Patents
Literature
52 results about "T cell immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The FDA approved CAR (chimeric antigen receptor) T-cell immunotherapy, a treatment in which a patient's T-cells, the soldiers of the immune system, are genetically reprogrammed to find and kill cancer cells. LLS recognized the early promise of this approach. Over more than two decades, LLS provided $40 million in funding...
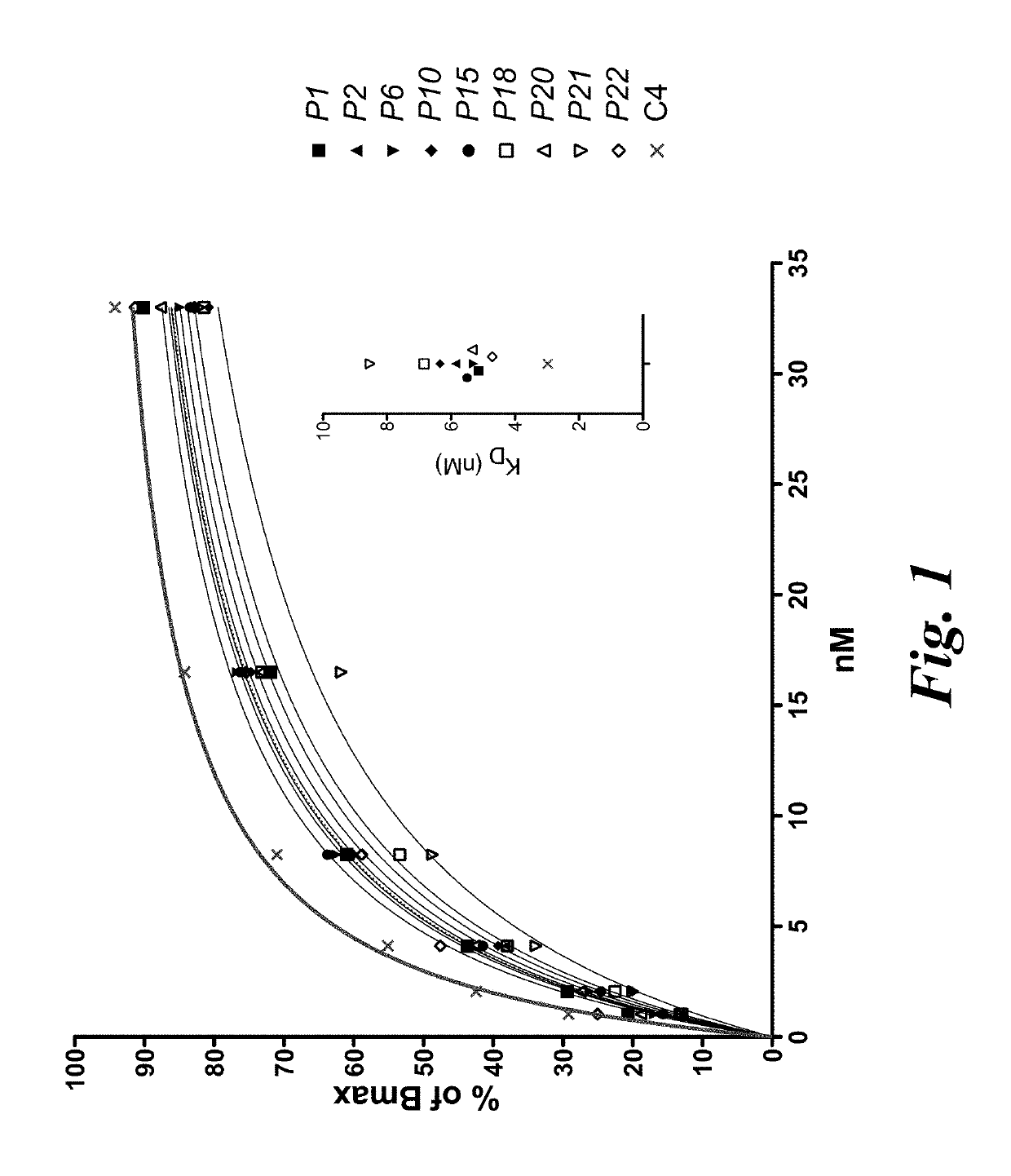
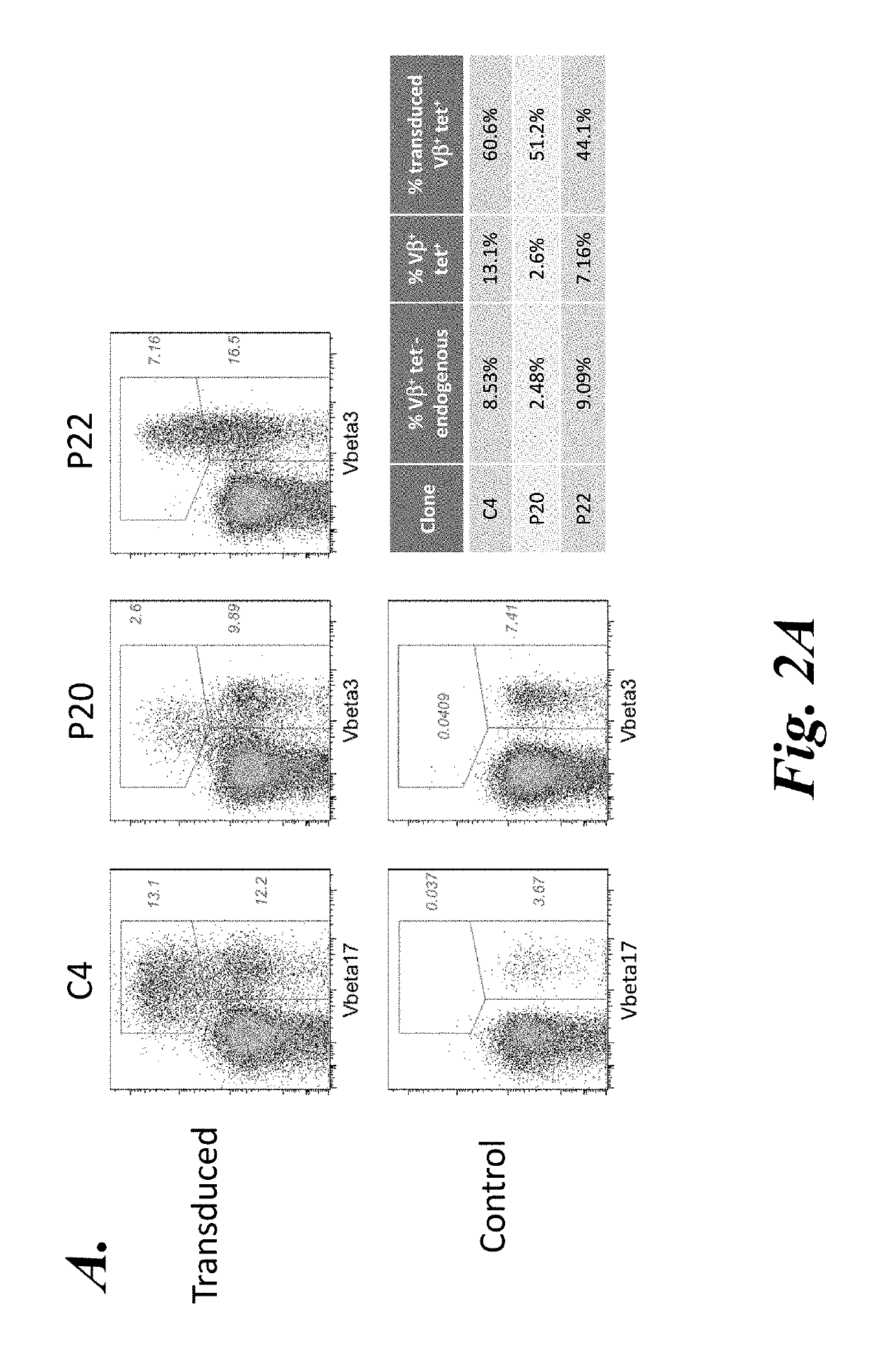
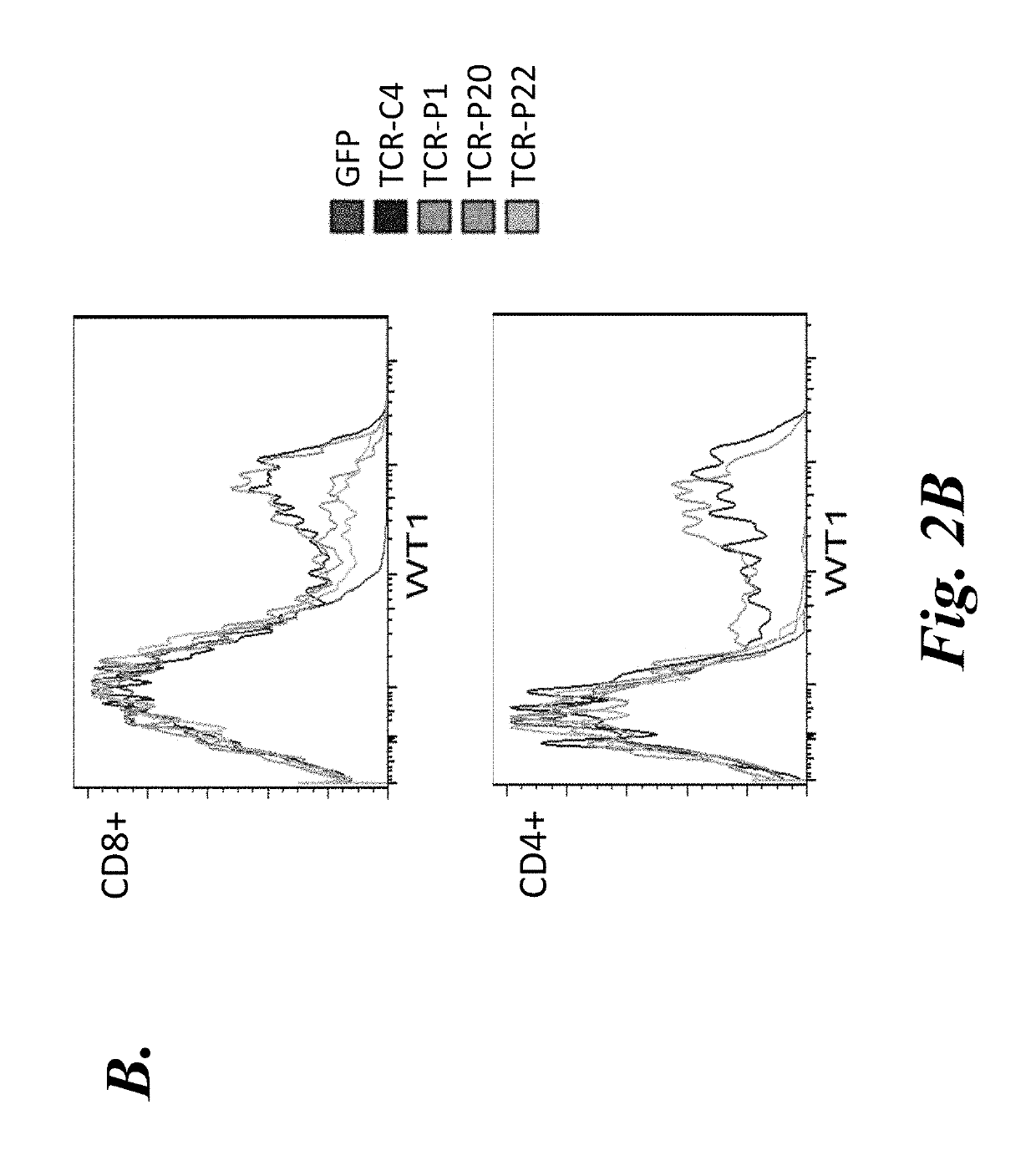
T cell immunotherapy specific for wt-1
The present disclosure provides high affinity and enhanced affinity T cell receptors specific for human Wilms tumor protein 1 (WT-1) epitopes for use in treating diseases or disorders, such as cancer cells that overexpress WT-1.
Owner:FRED HUTCHINSON CANCER CENT
Improved t cell compositions
The invention provides improved T cell compositions and methods for manufacturing T cells. More particularly, the invention provides methods of T cell manufacturing that result in adoptive T cell immunotherapies with improved survival, expansion, and persistence in vivo.
