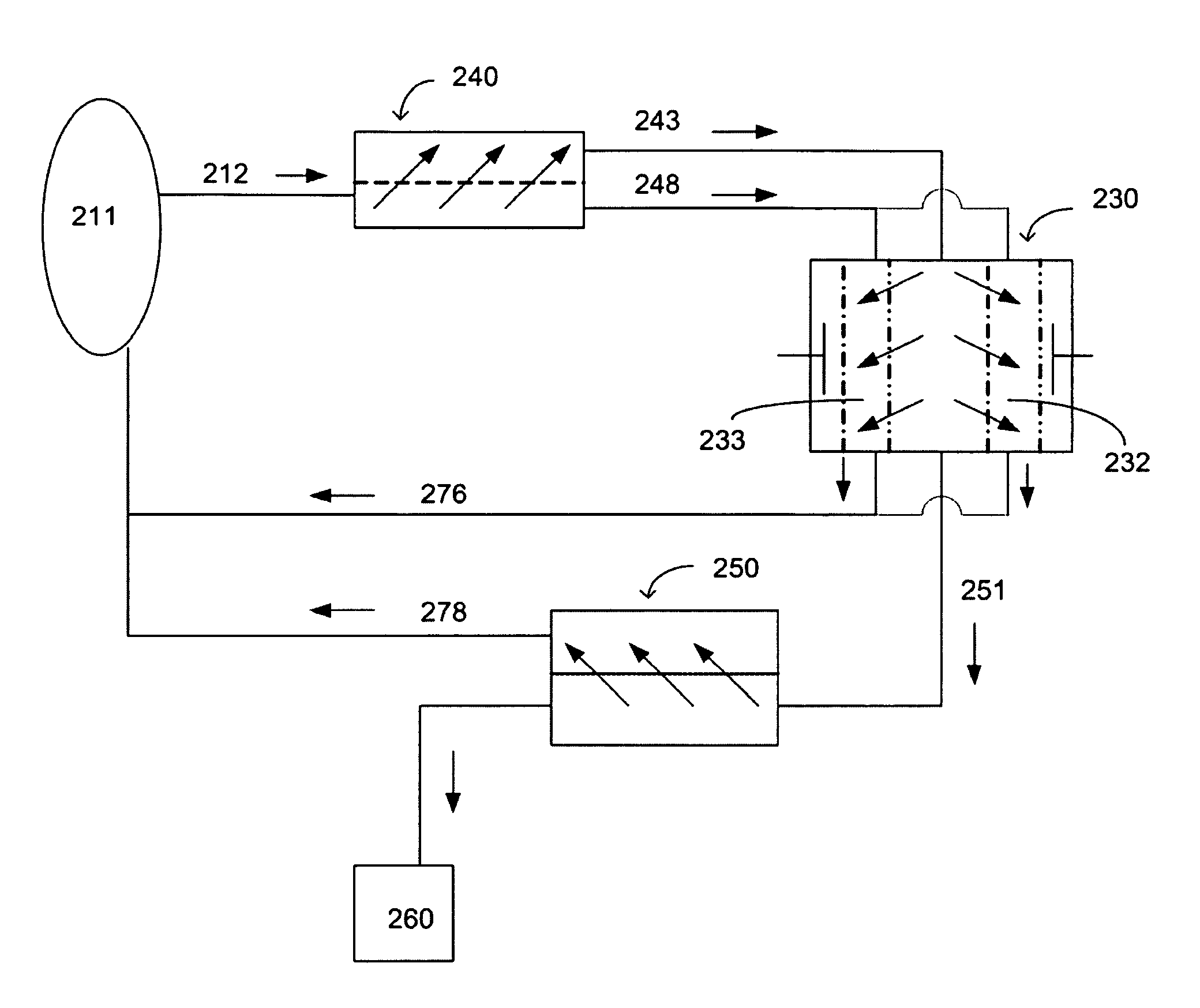
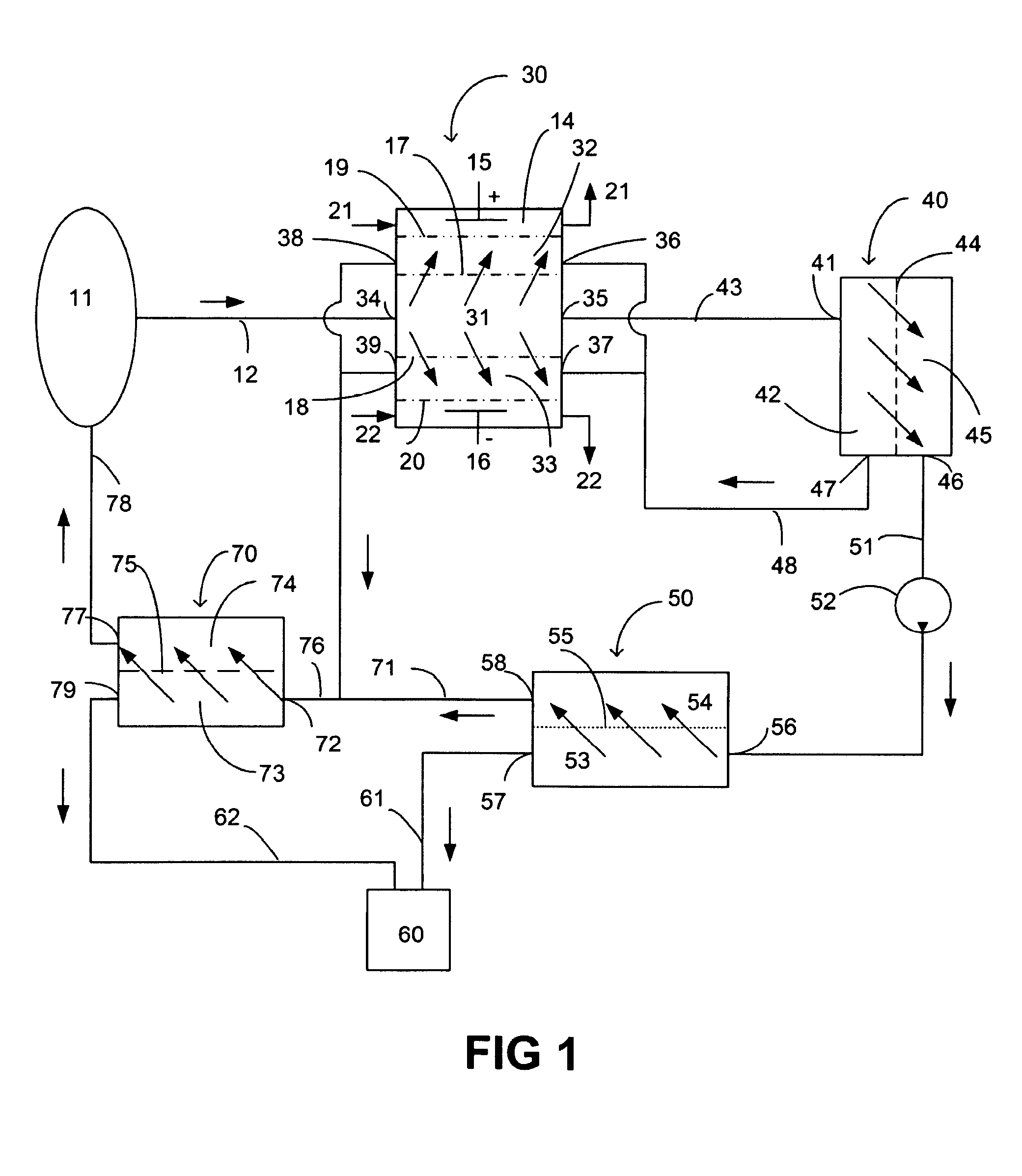
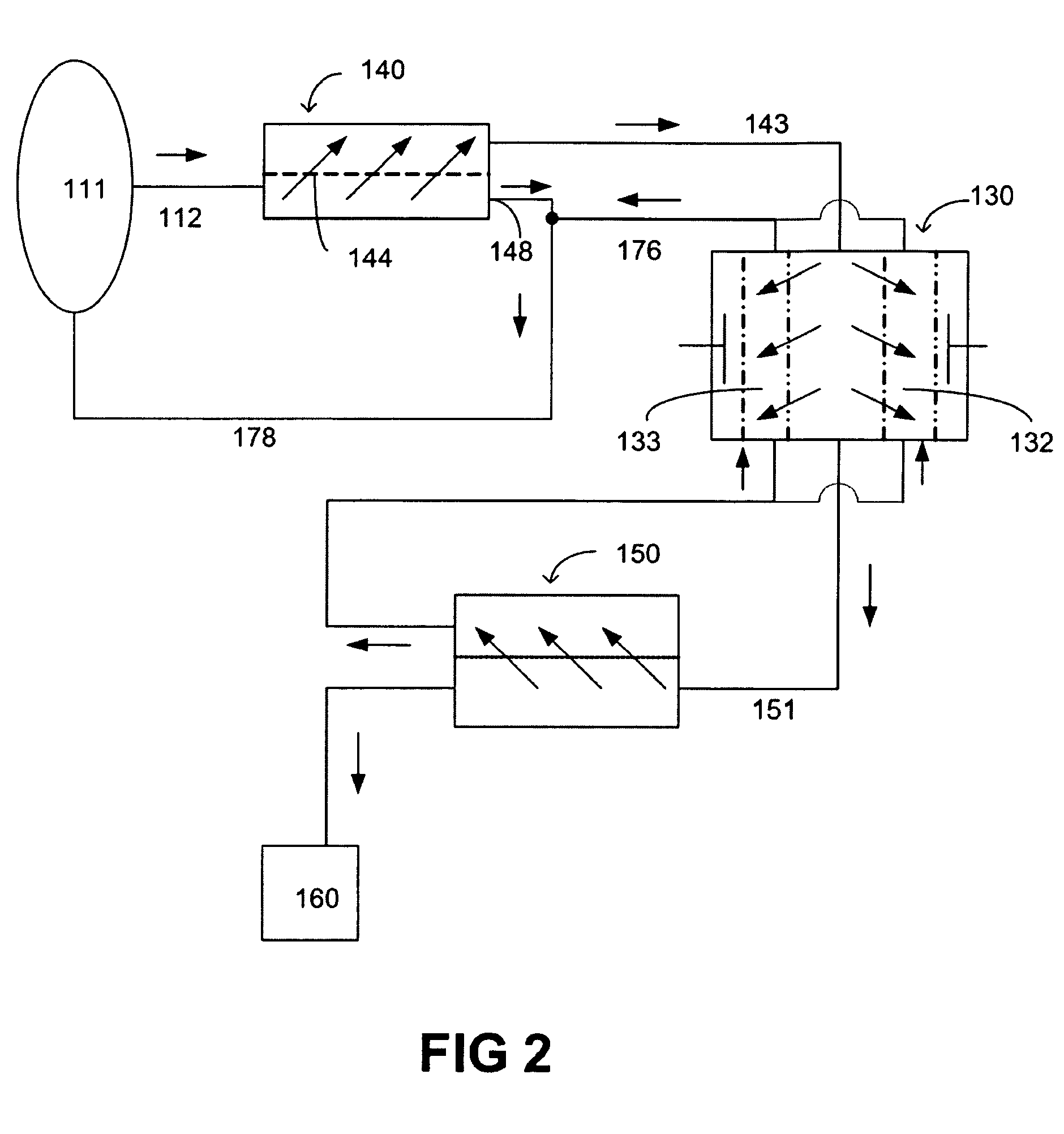
A method and a system for regenerating a body fluid, such as a peritoneal dialysis fluid. The body fluid is removed into an extracorporeal circuit comprising an electrofilter for removing charged ions from the body fluid, a nanofilter for removing large molecules, such as Dextran 40, and a reverse osmosis filter for concentrating the body fluid, for producing a synthetic urine to be discarded. The removed ions and large molecules are returned to the patient together with pure water from the reverse osmosis filter through an ultrafilter.
