Chimeric antigen receptor T cell preparation
A chimeric antigen receptor and preparation technology, which is applied in the fields of immunology and molecular biology, can solve the problems of restricting the application of chimeric antigen receptor T cell therapy, and achieve the effects of convenient clinical application, simplified preparation process, and simple ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] [Example 1] Construction of a chimeric antigen receptor lentiviral vector expressing a chimeric antigen receptor gene targeting CD19
[0041] (1) All artificially synthesized artificial CAR genes encoding chimeric antigen receptors (including the promoter of the human elongation factor 1α gene, with an EcoRV endonuclease site at the 5'-end and SalI at the 3'-end endonuclease sites).
[0042] (2) Use EcoRV and SalI (NEB Company) to cut the GFP gene and PGK promoter from the lentiviral vector pRRLSIN.cPPT.PGK-GFP.WPRE, and artificially synthesize the promoter containing the human elongation factor 1α gene The artificial CAR gene encoding the chimeric antigen receptor replaces the GFP gene and the PGK promoter to obtain a chimeric antigen receptor lentiviral vector that can express the chimeric antigen receptor gene for CD19.
Embodiment 2
[0043] [Example 2] Using the three-vector system to package pseudolentiviral particles containing recombinant vectors of artificial genes encoding chimeric antigen receptors in 293T cells
[0044] 293T was cultured in DMEM medium containing 10vol% FBS, and the consumables included DMEM (Gibco, C11995500BT), FBS (Gibco, 10099-141), trypsin (Gibco, 25200-056), Dulbecco's PBS (Hyclone, cat#SH30256. 01), PEI (1 mg / ml, Life Sciences, 23966-2), Opti-MEM (Gibco, 51985-034) and 10 cm cell culture dish (Fisher Scientific, 310109011).