Owner:2SEVENTY BIO INC
Medicine composition and application thereof
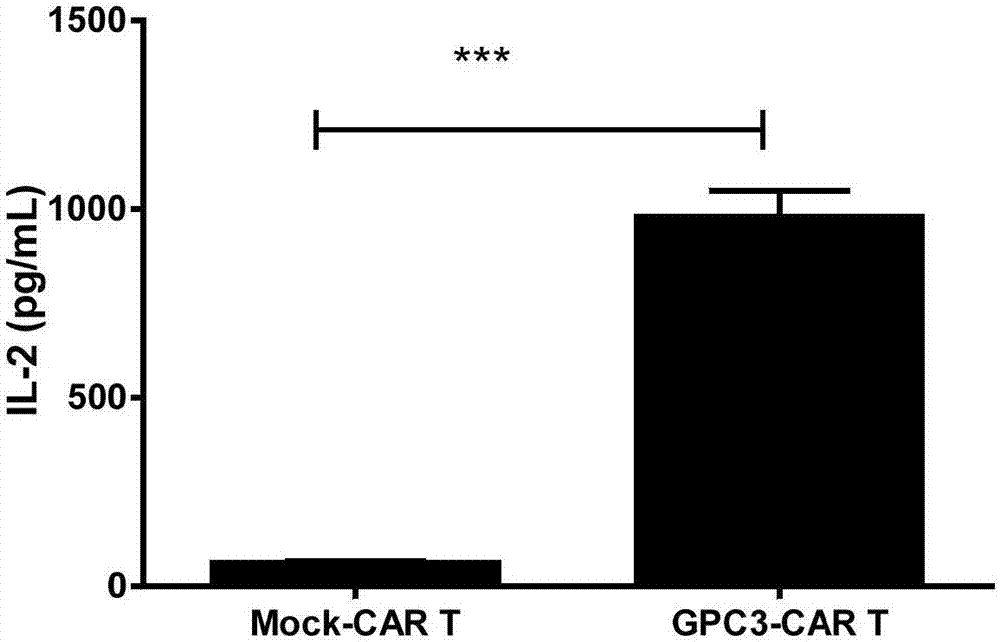
The invention relates to the field of cellular immunotherapy of tumor and in particular to a medicine composition and application thereof. The medicine composition comprises a medicine specifically targeting kidney tumor GPC3, wherein the medicine specifically targeting kidney tumor GPC3 comprises any one or a combination of at least two of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells, an anti-GPC3 antibody, TCR-T (T-lymphocyte Cell Receptor) cells targeting GPC3, and siRNA of DC-CIK (Dendritic Cell-Cytokine-Induced Killer) cells targeting GPC3 or targeting silenced GPC3. Testsshow that GPC3 can be new targeting antigen of nephroblastoma, and by adopting the medicine composition, nephroblastoma can be effectively killed and inhibited.
Owner:SHENZHEN IN VIVO BIOMEDICINE TECH LTD
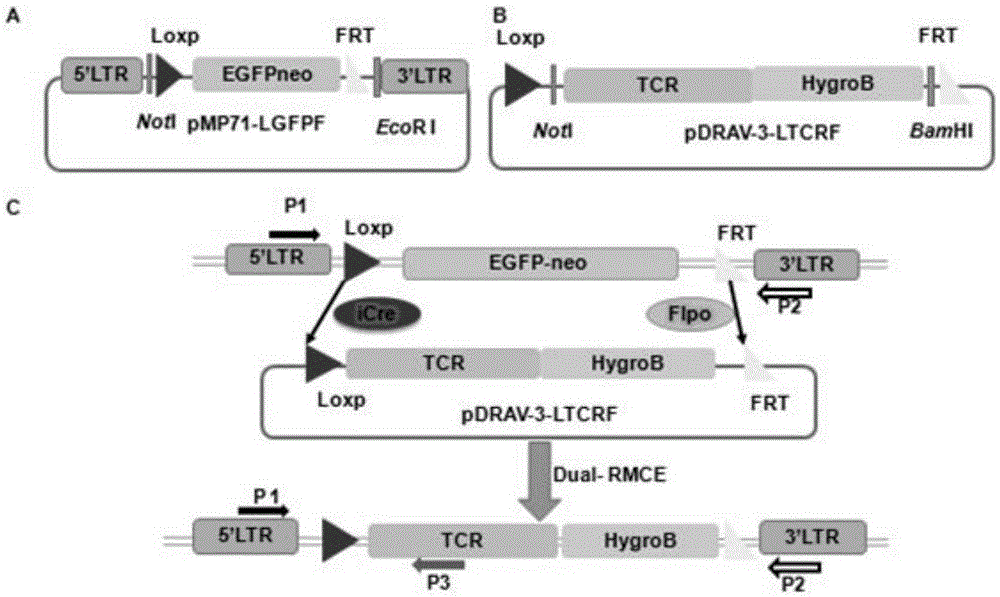
Dual-RMCE-mediated (dual-recombinase mediated cassette exchange-mediated) TCR (T cell receptor) gene replacement system and method
The invention discloses a TCR (T cell receptor) gene replacement system and method mediated by retrovirus gene transfer combining with the Dual-RMCE (dual-recombinase mediated cassette exchange) technology. The TCR gene replacement system comprises a retrovirus integration vector pMP71-LGFPF containing a replacement component Loxp-EGFP-FRT, and a replacement vector pDRAV-3-LTCRF containing TCR target genes. The TCR gene replacement system has the advantages that TCR gene replacement can be performed fast in a single site of a genome, a large amount of tumor antigen specificity T cells can be produced by using the in-vivo and in-vitro cell differentiation technology, and one tumor antigen specificity TCR molecule can be expressed; the problems that retrovirus random integration causes side effects and exogenous TCRs and endogenous TCRs combine to form self-reactive TCRs of traditional TCR gene treatment are solved, and the safe and reliable new technology is provided for clinical anti-tumor T cell immunotherapy.
Owner:JINAN UNIVERSITY
Methods of making T cell compositions
ActiveUS10479975B2Maintaining proliferationNervous disorderAntibody mimetics/scaffoldsImproved survivalIn vivo
The invention provides improved T cell compositions and methods for manufacturing T cells. More particularly, the invention provides methods of T cell manufacturing that result in adoptive T cell immunotherapies with improved survival, expansion, and persistence in vivo.
Owner:2SEVENTY BIO INC
T cell compositions
ActiveUS20190194615A1Maintaining proliferationCell receptors/surface-antigens/surface-determinantsGenetically modified cellsImproved survivalIn vivo
The invention provides improved T cell compositions and methods for manufacturing T cells. More particularly, the invention provides methods of T cell manufacturing that result in adoptive T cell immunotherapies with improved survival, expansion, and persistence in vivo.
Owner:2SEVENTY BIO INC
Anti-CD33 chimeric antigen receptor, coding gene, recombinant expression vector and construction method and application of recombinant expression vector
ActiveCN105820255AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsMammal material medical ingredientsSingle-Chain AntibodiesCD33
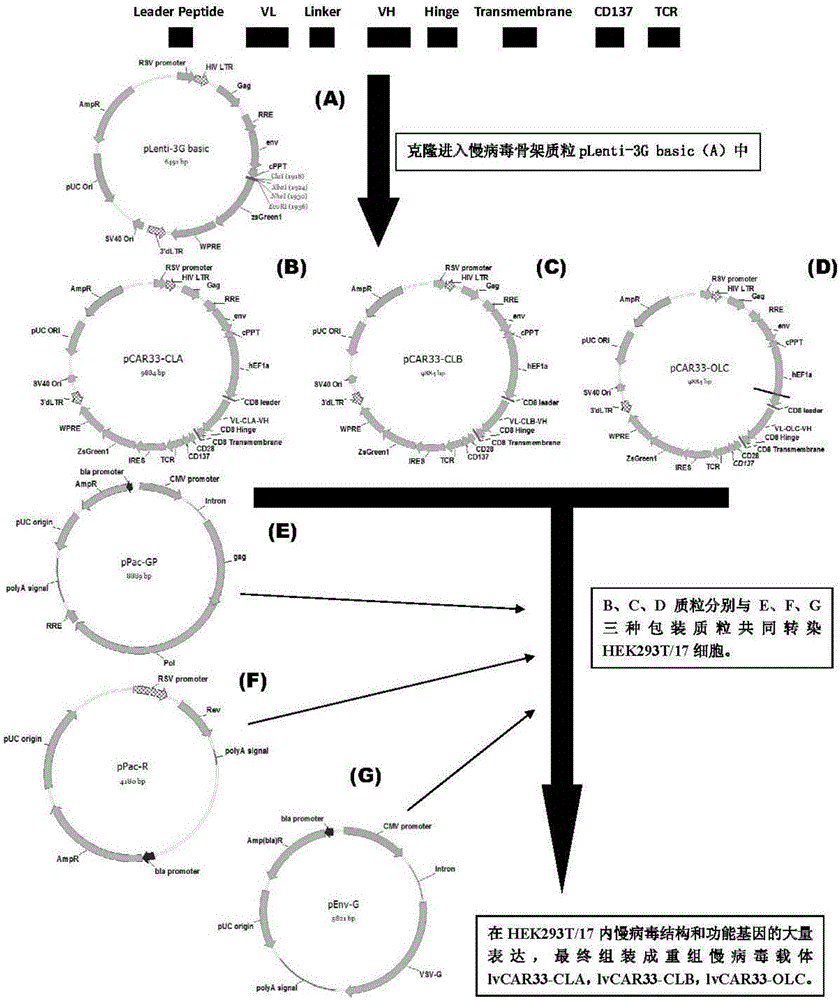
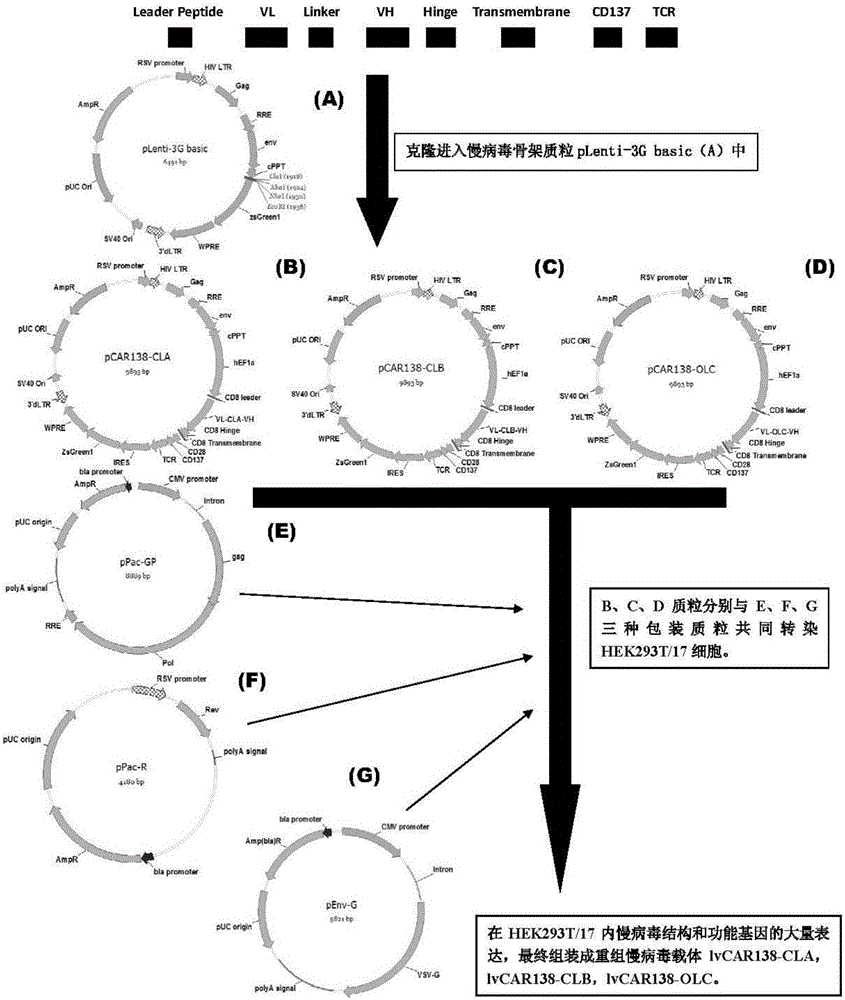
The invention discloses an anti-CD33 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD33 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a heavy chain VL of a CD33 single-chain antibody, an Optimal Linker C, a light chain VH of the CD33 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD33 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD33 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
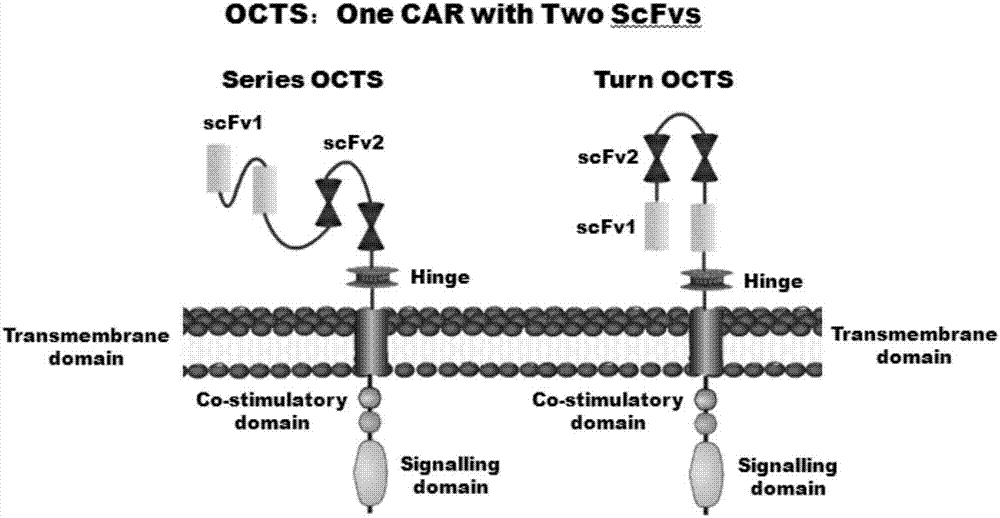
Lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on OCTS (One CAR with Two SeFvs) technology as well as constructing method and application of lymphoblastic leukemia CAR-T therapy carrier
ActiveCN107245500AExpand the scope of recognitionAvoid batch cultureMammal material medical ingredientsNGF-receptor/TNF-receptor superfamilySequence signalCD20
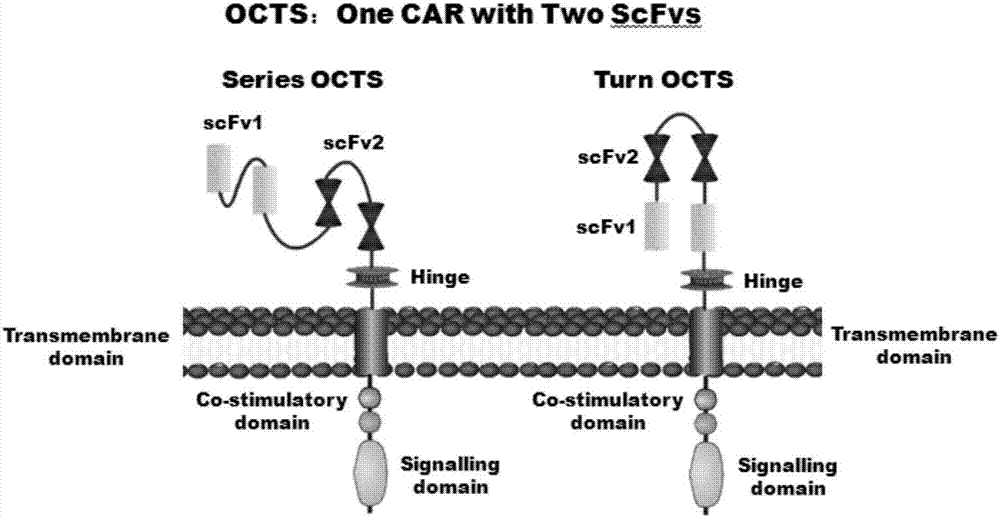