Owner:TRIOMED AB
Freezing medium for induced pluripotent stem cells, application of freezing medium, and freezing method of induced pluripotent stem cells
ActiveCN105087472AImprove securityTo ensure the effect of freezingEmbryonic cellsGerm cellsBiologyHuman Induced Pluripotent Stem Cells
The invention relates to the field of biology and provides a freezing medium for induced pluripotent stem cells, application of the freezing medium, and a freezing method of the induced pluripotent stem cells. The freezing medium uses an IMDM / F12 basal medium as substrate; each 100mL of the freezing medium comprises, by volume concentration, 2-20v / v% of DMSO, 0.5-5v / v% of dextran 40, 0.5-5v / v% of albumin, 1ng-1000ng of Thiazovivin, and the balance of IMDM / F12 basal medium. The freezing medium uses no animal serum, thus the risk of serum spreading of animal-origin pathogens is avoided. In addition, freezing with the freezing medium is more effective, and post-thawing survival rate of the cells is higher. After three months of freezing, the survival rate of thawed iPS (induced pluripotent stem) cells frozen with the freezing medium is higher than 91%, which is evidently higher than that of traditional freezing medium.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
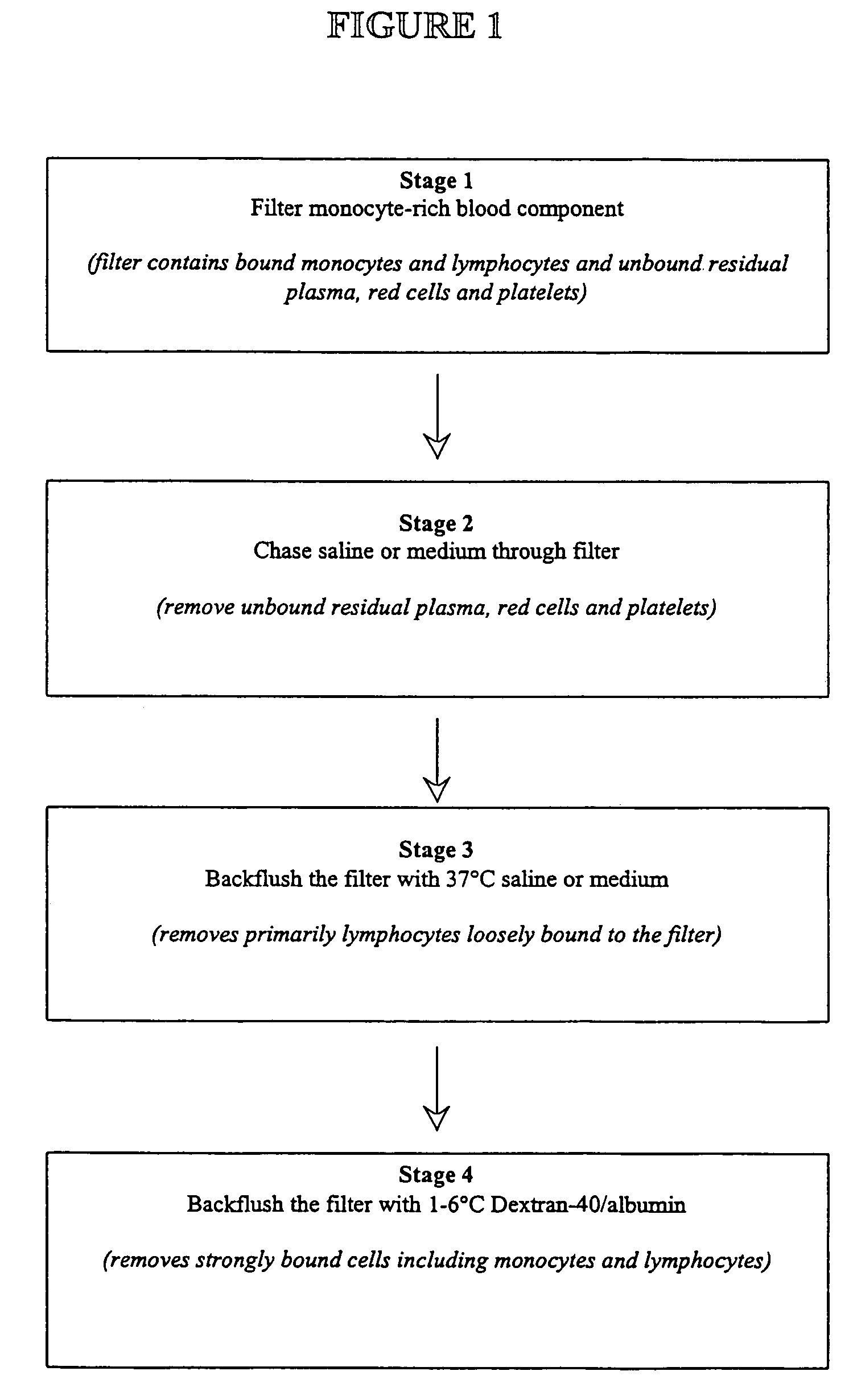
Method for enriching adherent monocyte populations
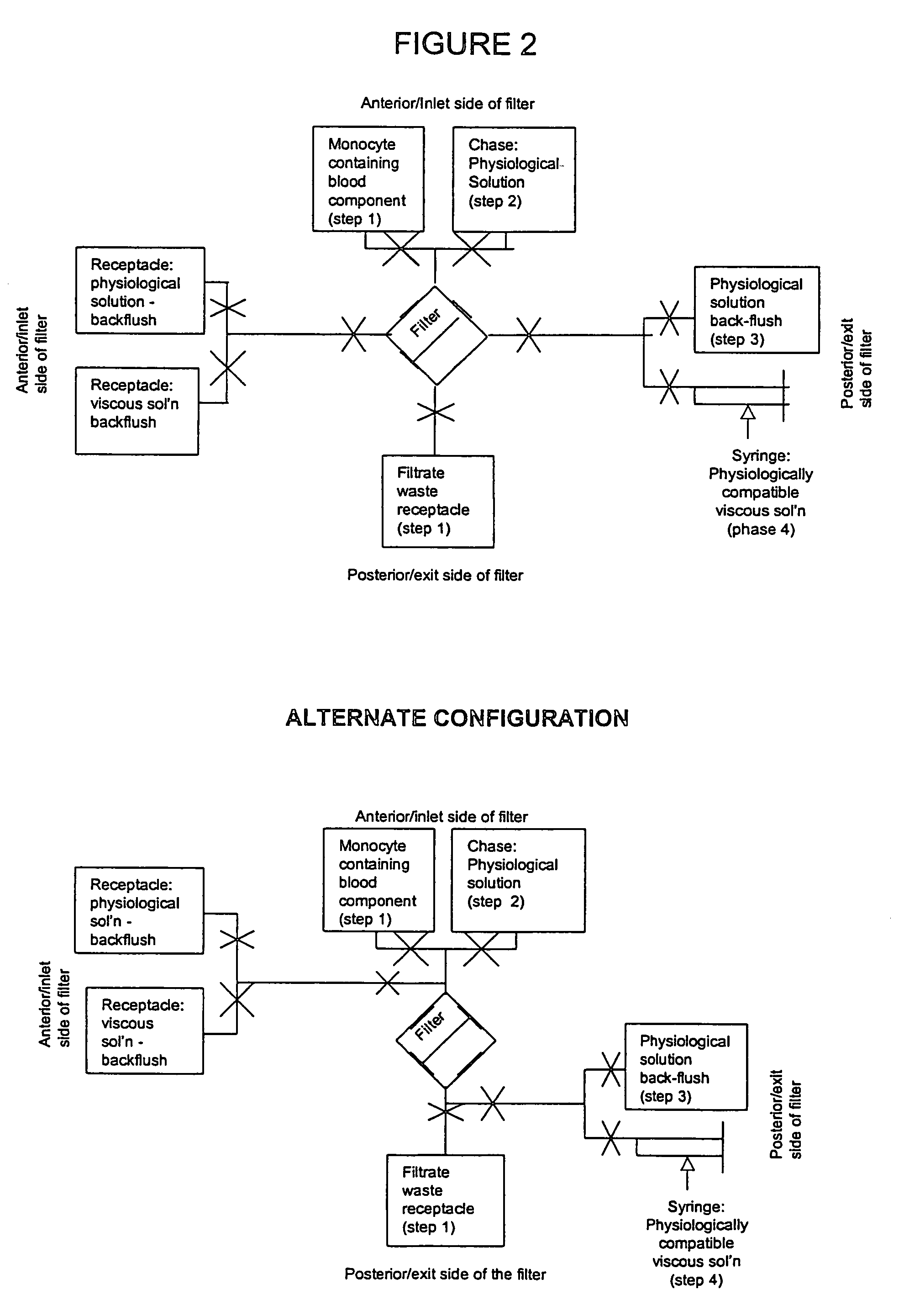
The present invention provides methods and apparatus for the rapid and efficient collection and purification of activated monocytes. The methods and apparatus of the present invention provide means for effecting the collection and purification in an aseptic environment. The method involves filtering a blood component mixture through a monocyte-adhering filter; washing the blood component mixture; backflushing the filter with a physiological solution; and backflushing the filter with Dextran-40 / serum albumin.