[0045] The preparation process of the pseudolentiviral particles packaging the recombinant vector containing the artificial gene encoding the chimeric antigen receptor is as follows:
[0046] Day 0: Plank
[0047] 24 hours before transfection, the 293FT cells in the logarithmic growth phase were digested with trypsin, the cell density was adjusted with seed medium (DMEM+10% FBS+500 μg / ml G418), and re-seeded in a 10cm cell culture dish at 37°C. 5%CO 2...
Embodiment 3
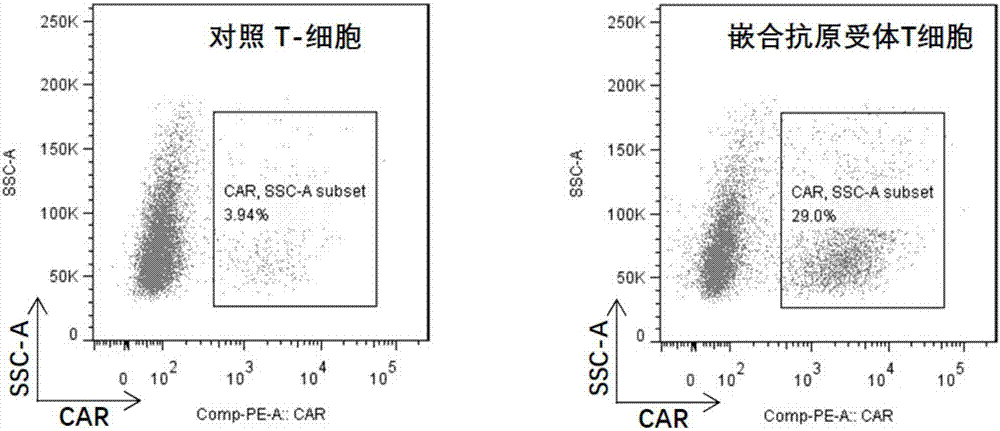
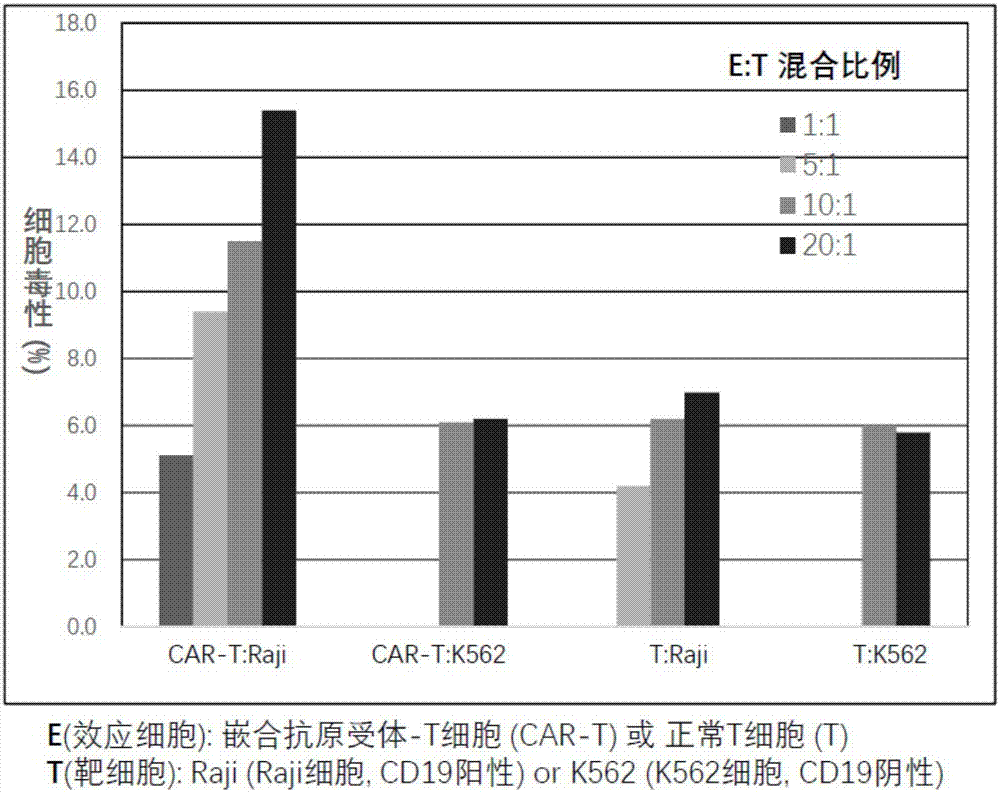
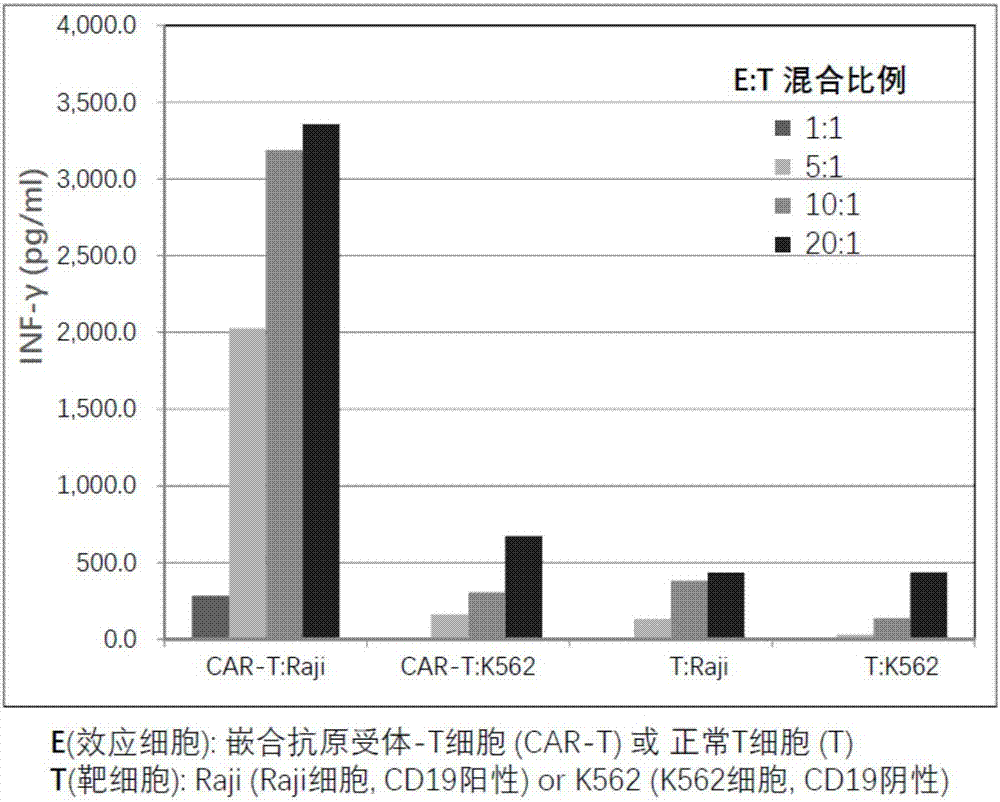
[0060] [Example 3] Preparation of T cells expressing chimeric antigen receptors (referred to as chimeric antigen receptor T cells) using pseudolentiviral particles containing recombinant vectors encoding artificial genes for CD19 chimeric antigen receptors
[0061] (1) Preparation of mononuclear lymphocytes (PBMCs)
[0062] Reagent consumables used include serum-free medium, serum-free freezing solution, PBS, Ficoll lymphocyte separation medium (GE Healthcare #17-5442-03), 5ml, 10ml and 25ml disposable sterile pipettes (Thermo), sterile Pipette tip (QSP), centrifuge tube (Thermo, 339652), cell freezing tube (Thermo, 375418).
[0063] Add the collected patient's autologous peripheral blood into a 50ml sterile centrifuge tube, put it in a horizontal centrifuge, centrifuge at 450g, 20°C for 8 minutes; the peripheral blood in the centrifuge tube will be divided into layers, the upper layer is plasma, and the lower layer is whole blood cells; Take the lower layer of whole blood ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com