The invention discloses a lymphoblastic leukemia CAR-T (Chimeric Antigen Receptor-T Cell Immunotherapy) therapy carrier based on an OCTS (One CAR with Two SeFvs) technology. The lymphoblastic leukemia CAR-T therapy carrier comprises a lentivirus skeleton plasma, a human EF1alpha promoter, an OCTS chimeric receptor structural domain and an IL6R single-chain antibody, wherein the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide and two groups of single-chain antibodies; the first group of single-chain antibodies is selected from any one of the following four groups of single-chain antibodies: a CD20 single-chain antibody light chain VL and a CD20 single-chain antibody heavy chain VH, a CD22 single-chain antibody light chain VL and a CD22 single-chain antibody heavy chain VH, a CD30 single-chain antibody light chain VL and a CD30 single-chain antibody heavy chain VH, and a CD123 single-chain antibody light chain VL and a CD123 single-chain antibody heavy chain VH; and the second group of the single-chain antibodies is a CD19 single-chain antibody light chain VL and a CD19 single-chain antibody heavy chain VH, an antibody Inner-Linker, a single-chain antibody Inter-Linker, a CD8-Hinge chimeric receptor linker, a CD8 Transmembrane chimeric receptor transmembrane zone, a TCR (T Cell Receptor) chimeric receptor T cell activation domain and a chimeric receptor co-stimulator zone. Besides, the invention discloses a constructing method for the carrier and application of the carrier to preparation of a drug for treating lymphoblastic leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Preparation method of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells, prepared CAR-T cells and application thereof
InactiveCN108103105APromote proliferationHigh proliferation rateGenetically modified cellsMammal material medical ingredientsNucleotideT lymphocyte
The invention discloses a preparation method of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells, the prepared CAR-T cells and application thereof. The preparation method comprises the following steps: S1, separating, activating and amplifying T lymphocytes: after activating separated CD3+CD8+T lymphocytes, culturing and amplifying; S2, infecting the T lymphocytes treated in step S1 bya recombinant slow virus carrying a CAR, so as to prepare the CAR-T cells, wherein a CAR gene carried in the recombinant slow virus carrying the CAR comprises a CD28 intracellular domain and a 4-1BBintracellular domain; nucleotide sequences of the CD28 intracellular domain and the 4-1BB intracellular domain are shown as SEQ ID7 and SEQ ID9 respectively. The CAR-T cells prepared by the method canbe widely applied to treatment of various cancers. Compared with the prior art, the proliferation rate of the prepared CAR-T cells is remarkably improved when compared with CAR-T cells prepared by aconventional method.
Owner:SHENZHEN WINGOR BIO TECH
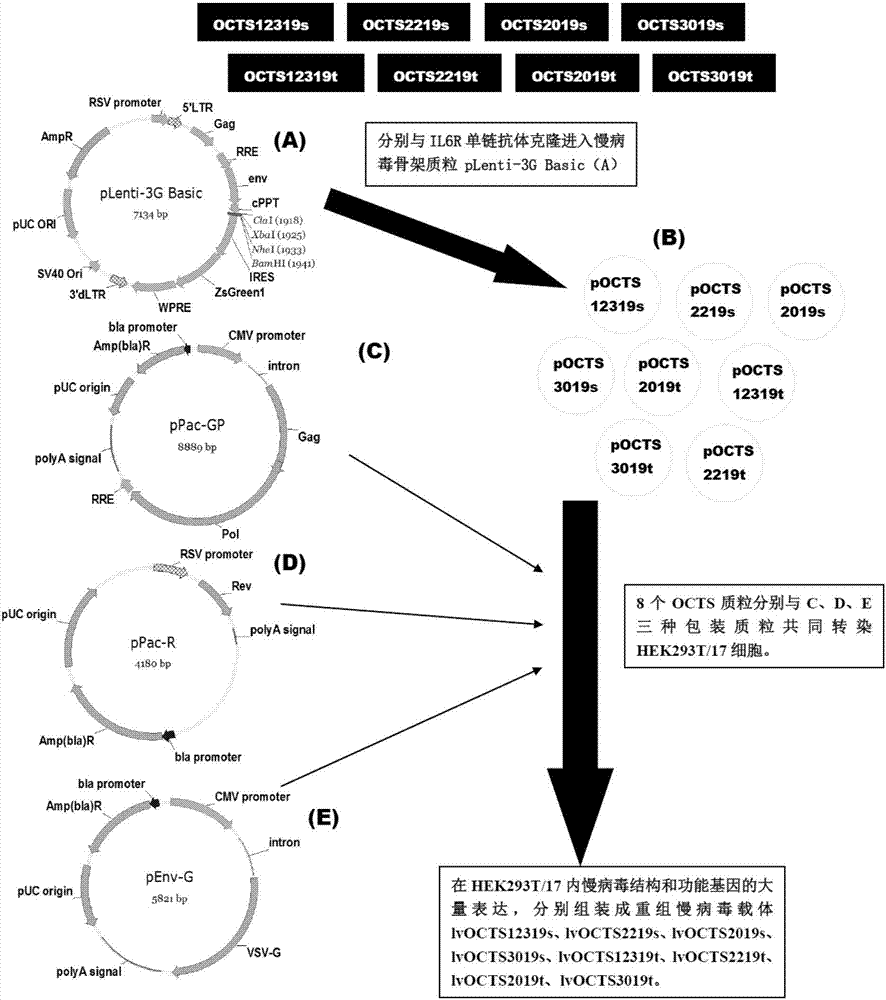
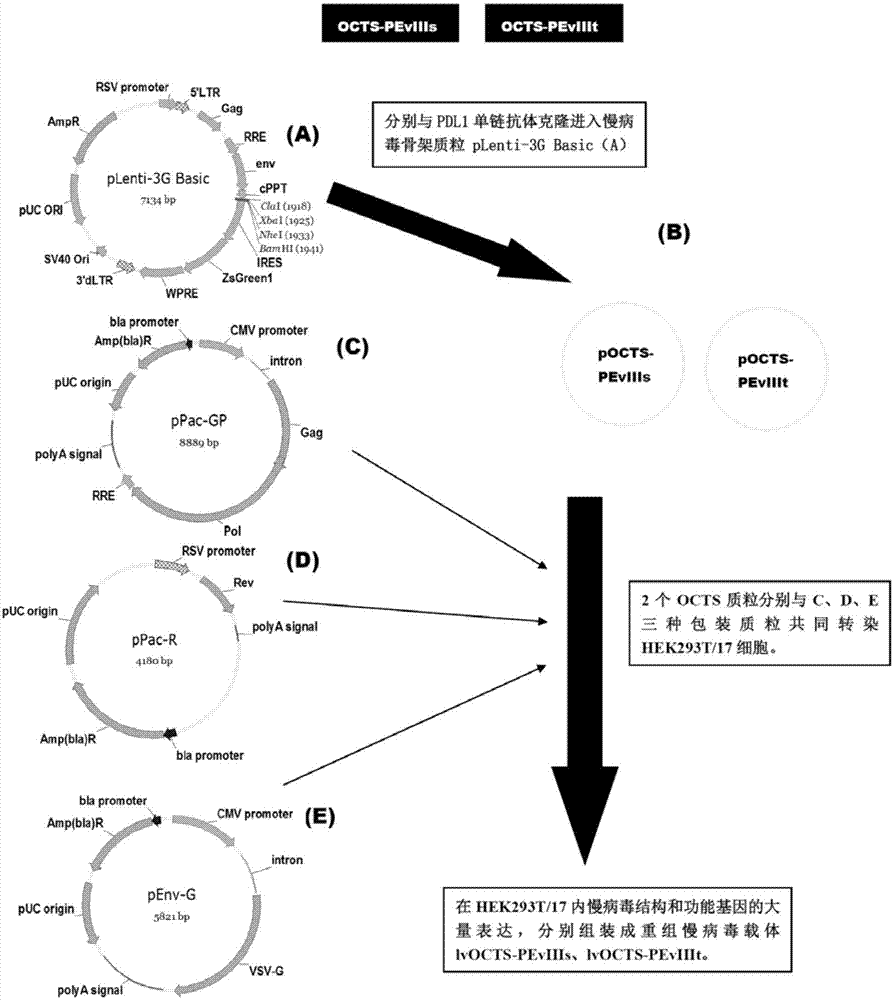
OCTS (One CAR (Chimeric Antigen Receptor) with two ScFvs (Single-chain variable Fragments)) technique based CAR-T (Chimeric Antigen Receptor-T cell immunotherapy) therapeutic vector for glioblastoma and construction method and application thereof
ActiveCN107267555ADirect and activate killingActivate killingVirusesPeptide/protein ingredientsSingle-Chain AntibodiesEukaryotic plasmids
The invention discloses an OCTS (One CAR (Chimeric Antigen Receptor) with two ScFvs (Single-chain variable Fragments)) technique based CAR-T (Chimeric Antigen Receptor-T cell immunotherapy) therapeutic vector for glioblastoma. The OCTS technique based CAR-T therapeutic vector comprises a lentiviral skeleton plasmid, a human EF1 [alpha] promoter (SEQ ID NO. 14), an OCTS chimeric receptor structural domain and a PDL1 single-chain antibody, wherein the OCTS chimeric receptor structural domain comprises a CD8 leader chimeric receptor signal peptide (SEQ ID NO. 15), a PDL1 single-chain antibody light chain VL (SEQ ID NO. 16), a PDL1 single-chain antibody heavy chain VH (SEQ ID NO. 17), an EGFRvIII single-chain antibody light chain VL (SEQ ID NO. 18), an EGFRvIII single-chain antibody heavy chain VH (SEQ ID NO. 19), an antibody Inner-Linker (SEQ ID NO. 20), a single-chain antibody Inter-Linker (SEQ ID NO. 21), a CD8 Hinge chimeric receptor linker (SEQ ID NO. 22), a CD8 Transmembrane chimeric receptor transmembrane domain (SEQ ID NO. 23), a TCR (T Cell Receptor) chimeric receptor T cell activation domain (SEQ ID NO. 26) and a chimeric receptor co-stimulator domain. In addition, the invention also discloses a construction method of the vector and application of the vector to the preparation of a medicine for treating the glioblastoma.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Anti-CD138 chimeric antigen receptor, coding gene, recombinant expression vector and construction method and application of recombinant expression vector