Owner:AMERICAN NAT RED CROSS
Cryoprotectant and method for cryopreserving placenta amnion and chorion
The invention relates to the technical field of tissue engineering and discloses a cryoprotectant and a method for cryopreserving placenta amnion and chorion. The cryoprotectant is composed of fetal calf serum, dimethyl sulfoxide and dextran 40, and the volume ratio of the fetal calf serum, the dimethyl sulfoxide and the dextran 40 is 7-9:1:1. On the basis of deep research of placenta amnion and chorion structures, the dimethyl sulfoxide, the dextran 40 and the fetal calf serum suitable for protecting placenta amnion stem cells and chorion stem cells are selected to form the cryoprotectant. The fetal calf serum, the dimethyl sulfoxide and the dextran 40 coordinate to protect activity of cells, the cells are prevented from forming ice crystal and being damaged, and the activity of recovered stem cells and the activity of newly prepared amnion chorion stem cells have no difference.
Owner:BOYALIFE
Method for preparing sotalol hydrochloride of injection
InactiveCN1481786AOvercome the shortcomings of slow oral absorption and slow onset of actionImprove stabilityPowder deliveryAmide active ingredientsFreeze-dryingSotalol Hydrochloride
The freeze dried sotalol hydrochloride for injection contains sotalol hydrochloride 20-80 g and excipient 40-100 g in each 1000 ampules. As freeze dried preparation for injection, the present invention has the advantages of fast absorption and action and high stability in the preservation period. It may be administrated via intravenous transfusion to further raise the medicine stability. The present invention is suitable for ventricular fast arythmia, etc.
Owner:吉林市卓怡康纳制药有限公司
Double enzyme method for preparing medicinal dextran with controllable molecular weight
The invention relates to a double enzyme method for preparing medicinal dextran with controllable molecular weight. The medicinal dextran comprises dextran 70, dextran 40 and dextran 20. The preparation steps of the medicinal dextran comprise two steps of: firstly, synthesizing high molecular dextran: firstly, preparing the high molecular dextran by catalyzing sucrose by adopting dextran, wherein the conversion rate of the sucrose converted into the dextran reaches over 95 percent; secondly, controllably degrading the high molecular dextran: carrying out catalytic degradation on the high molecular dextran into the medicinal dextran as the medicinal dextran by adopting dextranase with high enzyme activity and realizing the controllable degradation of the dextran; and after the reaction is ended, removing impure protein in the products by adopting a simple filtering method and obtaining dextrans with different molecular weights by adopting graded ethanol precipitation. The double enzyme method has the advantages of greenness, environment friendliness, low cost, simplicity and convenience; and in addition, according to the double enzyme method, the yield of products can be greatly improved and the medicinal dextran with high quality is obtained.
Owner:HEFEI UNIV OF TECH
Polyvinyl alcohol composite gel and its synthesis process
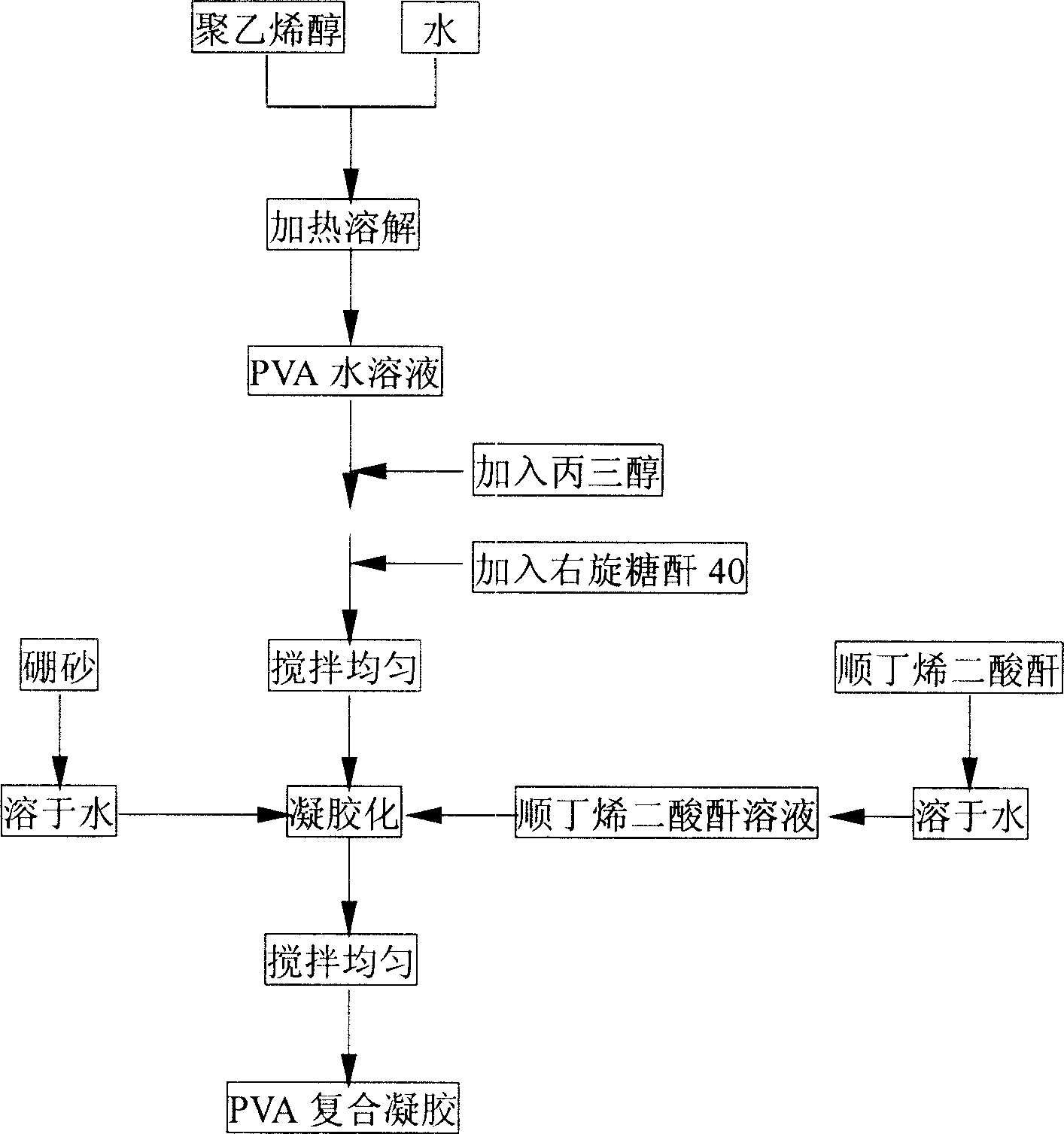
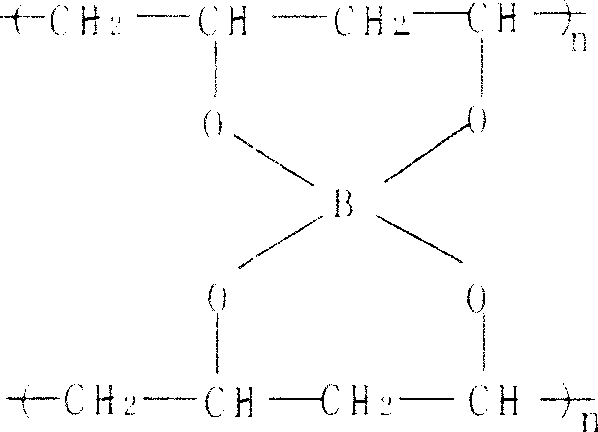
the invention discloses a polyvinyl alcohol plural gel and its producing method. It can be got by recombinating following materials as such ratios: polyvinyl alcohol or 8-15, optimized polyvinyl alcohol for 8-12; elasticizer glycerin for 3-5, optimized elasticizer glycerin for 3.8-5; dextran-40 for 0.5-1.5 or carbowax-4000 for 2-4, optimized dextran-40 for 1.0-1.5 or carbowax-4000 for 3-4; cross linker borax for 0.5-2, optimized cross linker borax for 0.5-1.5; maleic acid for 0.5-2; optimized maleic acid for 0.5-1.5. The materials are without toxin, and the polyvinyl alcohol plural gel by the invention is of good bioavailability, pretty high mechanical strength and high density void, which can be used as solidified carriers of enzyme, microbial cell and animal and plant cell.
Owner:GANSU ACAD OF SCI INST OF BIOLOGY
Nocardioactinomycetes cell wall skeleton preparation
InactiveCN1443542AImprove anti-tumor effectEnhance phagocytosisBacteria material medical ingredientsSexual disorderFreeze-dryingCurative effect
The present invention relates to a biological preparation, in particular, it is a red nocardia actinomycetes cell wall skeleton preparation. Firstly, culturing red nocardia actinomycetes CGMCC No.0712, making enrichment, culture, collecting thallus, breaking cell, enzyme purification, removing fat, blending, filling and freeze-drying so as to obtain the invented product containing red nocardia actinomycetes cell wall skeleton and dextran 40 according to a certain mixing proportion. The tests and clinical results show that its therapeutic effect is obvious for curing indications.