ActiveCN105820254AImprove in vitro killing effectGood clinical effectVirusesAntibody mimetics/scaffoldsSequence signalSingle-Chain Antibodies
The invention discloses an anti-CD138 chimeric antigen receptor, a coding gene, a recombinant expression vector and a construction method and an application of the recombinant expression vector. The anti-CD138 chimeric antigen receptor comprises a CD8leader chimeric receptor signal peptide, a light chain VH of the CD138 single-chain antibody, an Optimal Linker C, a heavy chain VL of a CD138 single-chain antibody, a CD8Hinge chimeric receptor hinge, a CD8Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulation factor and a TCR chimeric receptor T-cell activation domain, which are serially connected in sequence. In addition, the invention discloses the coding gene of the anti-CD138 chimeric antigen receptor, the recombinant expression vector and the construction method and the application of the recombinant expression vector. According to the anti-CD138 chimeric antigen receptor, the secretion of cell factors and the in vitro killing effect of CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy) cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
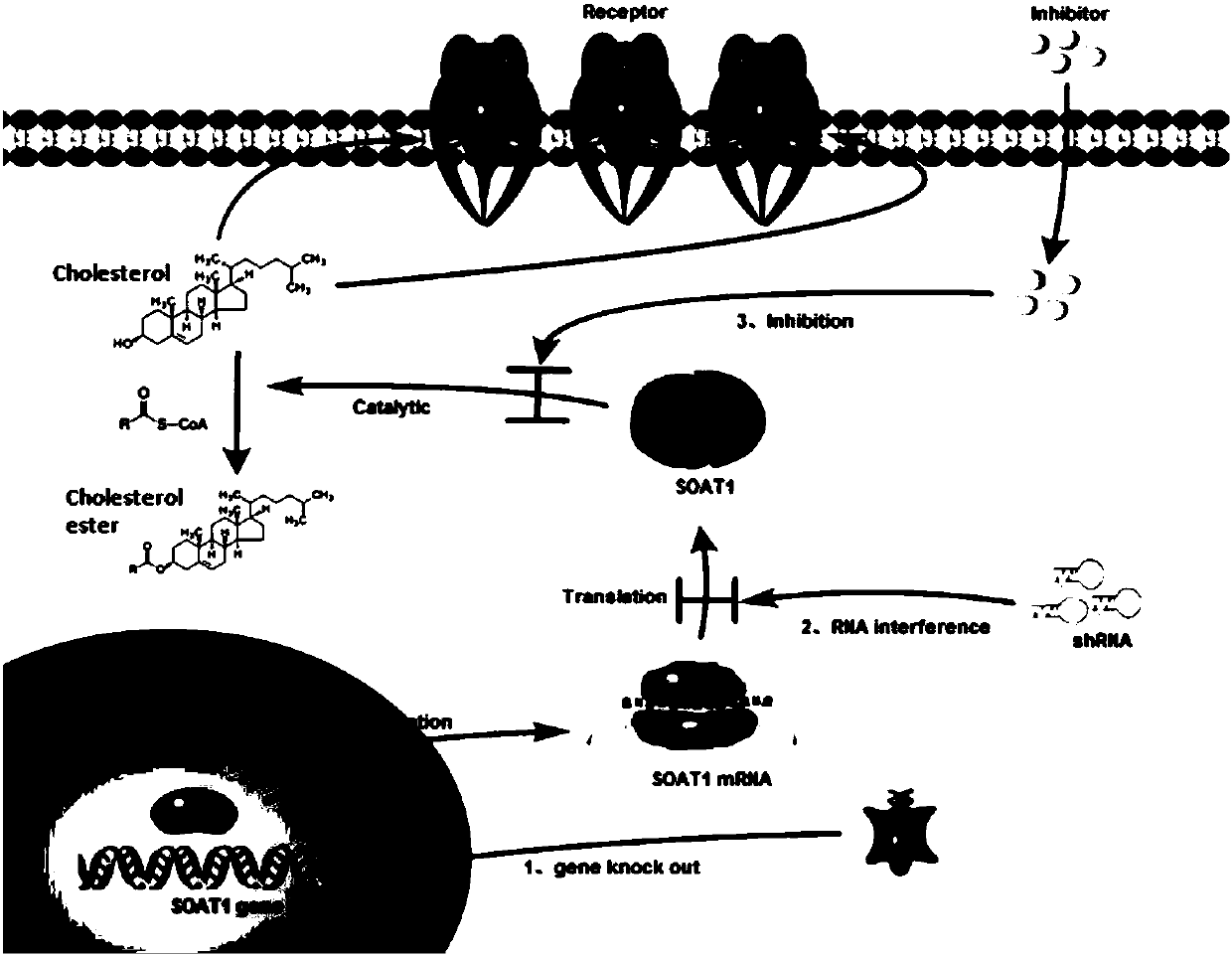
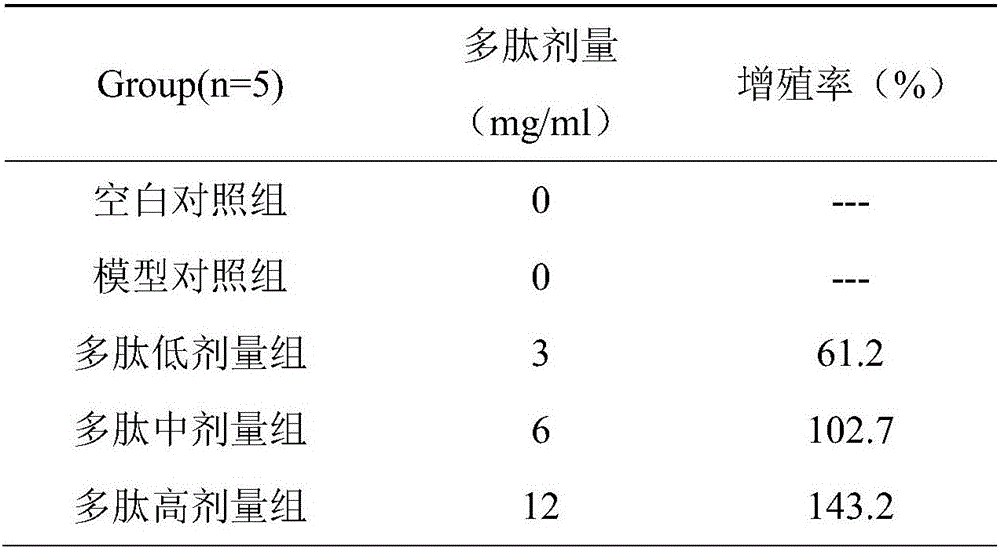
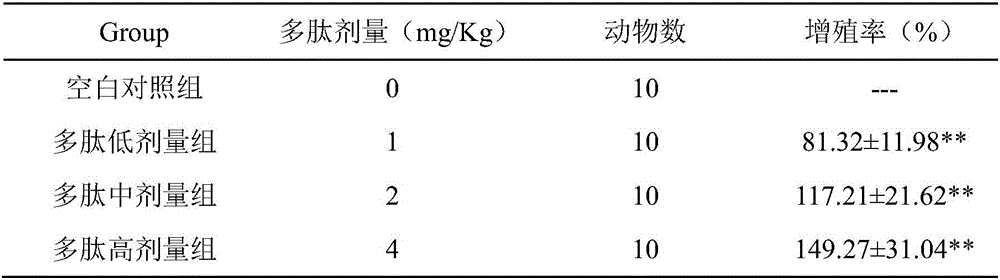
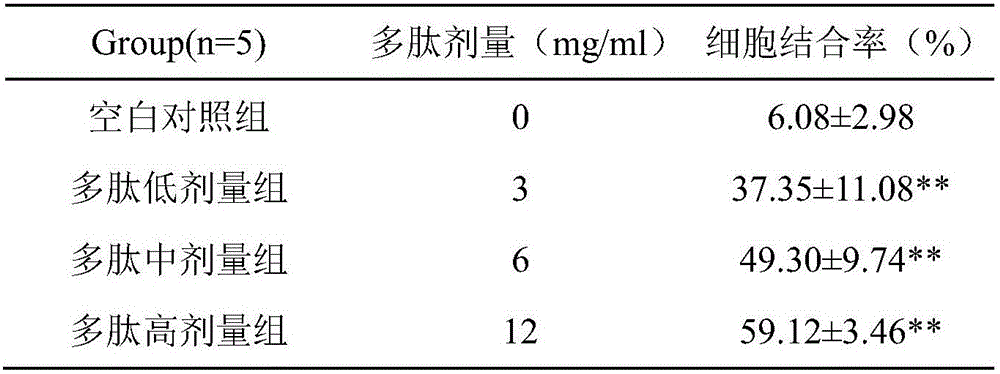
CAR-T cells with inhibited sterol o-acyltransferase 1 (SOAT1) as well as preparation method and application of CAR-T (Chimeric Antigen Receptor-T-cell Immunotherapy) cells
ActiveCN107058232ALimits the rate of conversion to cholesteryl estersIncrease contentImmunoglobulin superfamilyGenetically modified cellsCholesterolGene Knock-Down
The invention discloses CAR-T cells with inhibited sterol o-acyltransferase 1 (SOAT1). The CAR-T cells with inhibited SOAT1 comprise the following cells: a T cell subjected to SOAT1 gene knockout at DNA level and for expression of a hCAR19 receptor, a T cell subjected to SOAT1 gene knock-down at mRNA level and for expression of a hCAR19 receptor, and a T cell subjected to SOAT1 gene inhibiting effect by an inhibitor at protein level and for expression of a hCAR19 receptor. The invention also discloses a preparation method of the CAR-T cells with inhibited SOAT1 and application of the CAR-T cells in preparation of medicine for treating cells of tumors. A series of pre-clinical experiments prove that the CAR-T cells with inhibited SOAT1 have killing ability superior to that of CAR-T cells and have an extremely high application value in treatment of the cells of the tumors.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
CD19 immunogen polypeptide and application thereof
InactiveCN106008695AGrowth inhibitionEnhance immune responseImmunoglobulin superfamilyCancer antigen ingredientsCD8T cell immunity
The invention discloses a CD19 immunogen polypeptide, belongs to the field of anticancer vaccines, and particularly relates to an optimized polypeptide of CD19 immunogen used as an anticancer vaccine. The polypeptide can improve T cell immunologic response. The polypeptide has a brand new amino acid sequence and can be applied to preparation of tumor drugs, especially drugs for improving T cell immunologic response for tumor immunology cells, as well as drugs for CAR-T cell immunotherapy. The polypeptide has the advantages that the new sequence is adopted, the polypeptide can be applied to immunogen in the tumor immunology cell technology to promote T cell and CD8+ l immunologic response, to be effectively and specifically combined with MHC on the surfaces of T cells, to promote combination of T cells and tumor cells, and to inhibit the growth of tumor in tumor-bearing mice, and the polypeptide can be applied to CAR-T cell immunotherapy and the like as a target molecule of cell therapy.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
T cell immunotherapy specific for WT-1
The present disclosure provides high affinity and enhanced affinity T cell receptors specific for human Wilms tumor protein 1 (WT-1) epitopes for use in treating diseases or disorders, such as cancer cells that overexpress WT-1.
Owner:FRED HUTCHINSON CANCER CENT
Immune cell with anti-tumor function and application thereof
ActiveCN106754723AGrowth inhibitionAvoid damageGenetically modified cellsMammal material medical ingredientsAntigenSide effect
The invention belongs to the field of medical biotechnology, and particularly relates to a genetically modified immune cell with a malignant solid tumor treating function and application thereof. The immune cell is a specific immune cell which is subjected to gene modification so as to over-express lipid metabolism-related genes. The tumor growth inhibiting capacity of the immune cell is realized by reducing accumulation of lipid droplets of the immune cell itself in a tumor microenvironment, reducing expression of tumor promotion genes / protein, and improving the phagocytotic ability, antigen presentation ability and tumor killing ability of the immune cell. The immune cell is an in-vitro NKT cell, a DC cell, a macrophage, a monocyte, a granulocyte or a T cell. Overexpression of related metabolism regulation genes enables the anti-tumor capacity of the immune cell to be improved remarkably. Compared with a chimeric antigen receptor T cell immunotherapy (CAR-T) which receives much attention currently, toxic and side effects are small, cytokine storm can not be caused, and clinical requirements can be met.
Owner:NANJING UNIV
Preparation method and application of programmed death receptor 1 antibody magnetic bead
InactiveCN108503710AGuarantee structureOptimizing the Coupling ProtocolBlood/immune system cellsCarrier-bound/immobilised peptidesBiotechnology researchMagnetic bead
The invention discloses a preparation method of a programmed death receptor 1 (PD-1) antibody magnetic bead. The preparation method comprises the following steps: respectively coupling 14 anti-human PD-1 monoclonal antibodies with a carboxyl magnetic bead to prepare a PD-1 antibody magnetic bead, wherein the 14 anti-human PD-1 monoclonal antibodies are from the Canada YES company biotechnology research limited company, and the clone numbers of the 14 anti-human PD-1 monoclonal antibodies are respectively 7E5, 1H8, 1C4, 9A5, 9B4, 7C9, 3E5, 7G12, 9E11, 5B2, 5H7, 2H7, B1C4 and 2C4. The inventionalso discloses the application of the above PD-1 antibody magnetic bead in the field of CIK (cytokine induced killer), CART (Chimeric Antigen Receptor T-Cell Immunotherapy) and TIL (tumor infiltrationlymphocyte) immunotherapy for rejecting PD-1 positive cells. According to the preparation method disclosed by the invention, a coupling scheme of antibodies and magnetic beds is optimized, so that the coupling amount of the antibody on the surface of the magnetic bead can achieve 1ml of magnetic bead+1mg of antibody, and a fluorescence signal on the surface of the magnetic bead is enhanced afterthe antibody is coupled. The antibody magnetic bead prepared with the preparation method can be used for capturing PD-1 positive cells.
Owner:XUZHOU YES BIOTECH LAB
T cell receptor and application thereof
ActiveCN114213527APowerful killing effectImmunoglobulin superfamilyGenetically modified cellsDiseaseDendritic cell
The invention discloses a T cell receptor (TCR) and an application thereof, and the TCR can be specifically combined with a CT83 antigen short peptide CT8314-22 / HLA-A * 11: 01 compound; t cells transduced with the TCR can be specifically activated by dendritic cells expressing a CT8314-22 / HLA-A * 11: 01 compound, and have a powerful killing effect on target cells (such as cancer cells) positive to CT83 and HLA-A * 11: 01; the invention provides a new way for TCR-T cell immunotherapy of CT83 cancer testis antigen related diseases.
Owner:特赛免疫(广州)科技有限公司
Cell subset for immunotherapy of primary hepatocellular carcinoma and preparation method thereof
ActiveCN110484504AImprove tumor killing abilityGood effectBlood/immune system cellsCell culture active agentsAbnormal tissue growthCD137