Owner:LIAONING GREATEST BIO-PHARM CO LTD
Drug composition containing hydroxyfasudil compound
ActiveCN103040738ASimple prescriptionReduce symptoms of hypotensionPowder deliveryOrganic active ingredientsMethionine biosynthesisMedical prescription
The invention provides a drug composition containing a hydroxyfasudil compound, which comprises hydroxyfasudil, sodium dihydrogen phosphate, dextran 40, methionine and water for injection. A preparation method of the drug composition comprises the following steps of stirring and dissolving sodium dihydrogen phosphate, methionine, hydroxyfasudil and dextran with the water for the injection, adding active carbon for decoloration, filtering removing carbon, and then conducting filter sterilization and autoclaved sterilization. The drug composition containing hydroxyfasudil is simple in prescription and reduces the possibility of hypotension, the stability is improved by reasonable configuration, and application and popularization of a hydroxyfasudil injection is facilitated.
Owner:罗诚
Cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion
InactiveCN108552159AAvoid lostReduce workloadInorganic non-active ingredientsPharmaceutical delivery mechanismClinical gradeHydroxyethyl starch
The invention discloses a cryopreserving solution for clinical-grade CAR-T cryopreservation and capable of being directly reinfused through intravenous infusion. The cryopreserving solution comprisesthe following raw materials: dimethyl sulfoxide, a glycerol fructose sodium chloride injection, an invert sugar electrolyte injection, a dextran 40 glucose injection, a hydroxyethyl starch 130 / 0.4 electrolyte injection, a vitamin C injection, a human serum albumin injection and a 0.9 % sodium chloride injection; the cryopreserving solution has the pH value of 6.8-7.0. When a formula for the clinical-grade CAR-T cryopreservation and capable of being directly reinfused through the intravenous infusion is used for cryopreserving CAR-T cells, the thawed cells and the thawed cryopreserving solutiondo not need centrifugation, resuspension, fluid exchange and other processes and can be directly reinfused through the intravenous infusion, so that the loss of cell varieties due to pollution in-vitro repeated proliferation of the CAR-T cells, or changes of cell morphologies and cell functions are effectively avoided, medical equipment can be also significantly simplified and the workload of medical workers can be reduced.
Owner:WUHAN BIO RAID BIOTECH CO LTD
Freeze-dried live attenuated hepatitis A vaccine not containing gelatin or human albumin protective agent and preparation method for freeze-dried live attenuated hepatitis A vaccine
The invention belongs to the field of biological products, in particular to a freeze-dried live attenuated hepatitis A vaccine protective agent not containing gelatin or human albumin and used for preventing hepatitis A, and a preparation method for the freeze-dried live attenuated hepatitis A vaccine protective agent. The protective agent comprises trehalose, dextran 40, L-cysteine, arginine, glutamic acid, glycine, magnesium chloride, magnesium sulfate, sorbierite, mannitol and tris(hydroxymethyl)aminomethane. The protective agent with the formula is mixed with a hepatitis A vaccine stock solution to form a semi-finished product, the semi-finished product is packaged and freeze-dried, and virus infectious titers of the vaccine before and after freeze drying and the thermal stability after freeze drying are detected; after the formula is compared with the conventional production formula containing the gelatin, results show that the protective agent has good protective effect, the descent of the virus infectious titers of the vaccine in the freeze drying process is obviously decreased, and the endotoxin content in a finished product is obviously reduced (less than 0.25EU / ml); and after the freeze-dried vaccine with the formula is inoculated into a human body, results indicate that the vaccine has good immune effect and high safety.
Owner:ZHEJIANG PUKANG BIOTECH
Cryoprotectant and method for cryopreserving placenta amnion and chorion