The invention provides a cell subset for immunotherapy of primary hepatocellular carcinoma, namely a CD137 + CD3 + CD8 + CD45RO + T cell subset. The invention also provides a preparation method of theabove cell subset. The method comprises the following steps: firstly labeling a T cell subset in tumor tissues with a specific marker, and then carrying out flow cell sorting on the CD137 + CD3 + CD8+ CD45RO + T cell subset, and carrying out in-vitro expansion culture to obtain the cell subset for immunotherapy of primary hepatocellular carcinoma. The cell subset of the invention has stronger tumor-killing effect, anti-apoptosis capacity and multiplication capacity, and can survive in vivo for a long time. The invention aims to provide effective candidate cell populations for immunotherapy of liver cancer, especially T cell immunotherapy of tumor infiltration, and provide new ideas for treatment of other tumors.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
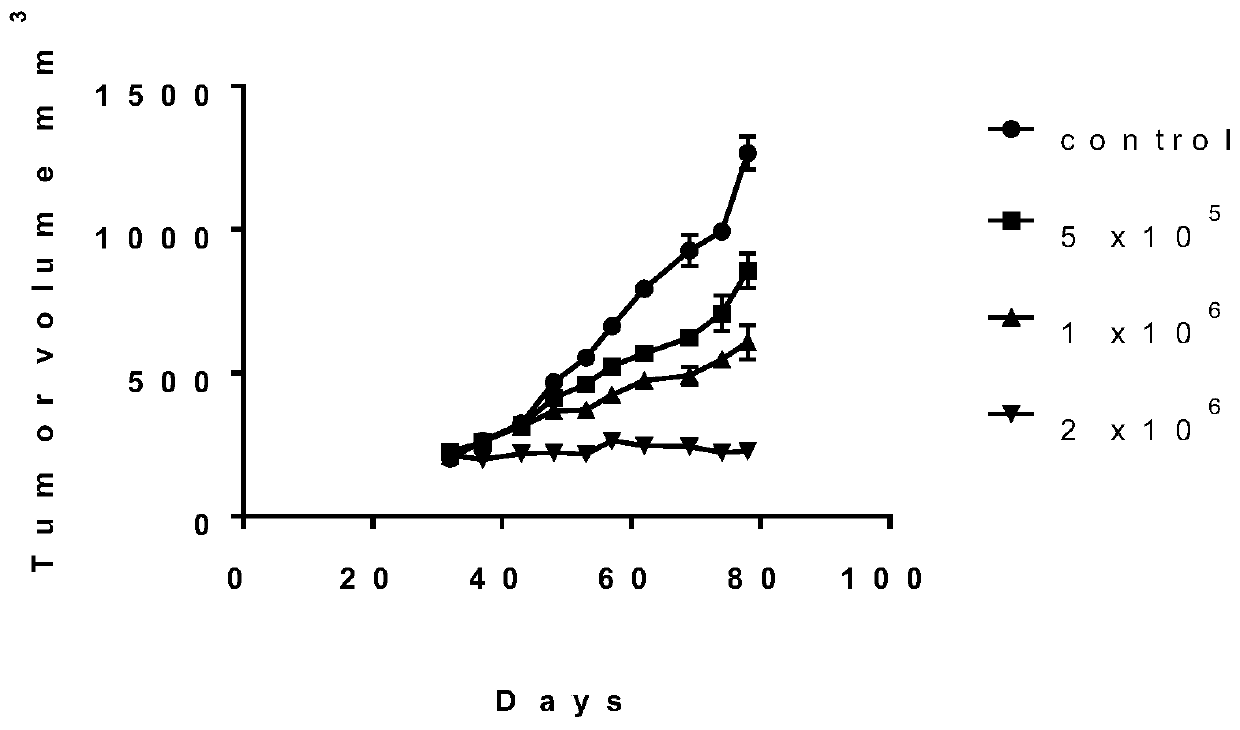
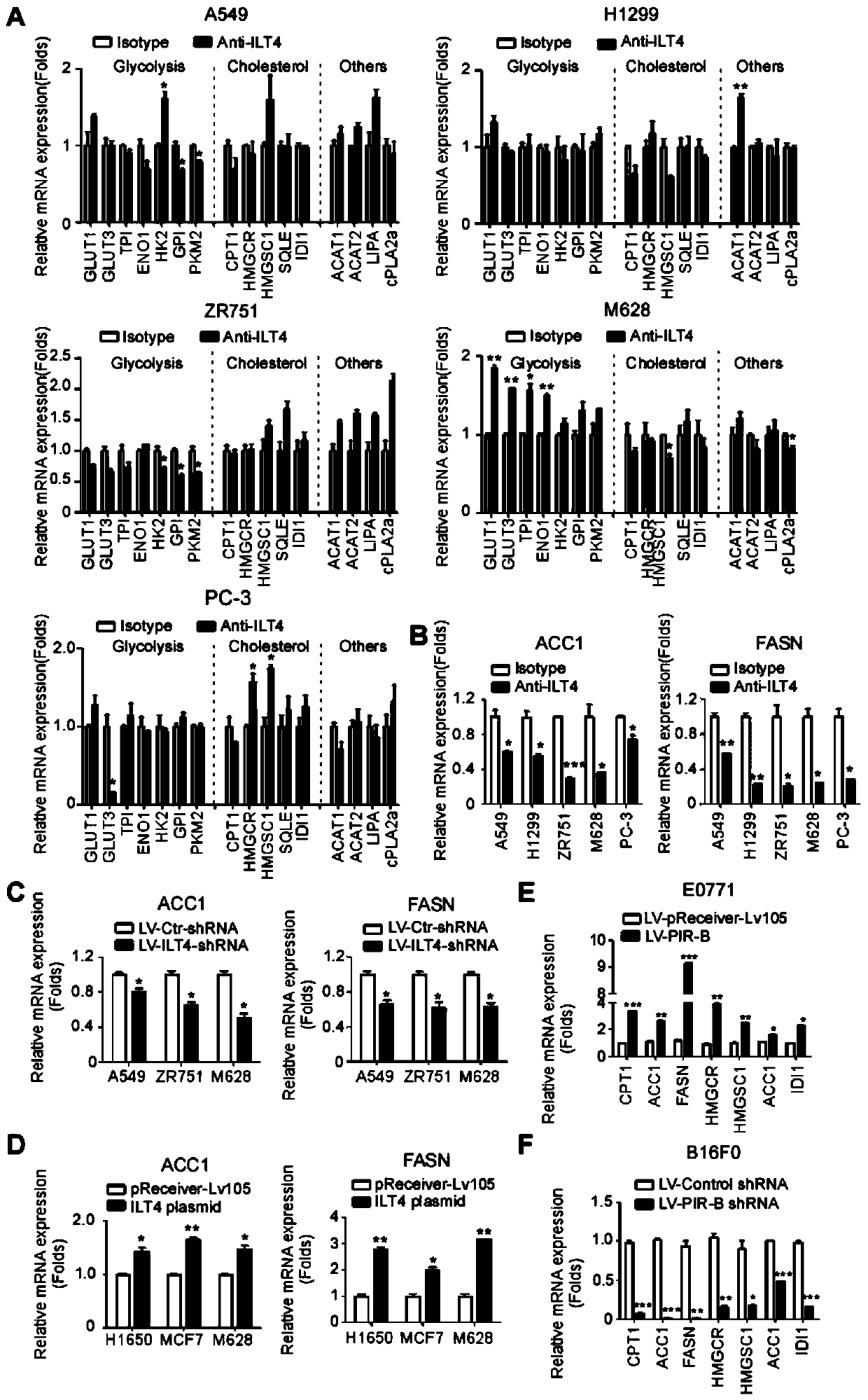
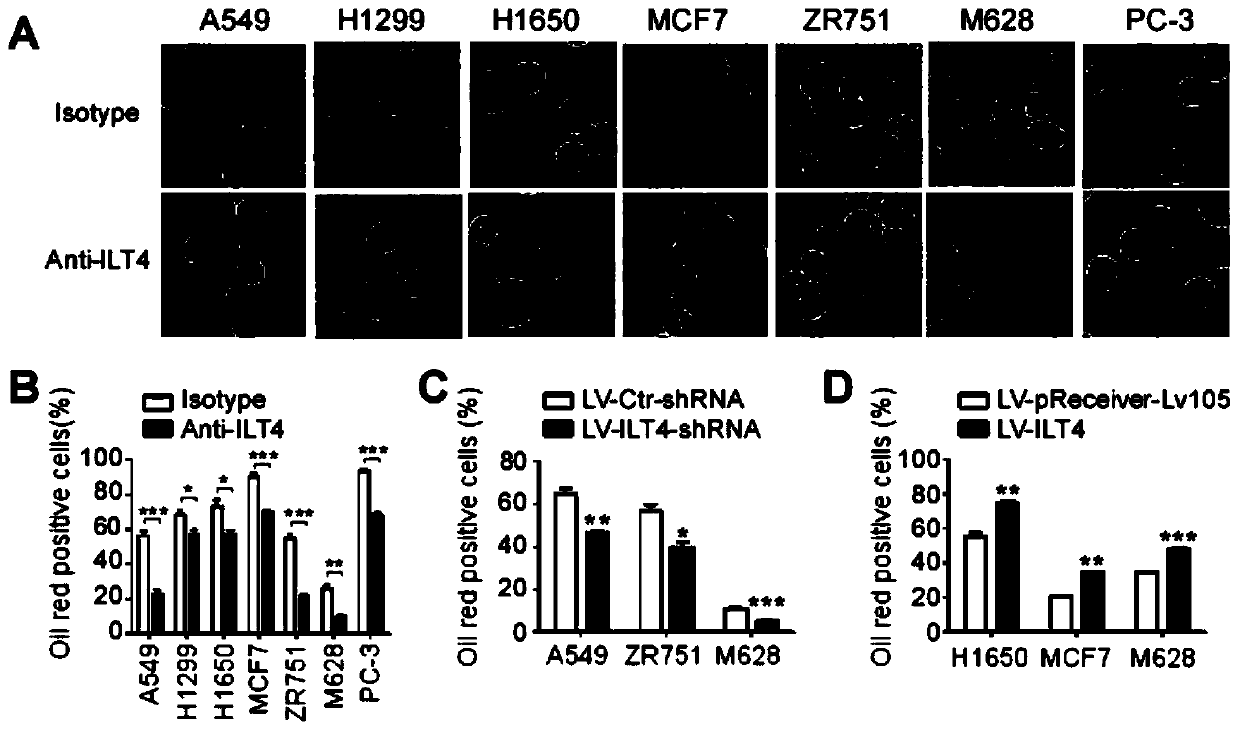
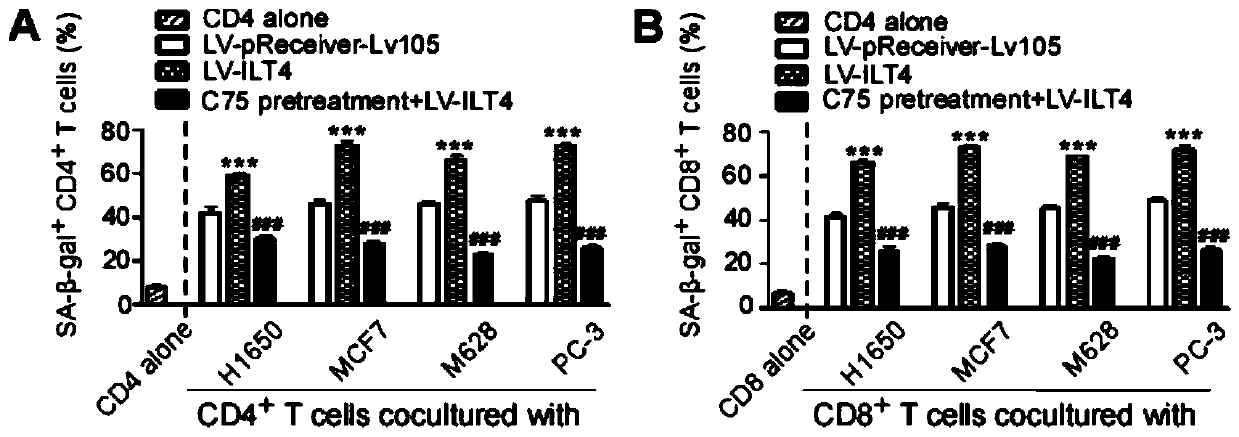
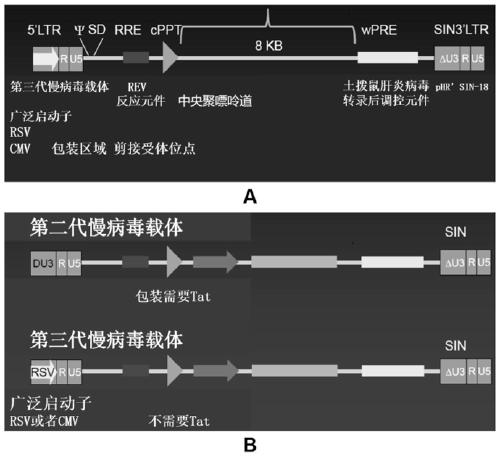
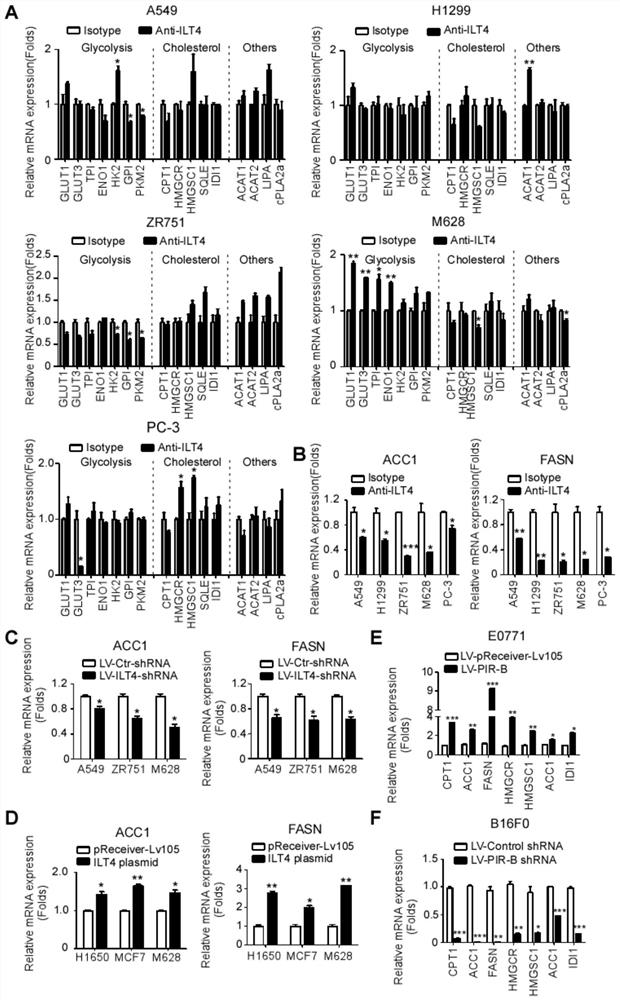
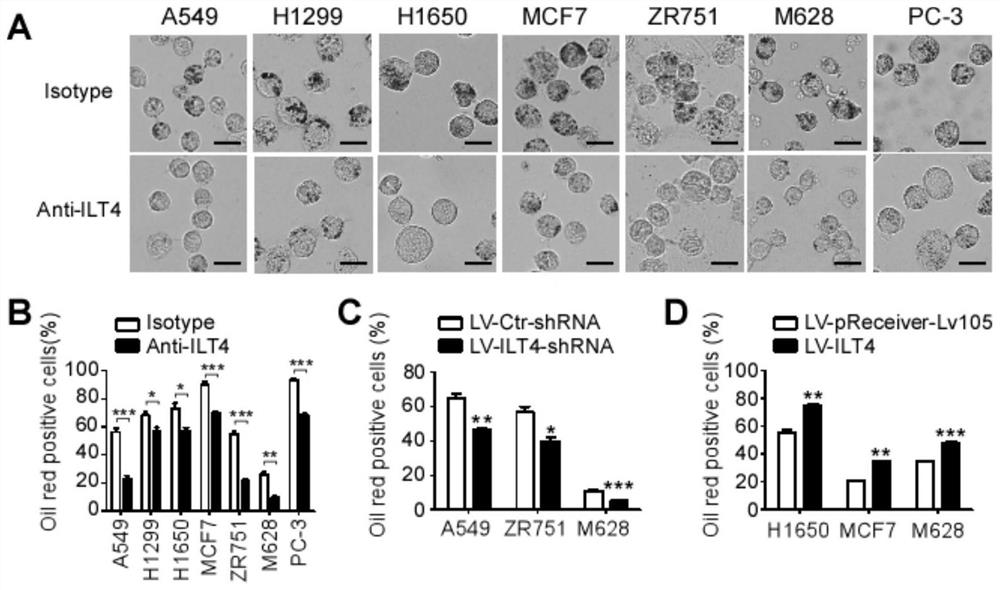
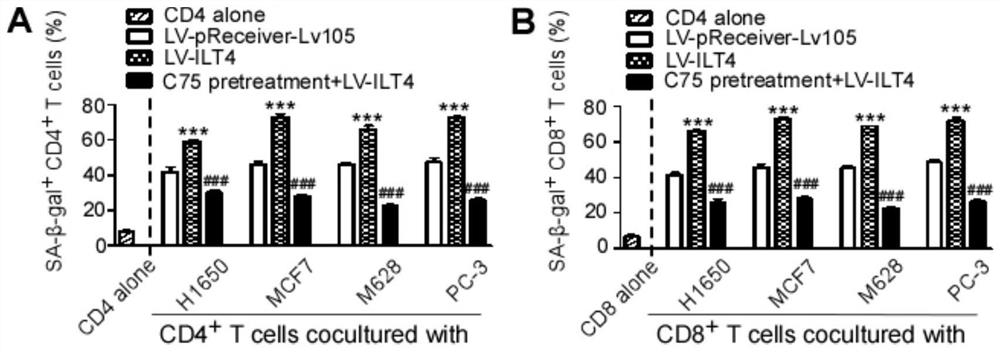
Application of blocking tumor-derived ILT4 in adoptive T cell therapy
ActiveCN110917356AHigh benefitIncrease lethalityImmunological disordersAntineoplastic agentsMelanomaOncology
The invention provides an application of blocking tumor-derived ILT4 in adoptive T cell therapy. Researches find that the tumor-derived ILT4 has an induction effect on microenvironment T cell aging, molecular and metabolic mechanisms of the tumor-derived ILT4 are explored in detail, and it is proved from a preclinical model that the targeted ILT4 can reverse the immunosuppressive microenvironmentof tumors and participate in targeted therapy of malignant tumors, and a B6 mouse malignant melanoma adoptive T cell immunotherapy model is further constructed, and the feasibility of ILT4 blocking combined ACT therapy is studied. An effective means is provided for overcoming ACT treatment drug resistance.
Owner:JINAN CENTER HOSPITAL
B and T lymphocyte attenuator immunogen polypeptide and application thereof
InactiveCN105968189AGrowth inhibitionEnhance specific bindingCell receptors/surface-antigens/surface-determinantsVertebrate antigen ingredientsT lymphocyteWilms' tumor
The invention discloses a B and T lymphocyte attenuator immunogen polypeptide, belongs to the field of anti-cancer vaccines, and particularly relates to optimized polypeptide of B and T lymphocyte attenuator immunogen serving as an anti-cancer vaccine, and T cell immune responses are enhanced. The amino acid sequence is a brand-new sequence. The invention relates to application of the polypeptide in preparation of drugs for treating tumors, in particular to application in preparation of drugs which are used for tumor immune cells and used for enhancing the T cell immune responses and application in preparation of drugs for CAR-T cell immunotherapy. The B and T lymphocyte attenuator immunogen polypeptide has the advantages that the immunogen polypeptide has the brand-new amino acid sequence, can be used in tumor immune cell technologies, can promote T cell proliferation, can promote specific binding of T cells and tumor surface antigens, can inhibit tumor growth in tumor-burdened mouse bodies and is applied to cell therapies of CAR-T and the like by serving as target molecules of the cell therapies.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Universal PCR primer combination for detecting chimeric antigen receptor genes, and application thereof
InactiveCN109722472AStrong specificityHigh sensitivityMicrobiological testing/measurementSignalling moleculesAntigen receptors
The present application discloses a universal PCR primer combination for detecting chimeric antigen receptor genes, and an application thereof. The primer combination comprises a primer pair for specifically detecting the chimeric antigen receptor genes, sequences of the primer pair are shown in SEQ ID No.1-2, chimeric antigen receptors comprise a costimulatory signal molecule ligand CD3-zeta, anda target of the primer pair is located on a coding gene of the costimulatory signal molecule ligand CD3-zeta. The primer pair provided by the present application has advantages of good specificity, high sensitivity and high accuracy, can be widely applied to qualitative and quantitative detections of one or more of the chimeric antigen receptor genes, and has important application values in the fields of T cell immunotherapy of CAR-T cell quality control, companion diagnostics and detection of CAR-T in blood during a treatment process of clinical patients using one or more of the CAR-T, etc.
Owner:BIORAY LABORATORIES INC
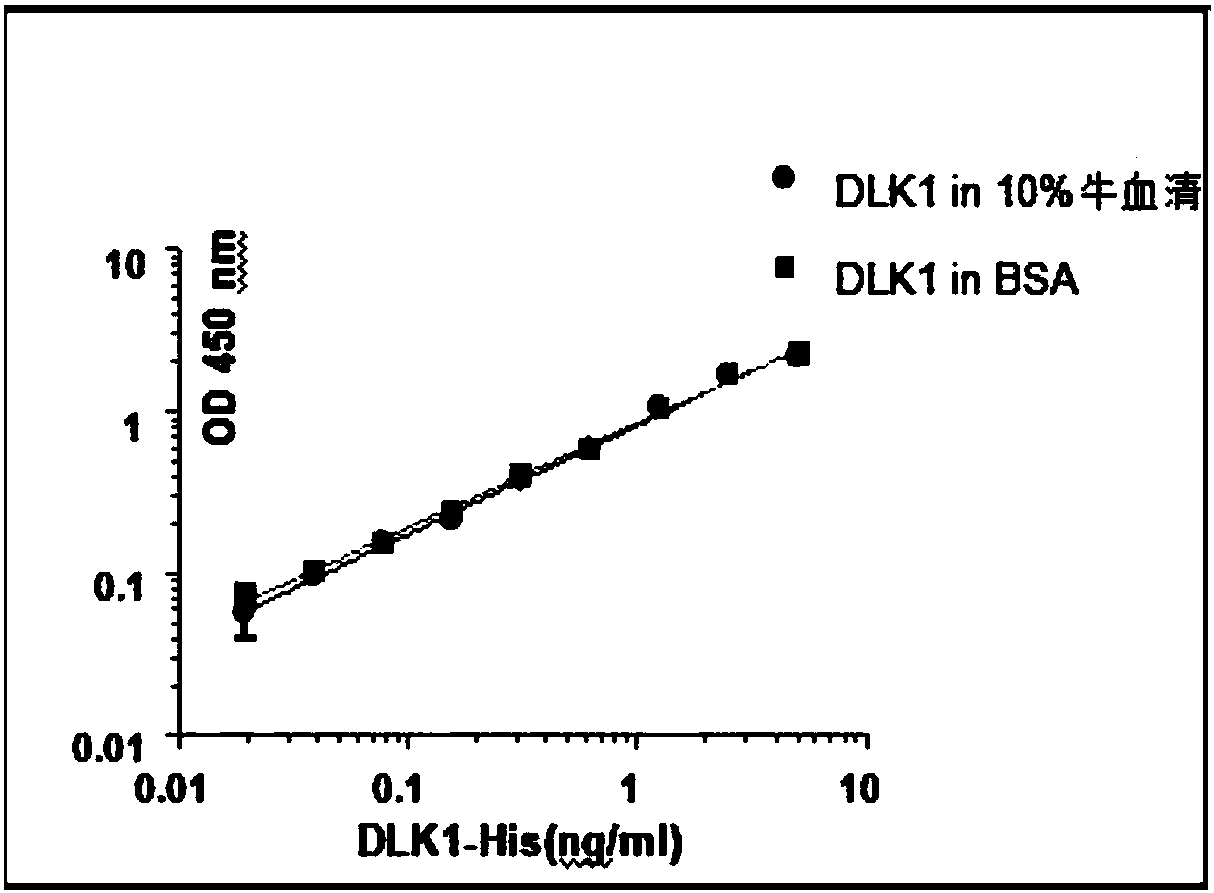
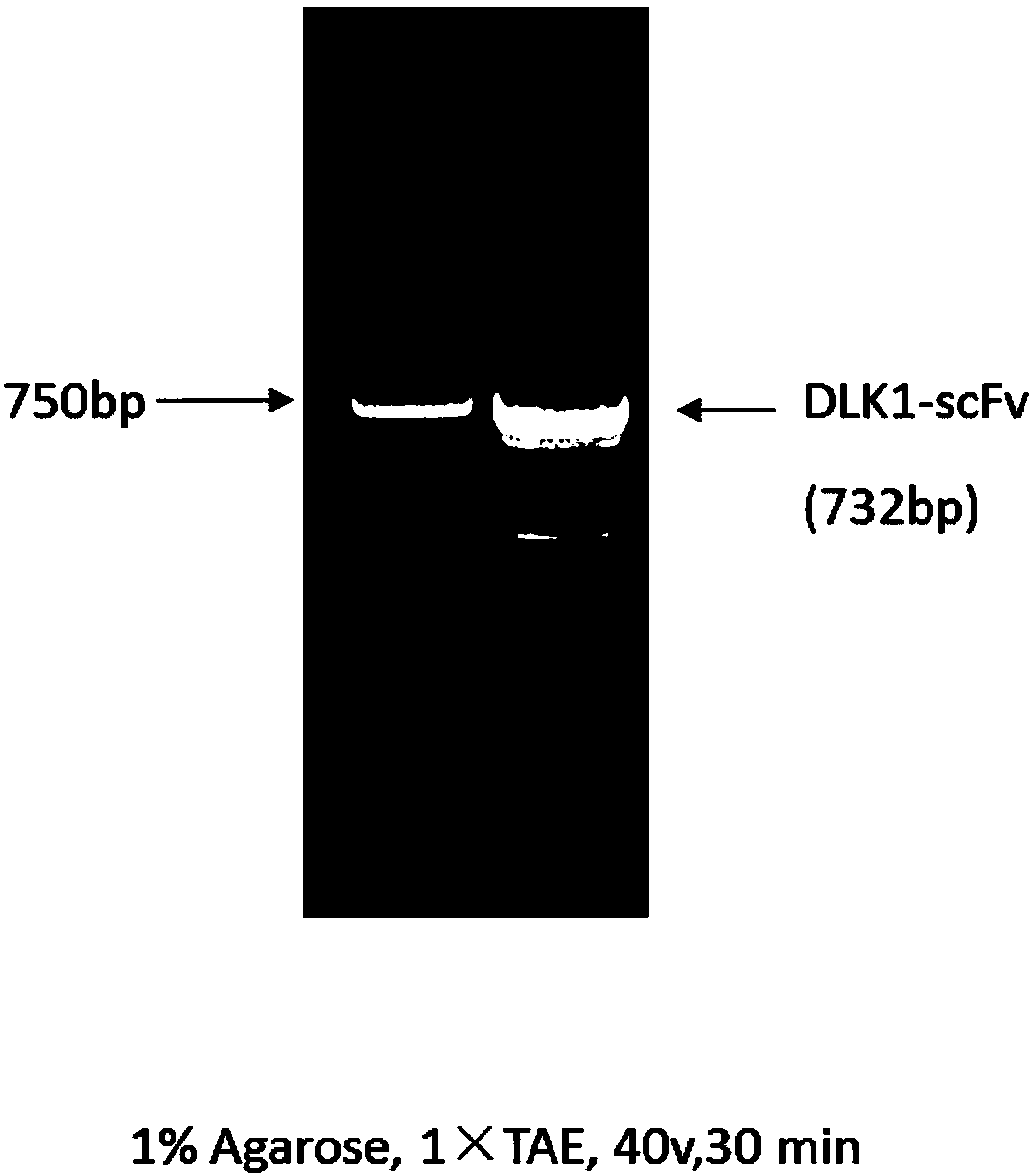
CAR-T construction method with antigens DLK1 related to liver cancers as targets
ActiveCN108456662AImprove enduranceLittle side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsAntigenSingle-Chain Antibodies
The invention discloses a CAR-T construction method with antigens DLK1 related to liver cancers as targets. According to the method, single-chain antibodies of the antigens DLK1 related to the liver cancers are selected to be recombined into T cells, the genetically-engineered T cells are further activated, and therefore the lethality of the T cells on liver cancer cells is improved; according tothe single-chain antibodies of DLK1, RNA is obtained from a hybridoma cell line which secretes monoclonal antibodies of DLK1, and then cDNA is obtained after reverse transcription; PCR primers for a heavy-chain variable area and a light-chain variable area are designed; with cDNA as a template, the single-chain antibodies of DLK1 are obtained by amplifying and splicing the heavy-chain and light-chain variable areas. A detection method has relative sensitivity and specificity, and the side effects of recognizing normal cells by the T cells can be reduced. The CAR-T construction method providesa certain research basis for a CAR-T cell immunotherapy in the aspect of the immunotherapy of the liver cancers.