ActiveCN102763642BHigh activityNo freeze damageArtificial cell constructsVertebrate cellsBiologyCattle calf
The invention relates to the technical field of tissue engineering and discloses a cryoprotectant and a method for cryopreserving placenta amnion and chorion. The cryoprotectant is composed of fetal calf serum, dimethyl sulfoxide and dextran 40, and the volume ratio of the fetal calf serum, the dimethyl sulfoxide and the dextran 40 is 7-9:1:1. On the basis of deep research of placenta amnion and chorion structures, the dimethyl sulfoxide, the dextran 40 and the fetal calf serum suitable for protecting placenta amnion stem cells and chorion stem cells are selected to form the cryoprotectant. The fetal calf serum, the dimethyl sulfoxide and the dextran 40 coordinate to protect activity of cells, the cells are prevented from forming ice crystal and being damaged, and the activity of recovered stem cells and the activity of newly prepared amnion chorion stem cells have no difference.
Owner:BOYALIFE
Alprostadil freeze-dried powder injection and preparation method thereof
InactiveCN102688203AHas an enveloping effectLow hemolytic activityOrganic active ingredientsPowder deliveryMedicineFreeze-drying
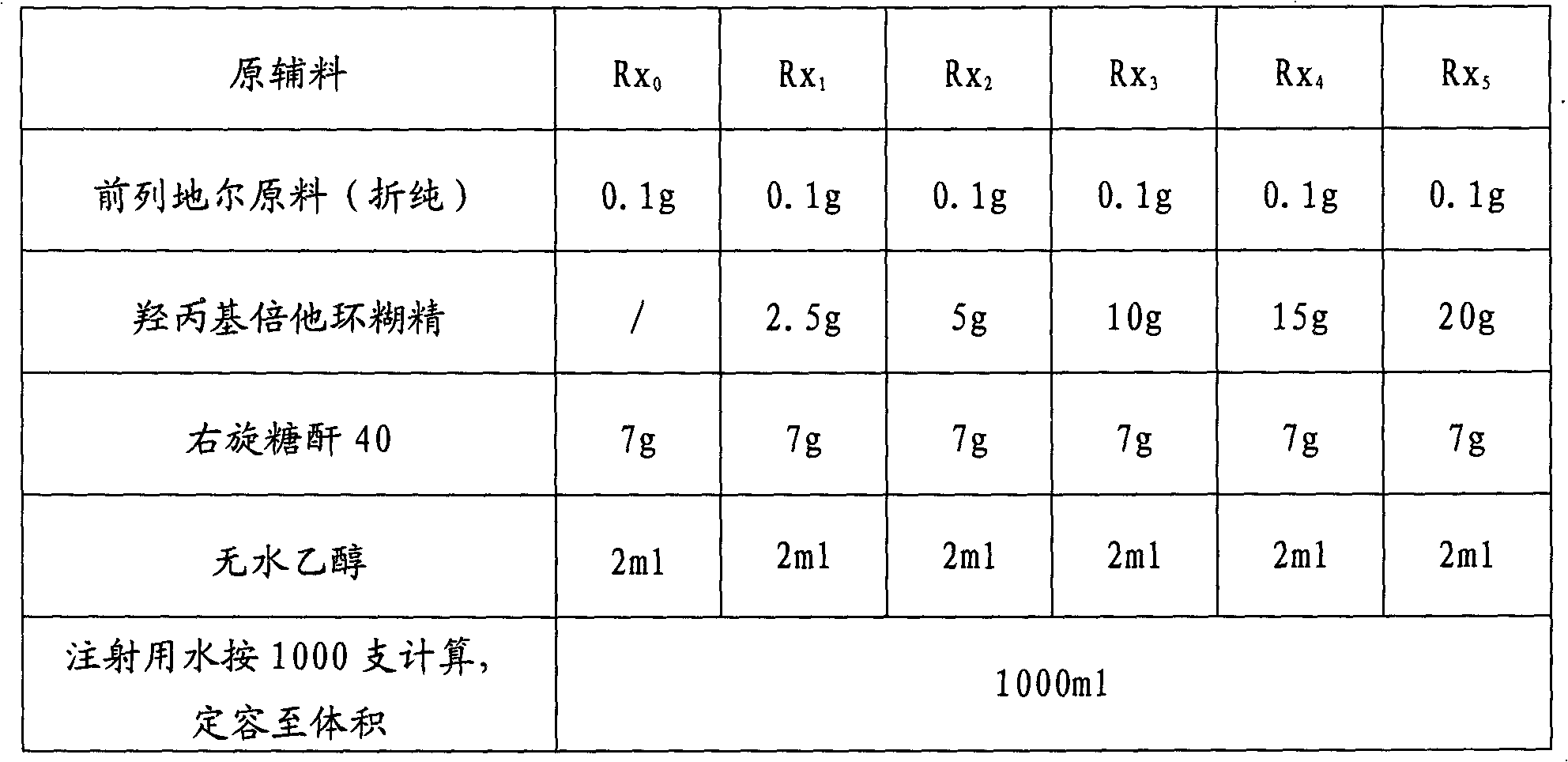
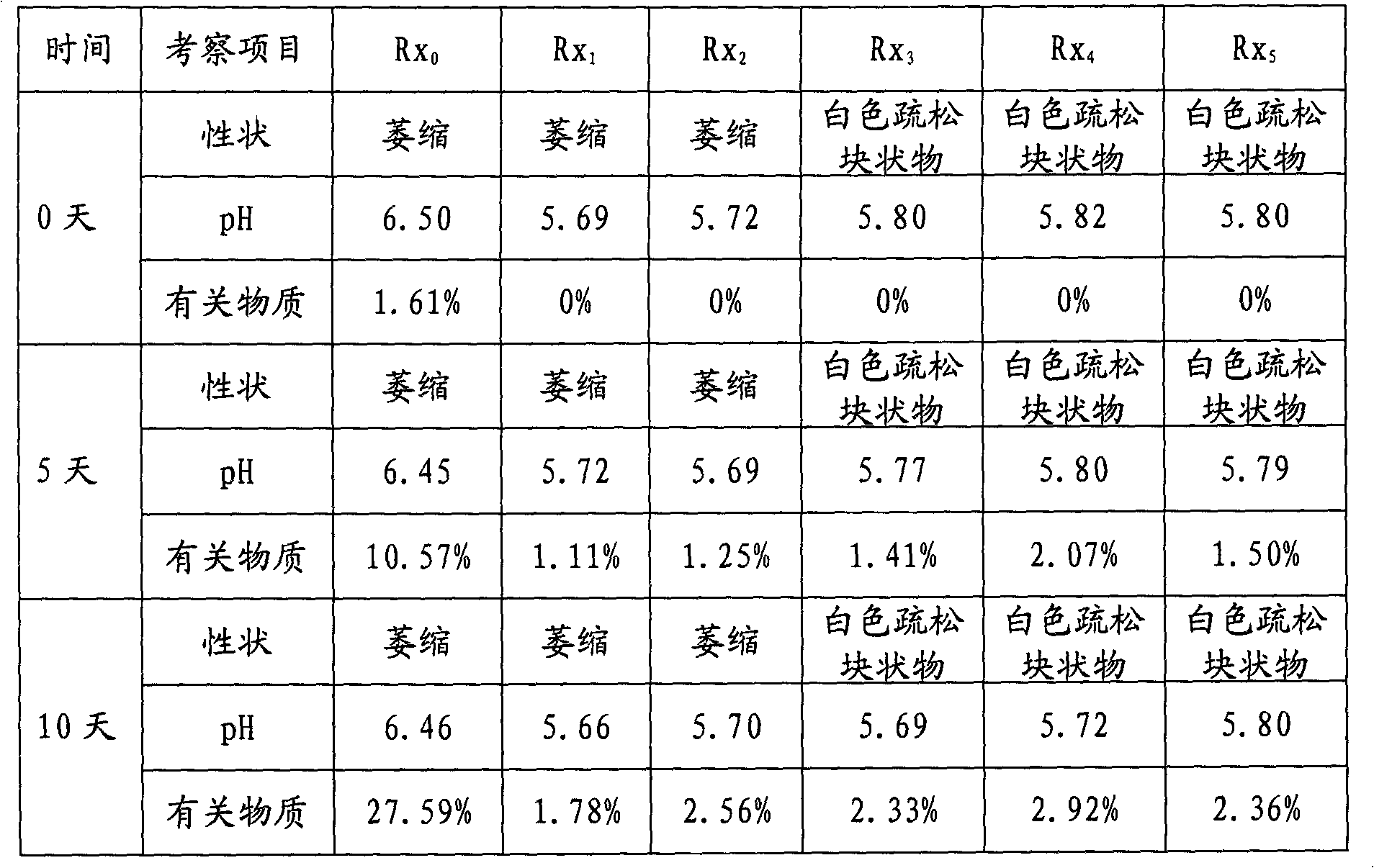
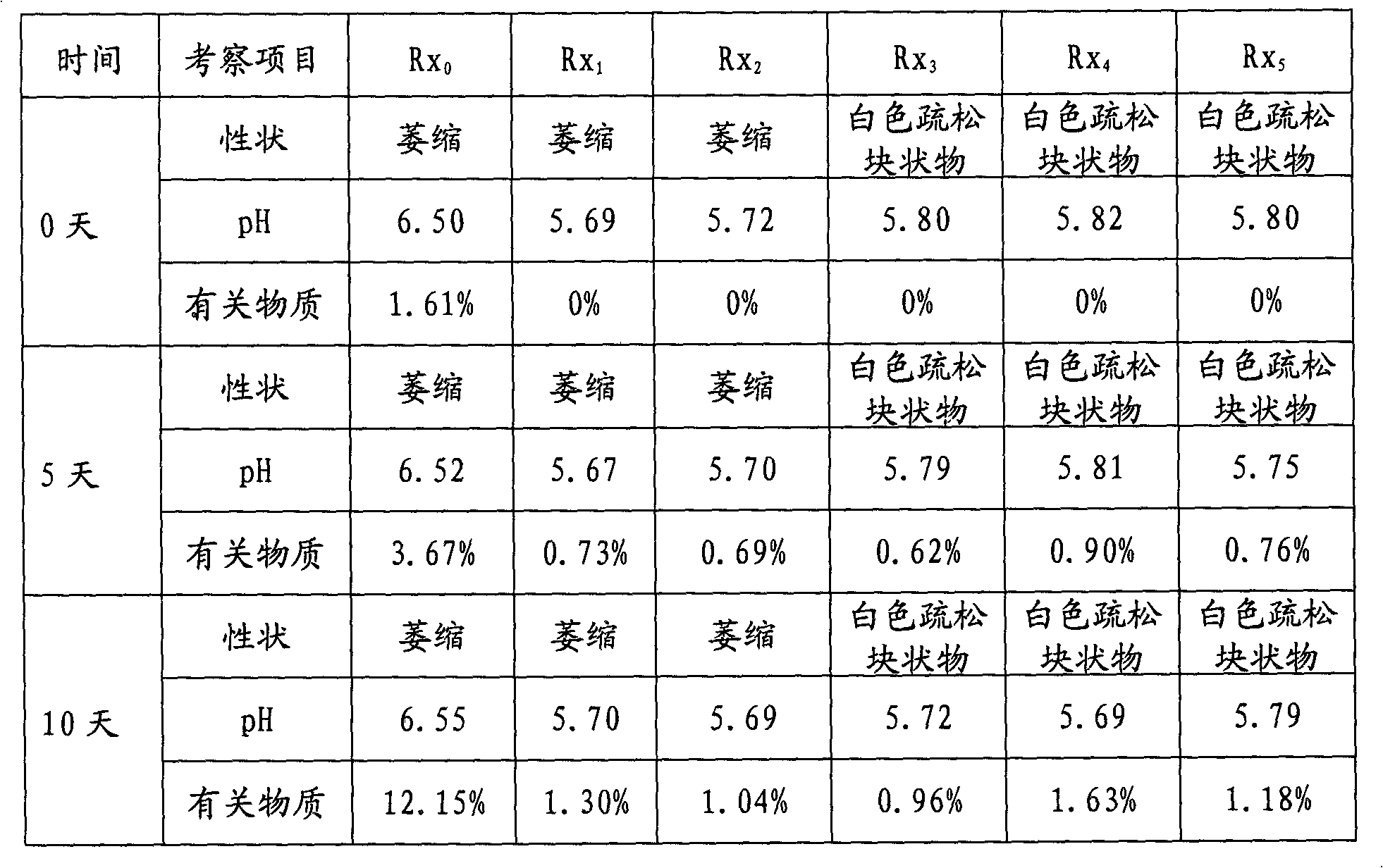
The invention discloses an alprostadil freeze-dried powder injection and a preparation method thereof. The alprostadil freeze-dried powder injection comprises the following components: alprostadil, hydroxypropyl beta-cyclodextrin and dextran 40, wherein the weight ratio of the alprostadil to the hydroxypropyl beta-cyclodextrin to the dextran 40 is 1:(50-150):70. According to the technical scheme disclosed by the invention, the hydroxypropyl beta-cyclodextrin has an enveloping effect, is low in hemolytic activity, has no irritation to muscles, is capable of enhancing the stability of medicines and can be used for improving the stability and the dissolvability of the alprostadil, and the water solubility of the hydroxypropyl beta-cyclodextrin is greater than 50 percent. The dextran 40 is used for improving the freeze-dried forming characteristics of the alprostadil for injection. The pH value is regulated to be 4.0-4.5, and the alprostadil freeze-dried powder injection is higher in stability within the range of 4.0-4.5. The alprostadil freeze-dried powder injection obtained by adopting the technical scheme disclosed by the invention is safe and stable and is exact in curative effect and high in clinical adaptability of patients.
Owner:杭州澳亚生物技术股份有限公司
Freeze-dried rabies vaccine for human use and preparation method thereof
The invention discloses a freeze-dried rabies vaccine for human use and a preparation method thereof. The preparation method comprises the following steps: adding cane sugar and dextran 40 into water for injection respectively, stirring to a fully-dissolved state, and performing steam sterilization at the temperature of 115 DEG C under the pressure of 0.09-0.10 MPa for 45 minutes; adding glycine into water for injection of which the temperature is 15-30 DEG C, stirring to a fully-dissolved state, and degerming and filtering with a microfiltration membrane of 0.22 mu m; preparing a dose of which the total protein content does not surpass 80 mu g according to the measured protein content or antigen content of an early vaccine stock solution, and adding cane sugar of which the final concentration is 4-10 percent by weight, dextran 40 of which the final concentration is 1-4 percent by weight and glycine of which the final concentration is 0.5-2 percent by weight to obtain a semi-finished product; performing split charging on the semi-finished product, semi-plugging, putting into a freezer drier box, setting a freeze drying parameter, and performing freeze drying; performing pre-freezing, vacuum pumping, sublimation, secondary drying and vacuum plugging, and ending freeze drying to obtain the freeze-dried rabies vaccine for human use. Active ingredients in the vaccine disclosed by the invention can be well protected, the vaccine is high in thermal stability, and the period of validity can be at least up to 24 months.
Owner:DALIAN HISSEN BIO-PHARM CO LTD
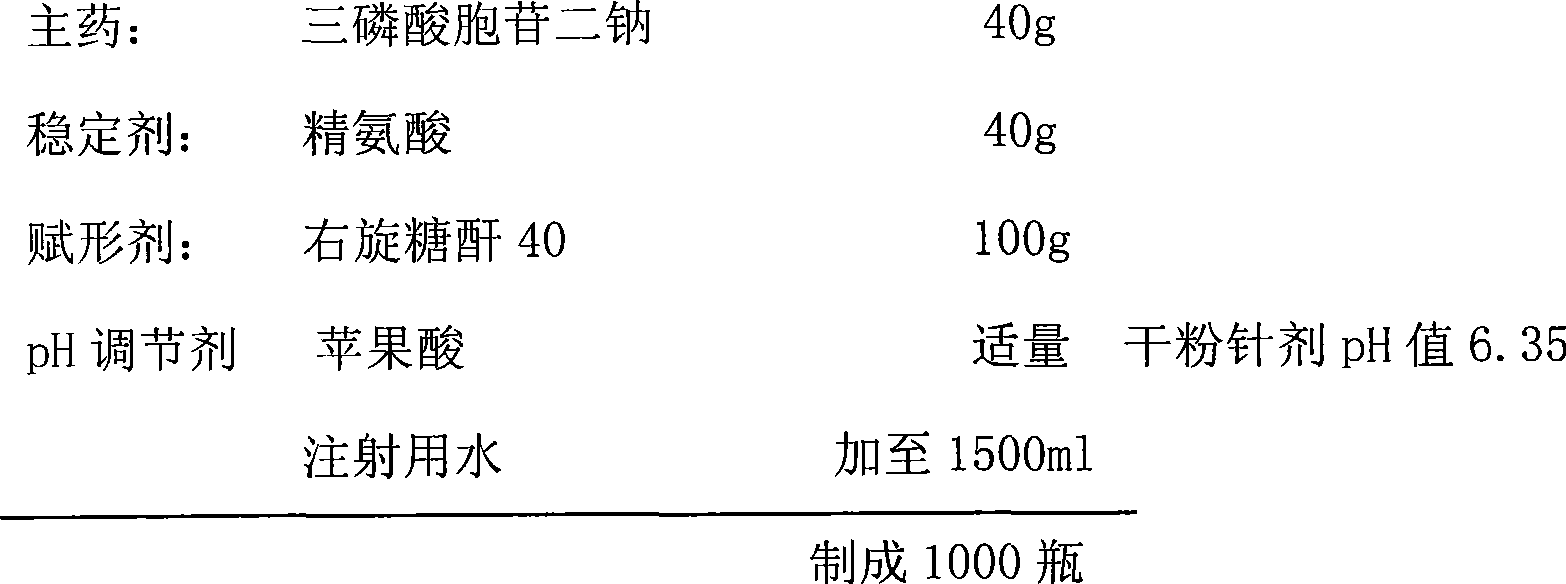
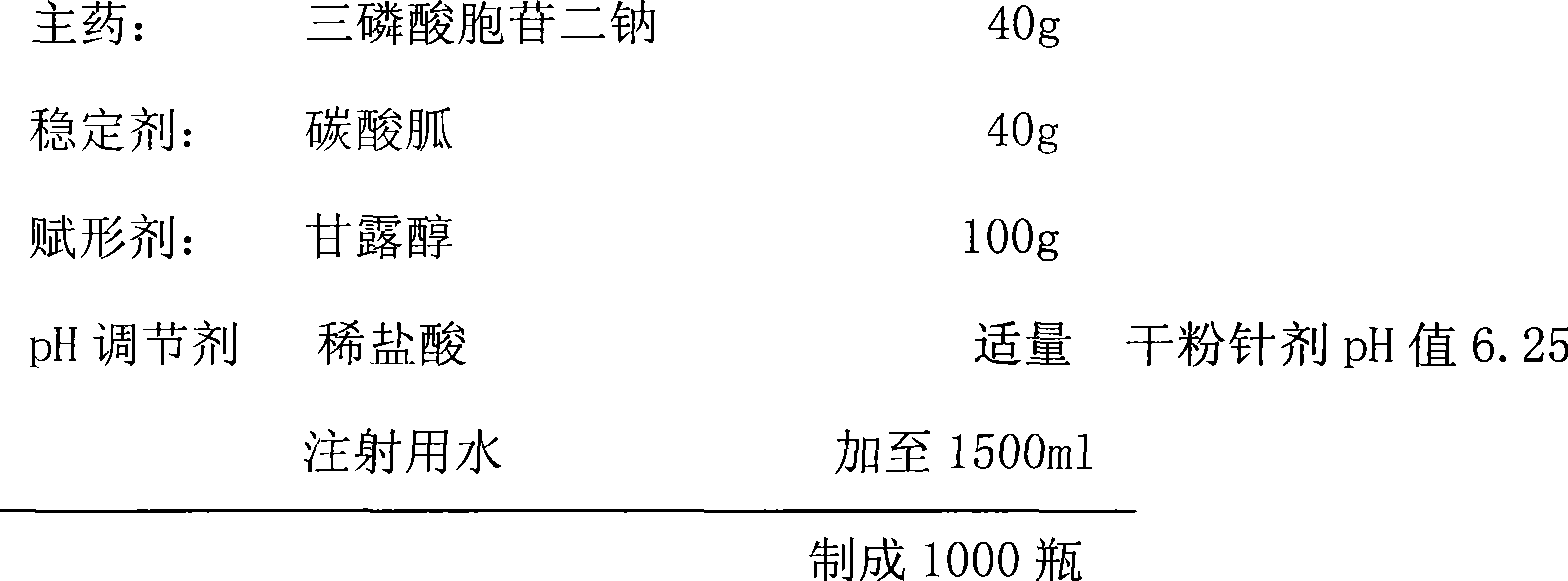
Cytidine disodium triphosphate freeze-dried powder-injection for injection
InactiveCN101518515AImprove quality stabilityImprove safety and effectivenessOrganic active ingredientsPowder deliveryArginineFiltration
The invention discloses a prescription of a cytidine disodium triphosphate freeze-dried powder-injection for injection and a process for preparing the same. The powder-injection consists of an active component of cytidine disodium triphosphate, a stabilizer of guanidine carbonate and / or arginine, an excipient of mannitol or dextran 40, as well as a pH regulator, wherein the weight ratio of the active component to the stabilizer to the excipient is 1:0.5-5:0.5-5, and the weight ratio of the guanidine carbonate to the arginine in the stabilizer is 2-4:1; and the addition of the pH regulator is standardized to control the pH value of the powder-injection between 6.0 and 6.5. The process for preparing the cytidine disodium triphosphate freeze-dried powder-injection comprises the steps of dissolving the prescription quantity of each component into certain amount of injection water, and then performing filtration, filling, pre-freezing, and freeze drying. The cytidine disodium triphosphate freeze-dried powder-injection and the method improve the stability of the active component, and solve the problems that the cytidine disodium triphosphate in the prior freeze-dried powder-injection is decomposed to a large extent in the process of preservation and the effectiveness of the medicament is reduced. Besides, the cytidine disodium triphosphate freeze-dried powder-injection and the method improve the using safety of the medicament and are advantageous for the long-time storage of medicaments.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid
PendingCN112772637ASo as not to damageReduce adverse effectsDead animal preservationSodium Chloride InjectionMesenchymal stem cell
The invention discloses a DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid, which is characterized by being prepared from the following raw materials in parts by volume: 50 to 60 parts of compound electrolyte injection, 20 to 40 parts of dextran 40 glucose injection, 1 to 10 parts of sodium chloride injection, 1 to 10 parts of glucose injection, 30 to 50 parts of human serum albumin, and 1 to 10 parts of a mesenchymal stem cell serum-free medium. The cryopreservation liquid does not contain DMOS or serum, so that the risk of clinical use is reduced, the influence of the uncertainty of serum components and the instability of serum culture on the normal induced differentiation function of the mesenchymal stem cells is avoided, and the cryopreservation liquid enables the human umbilical cord mesenchymal stem cells to keep a good cryopreservation effect, and the human umbilical cord mesenchymal stem cells have high survival rate after cryopreservation and resuscitation. In addition, the cells cryopreserved by the cryopreservation liquid can be directly diluted and then applied clinically, components of the cryopreservation liquid do not need to be removed through centrifugation, and the cryopreservation liquid can be used as an auxiliary material and directly applied to clinical administration, so that the cryopreservation liquid is more convenient to use.
Owner:朱灏
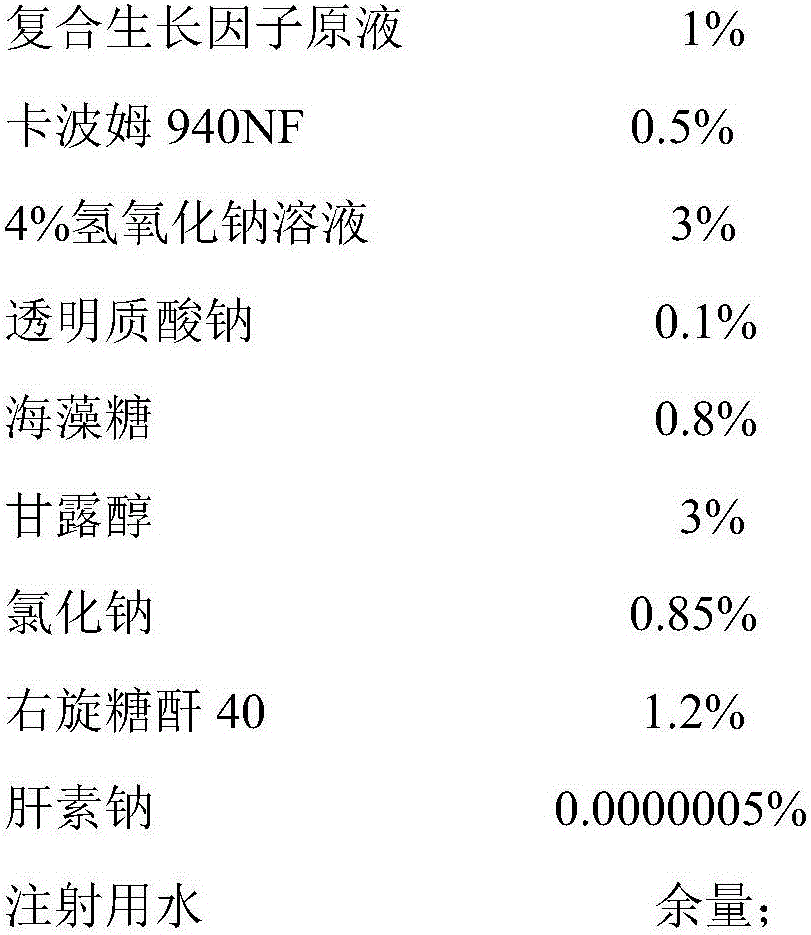
Composite growth factor skincare gel and preparation method thereof
InactiveCN106038350AGood skin careLong-lasting skin careCosmetic preparationsToilet preparationsIrritationMannitol
The invention discloses composite growth factor skincare gel. The composite growth factor skincare gel is prepared from, by mass, 1-50% of composite growth factor stock solution, 0.5-1.5% of carbomer 940NF, 1-10% of 4% sodium hydroxide solution, 0.1-2% of sodium hyaluronate, 0.1-10% of trehalose, 1-10% of mannitol, 0.85-0.95% of sodium chloride, 0.1-5% of dextranum 40, 0.00003-0.3% of heparin sodium and the balance injection water. Rh-bFGF and KGF in the composite growth factor stock solution are compounded according to a specific proportion and used for a skincare gel formula; on the basis of moisture preservation, skin redness and swelling can be effectively relieved, and skin wounds and scars can be effectively restored; skin is prevented from bearing toxic irritation of other skincare products, and the composite growth factor skincare gel mainly aims at people suffering from severe skin aging, skin redness and swelling and skin local wounds or breakage, such as hormone face patients for long-term skincare.
Owner:朗肽生物制药股份有限公司
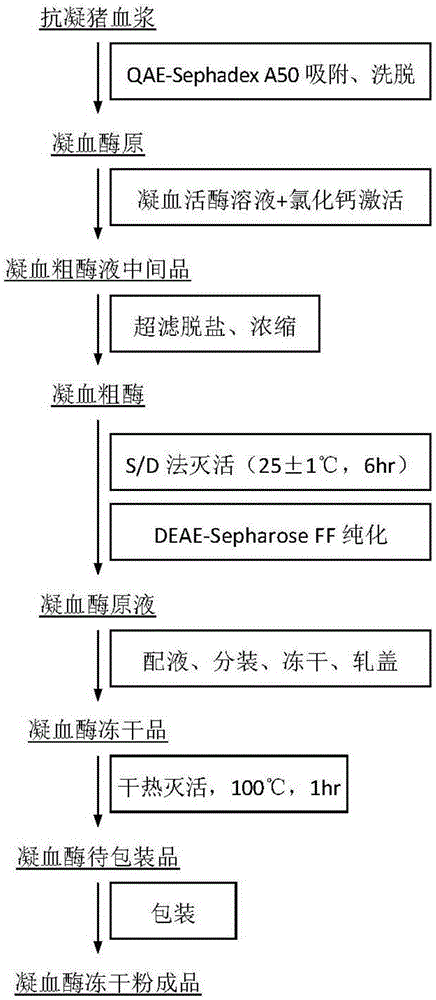
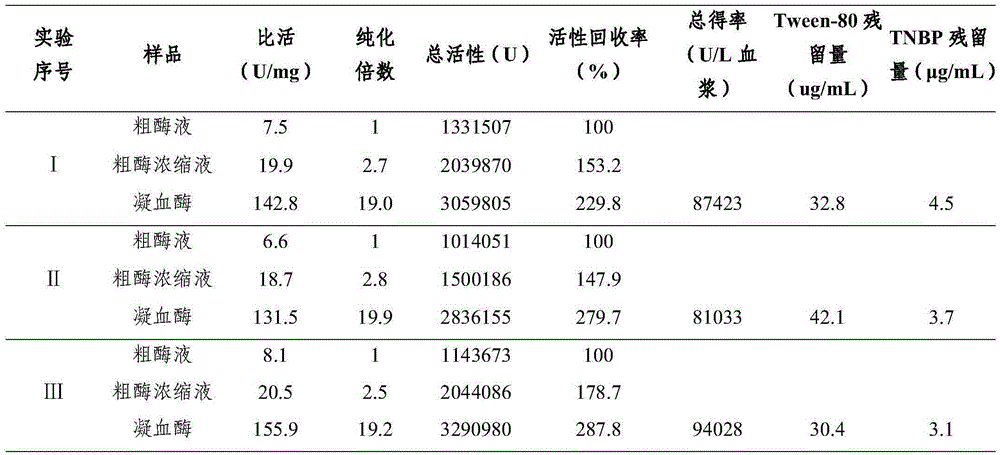
Preparing method for pig thrombin freeze-dried powder
ActiveCN105250226AEffective inactivationImprove securityPowder deliveryPeptide/protein ingredientsFreeze-dryingDEAE-Sepharose
The invention discloses a preparing method for pig thrombin freeze-dried powder. The preparing method comprises the following steps that anticoagulant pig plasma and gel are mixed and stirred for adsorption and pass through a column for elution, and a prothrombin solution is obtained; normal saline is added to rabbit brain powder, then the prothrombin solution and CaCl2 are added for zymogen activation, a crude thrombin enzyme solution is obtained, subjected to ultrafiltration for desalination and concentrated, viral inactivation is carried out, an obtained crude enzyme solution passes through DEAE-Sepharose Fast Flow chromatographic column, elution is carried out, target peaks are collected, and pig thrombin is obtained; the pig thrombin is added with mannitol or dextran 40, filtering, sterilizing, subpackaging, freeze drying and vacuum tamponing are carried out, an aluminum plastic combined cover is rolled, and a pig thrombin freeze-dried product is obtained; the freeze-dried product is subjected to dry heat treatment at 100 DEG C for secondary virus inactivation, and the pig thrombin freeze-dried powder is obtained after packaging. The specific activity of pig thrombin in the product is not lower than 130 U / mg, the whole process is simple in step and easy to implement, the product safety is improved, and the preparing method is suitable for industrial production.
Owner:WUHAN HITECK BIOLOGICAL PHARMA
Preparation method of organ preservation solution
ActiveCN102524242ASolve the problem of oxidation decompositionStable in natureDead animal preservationAdenosineAcetylcysteine
The invention provides a preparation method of organ preservation solution, which is characterized by comprising carrying out combination preparation to dextran 40, acetylcysteine and sodium hydroxide, filling, sterilizing, and obtaining product solution A; performing combination preparation to potassium citrate, mannitol, potassium chloride, sodium hydrogen phosphate, sodium dihydrogen phosphate, adenosine and magnesium sulfate, filling, sterilizing, and obtaining product solution B, evenly mixing the solution A and the solution B which are of the same volume, and obtaining the organ preservation solution. First acetylcysteine and sodium hydroxide which are equimolar are dissolved with each other to form sodium acetylcysteine, raw materials are dissolved easily, preparation time is reduced, and the raw materials existing in the form of sodium acetylcysteine are stable in nature. The preparation method solves the problem of oxygenolysis of acetylcysteine in the organ preservation solution in the preparation and storage process, is simple and good in stability.
Owner:HUAREN PHARMACEUTICAL CO LTD
Hair restorer
InactiveCN110339338AAchieve hair growth effectPromote regenerationPowder deliveryOrganic active ingredientsAlopecia seborrhoeicaUvb irradiation
The invention provides a hair restorer. The hair restorer comprises an adipose tissue-derived stromal cell conditioned medium, a fibroblast exosome, mannitol, trehalose and dextran 40, wherein adiposetissue-derived stromal cells are irradiated according to the UVB irradiation dosage of 20-100 mJ / cm<2>, the irradiated adipose tissue-derived stromal cells secrete various types of bioactive factors,hair papilla can be stimulated, hair follicle stem cells can be activated, and the function of hair restoration from inside to outside is realized. The invention further provides a hair restoration composition with a nutritional agent and the hair restorer. The hair restoration composition has a remarkable treatment effect on androgenic alopecia, alopecia seborrhoeica and alopecia areata and is easy to use and popularize.
Owner:陕西佰瑞衡健康科技有限公司
Long-term preservation liquid for corneal tissue and preparation method thereof
InactiveCN109221093AFacilitate long-term maintenance of structureFacilitates long-term transparencyDead animal preservationFiberSodium bicarbonate
The invention discloses long-term preservation liquid for corneal tissue and a preparation method thereof. The long-term preservation liquid is prepared from the following components: 15 to 25g / L of sodium chondroitin sulfate, 0.5 to 4g / L of sodium hyaluronate, 0.5 to 3 g / L of dextran 40, 15 to 30 g / L of glycerin, 0.001 to 0.3 g / L of sodium pyruvate, 0.002 to 2 g / L of vitamin C, 0.05 to 0.7 g / L ofgentamicin sulfate, 1.4 to 2.2 g / L of sodium bicarbonate, 5.0 to 6.5 g / L of 4-hydroxyethylpiperazine ethanesulfonic acid, and 1 L of water for injection. A combination system of sodium chondroitin sulfate, sodium hyaluronate and dextran is adopted to obtain the preservation liquid which can be used for long-term preservation of corneal tissue, thereby being particularly advantageous for maintaining an original collagen fiber structure and transparency of a corneal lens for a long term; after the cornea tissue is preserved by the long-term preservation liquid, the cornea tissue can be used directly after rewarming, the operation is simple, and rehydration is not needed.
Owner:镇江雷音再生医学科技有限公司
Stem cell cryopreservation liquid and production method thereof
InactiveCN109430252AGuaranteed activityGuaranteed biological activityDead animal preservationGlycerolTREHALOSE DIHYDRATE
The invention provides stem cell cryopreservation liquid and a preparation method thereof. The stem cell cryopreservation liquid is prepared from the following substances in percentage by volume: 35 to 55 percent of low-sugar type DMEM (Dulbecco's Modified Eagle Medium), 15 to 35 percent of Ultroser G serum replacement, 5 to 20 percent of glycerol, 5 to 10 percent of recombinant human serum albumin and 10 to 20 percent of dextran 40; the stem cell cryopreservation liquid further comprises 4 to 10mg / L of a bFGF (basic Fibroblast Growth Factor) and 250 to 700mmol / L of non-crystalline amorphous trehalose; the invention further provides the production method of the stem cell cryopreservation liquid. The stem cell cryopreservation liquid provided by the invention has the beneficial effects thatthe stem cell cryopreservation liquid is stable and reliable, a differentiation potential of cells can be effectively protected for long time and the cell bioactivity is ensured; exogenous microorganism infection and allergic risks are reduced; the production method is simple to operate and feasible, and has relatively good practical value.
Owner:成都赋智未来科技有限公司
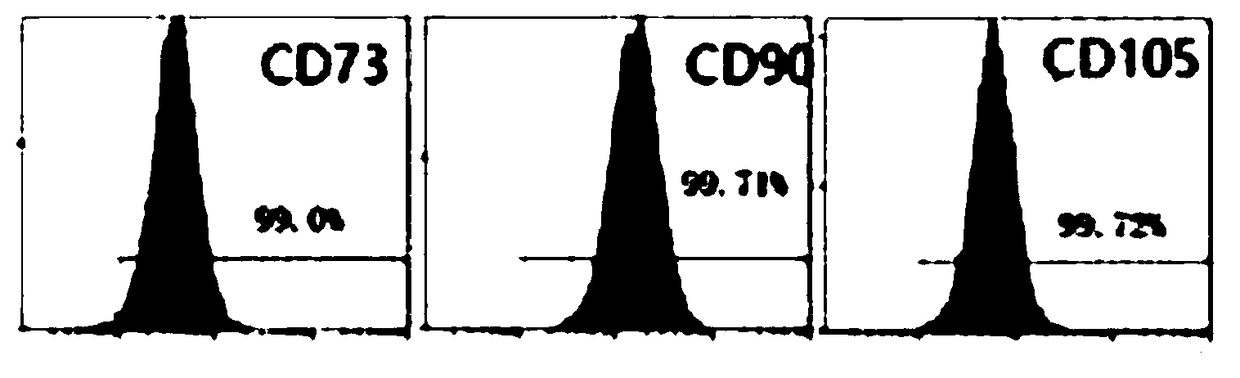
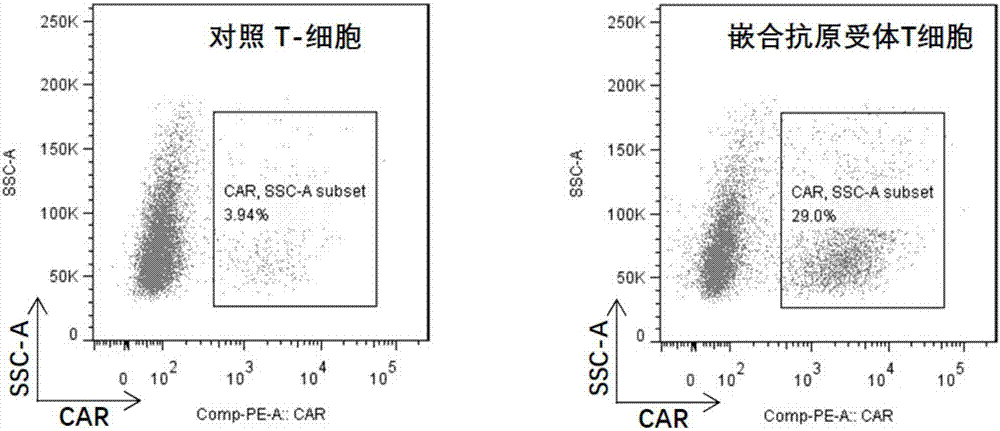
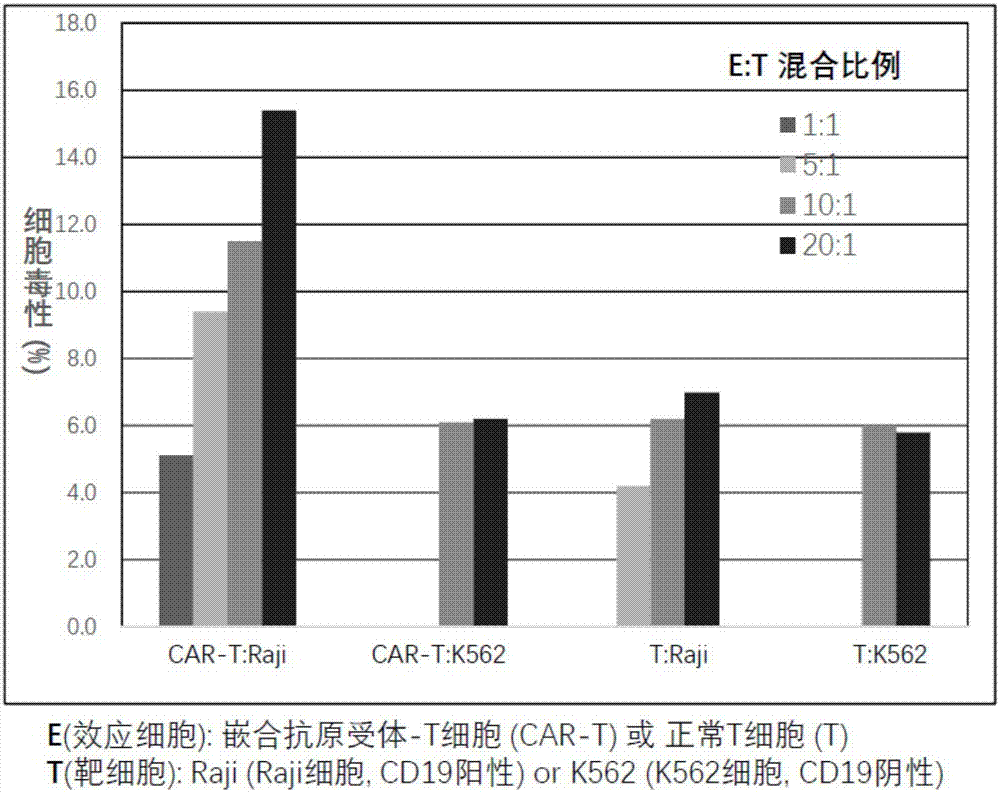
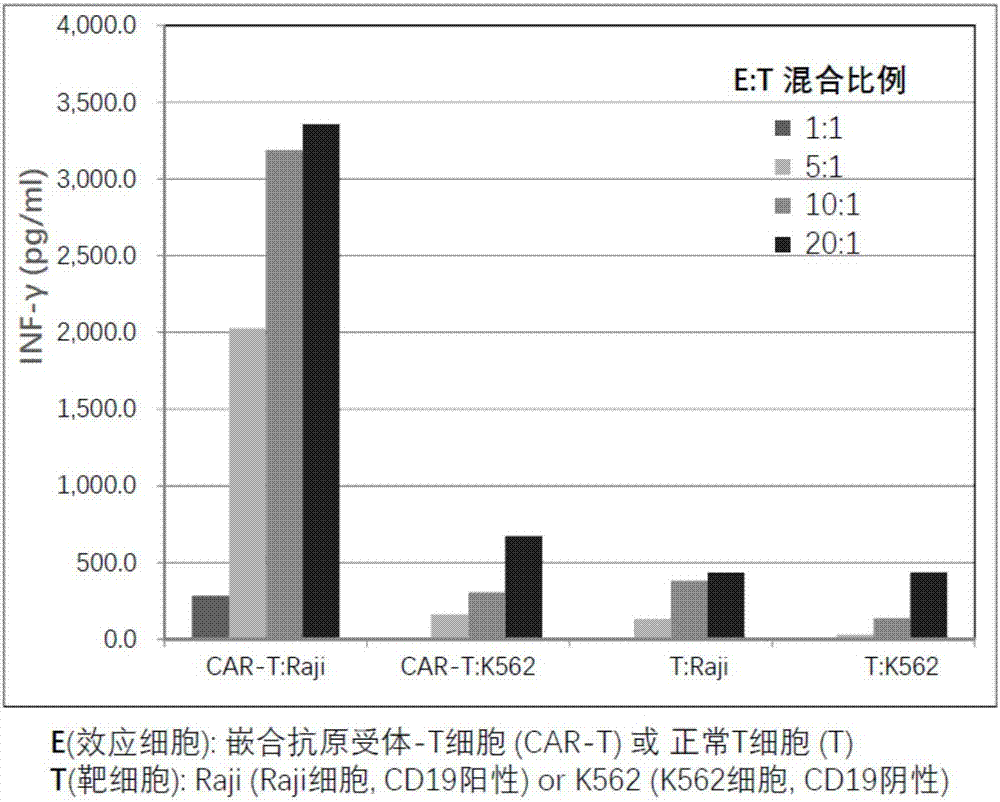
Chimeric antigen receptor T cell preparation
ActiveCN107970258AProlong survival timeReduce vitalityGenetically modified cellsPharmaceutical delivery mechanismRingers solutionSodium Chloride Injection
The invention discloses an immune cell preparation. The preparation consists of immune cells and and an immune cell preservation re-infusion liquid which is free from dimethyl sulfoxide and dextran 40, wherein the immune cell preservation re-infusion liquid consists of the following components: an electrolyte solution which is isosmotic with plasma, a human serum albumin injection and a glucose injection, wherein the electrolyte solution is any one of a sodium chloride injection, a compound sodium chloride injection, a compound electrolyte injection and a lactated Ringer's solution. The immunecell preparation provided by the invention conforms to the characteristic that China is concentrated and dense in population; and the immune cell preparation is applicable to an immune cell preparation center close to a treatment agency, and the immune cell preparation only needs to be preserved at 4-15 DEG C. With the application of the immune cell preparation provided by the invention, in particular the chimeric antigen receptor T cell preparation, a preparation process can be simplified, logistics cost can be greatly reduced, the safety of the preparation can be improved and a treatment cycle can be shortened; therefore, treatment cost of the chimeric antigen receptor T cell preparation can be reduced, so as to benefit broad patients.
Owner:英普乐孚生物技术(上海)有限公司
Sodium bialginate for injection and its preparation method
InactiveCN1481809AOvercome the disadvantage of low bioavailabilitySolve easy discolorationPowder deliveryOrganic active ingredientsSodium bicarbonateFreeze-drying
The present invention is one kind of freeze dried polysaccharide sulphate for injection, and each 1000 ampules of the injection contains polysaccharide sulphate 25-200 g and excipient 25-200 g. The injection preparation is prepared through advanced low temperature freeze drying process, and is stable in the preserving period and ever suitable for clinical application. The present invention has the functions of lowering blood viscosity, resisting blood coagulation, lowering blood fat, improving micro circulation, etc. and is used in preventing and treating ischemic cardiac and cerebral vascular diseases and hyperlipemia.
Owner:吉林市卓怡康纳制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com