Owner:SHANGHAI JIAO TONG UNIV
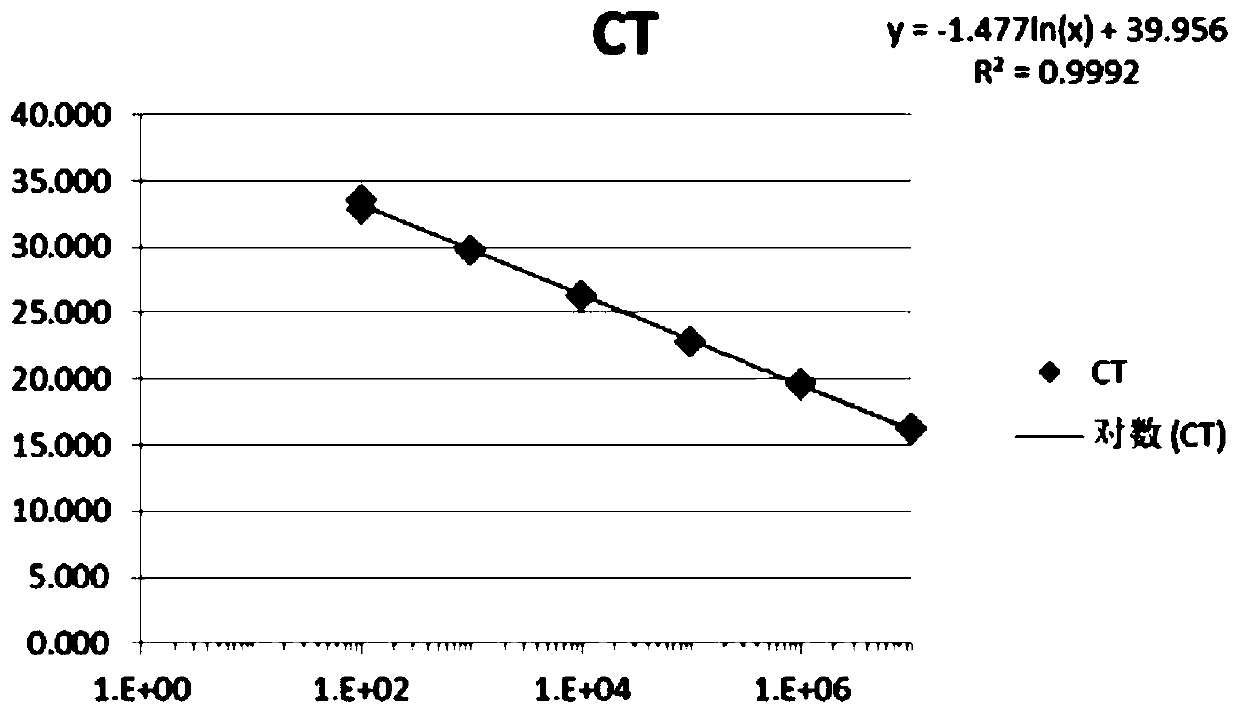
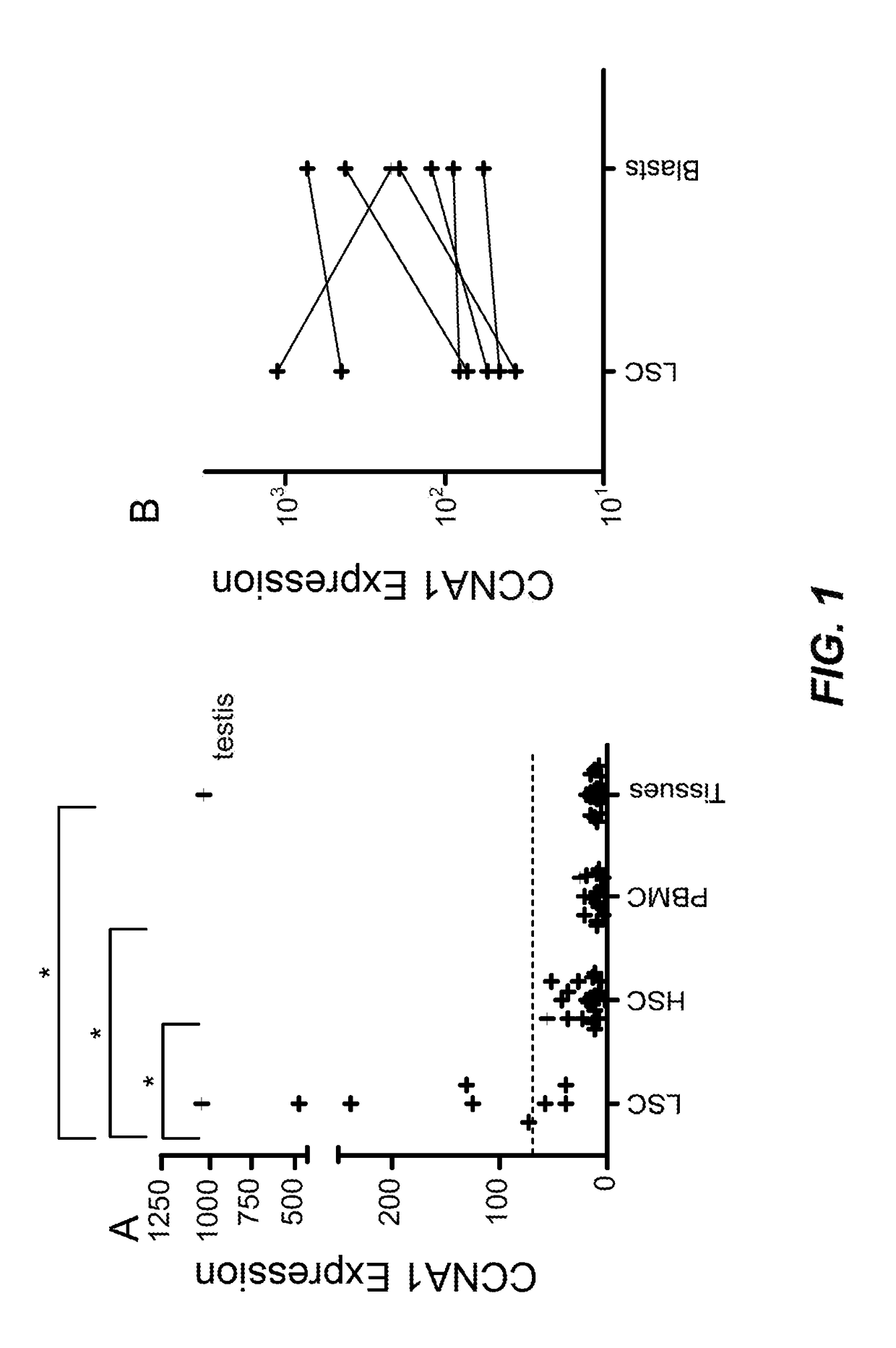
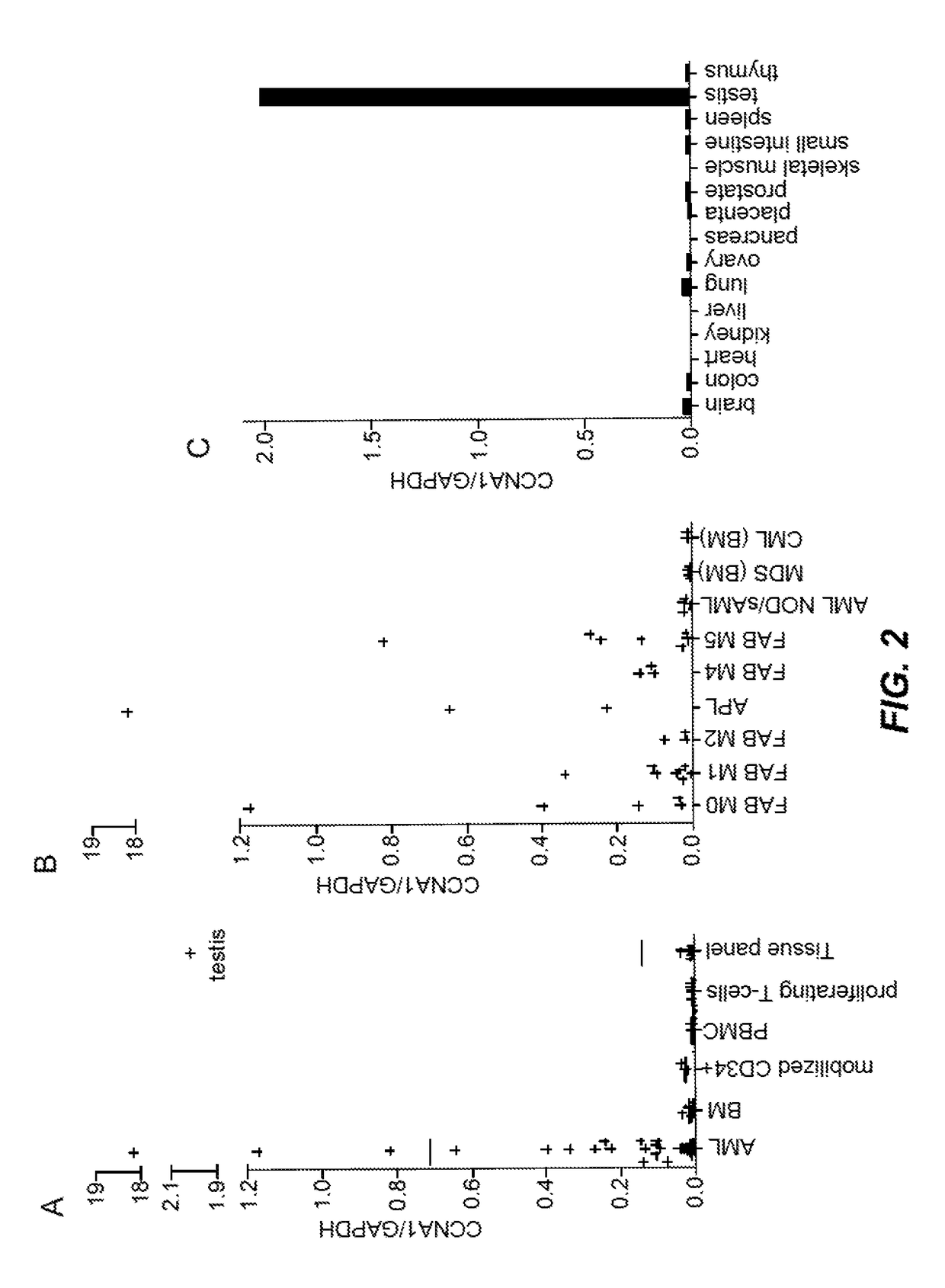
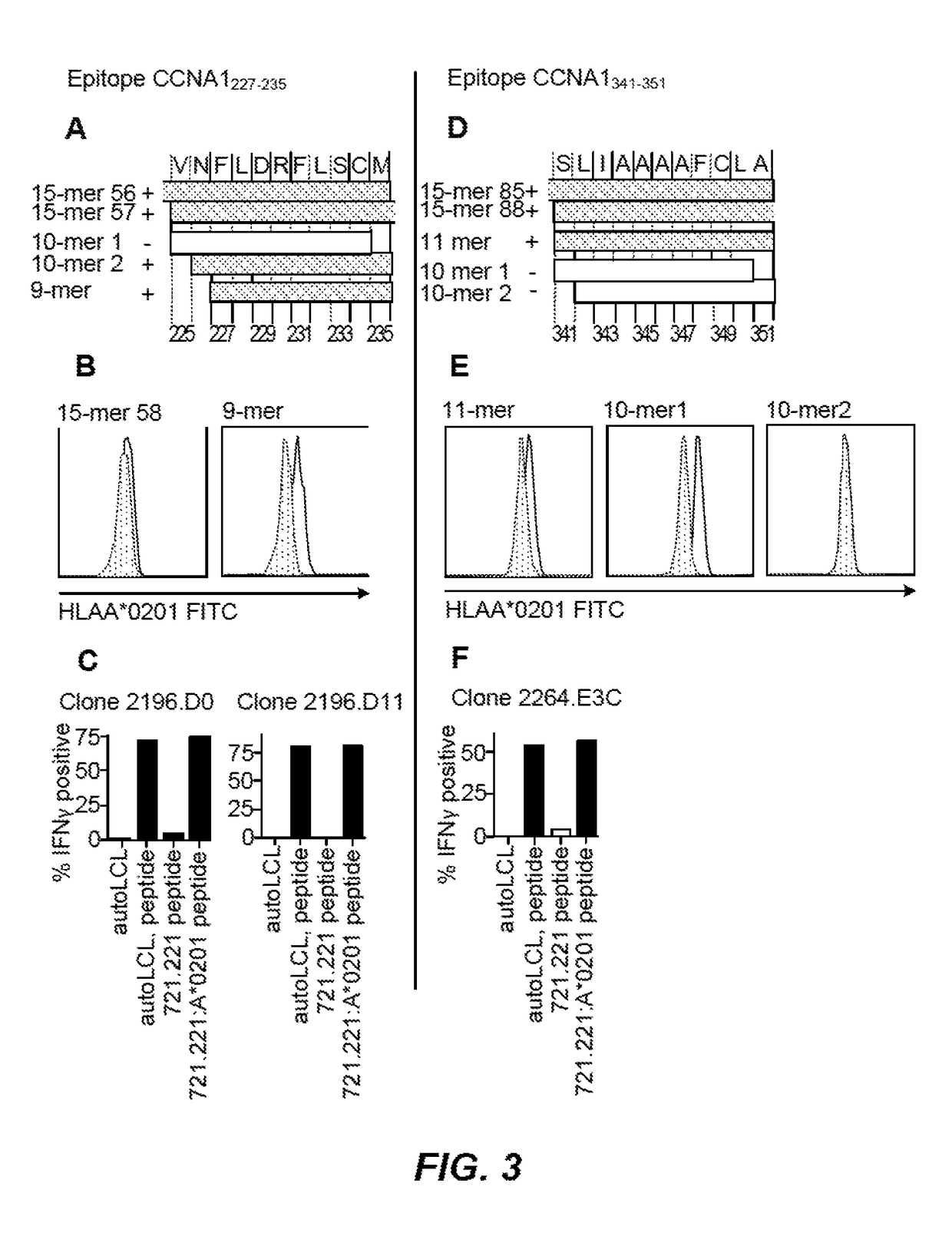
Cyclin A1-targeted T-cell immunotherapy for cancer
ActiveUS10208086B2Peptide/protein ingredientsGenetically modified cellsLeukemia associated antigensMyeloid leukemia
Compositions and methods are provided for eliciting antigen-specific T-cell responses against human cyclin A1 (CCNA1), which is herein identified as a leukemia-associated antigen based on its overexpression in acute myeloid leukemia (AML) including leukemia stem cells (LSC) and in immunologically privileged testis cells, but not in other normal cell types. CCNA1-derived peptide epitopes that are immunogenic for T-cells including CTL are disclosed, as are immunotherapeutic approaches using such peptides for vaccines and generation of adoptive transfer therapeutic cells.
Owner:FRED HUTCHINSON CANCER CENT
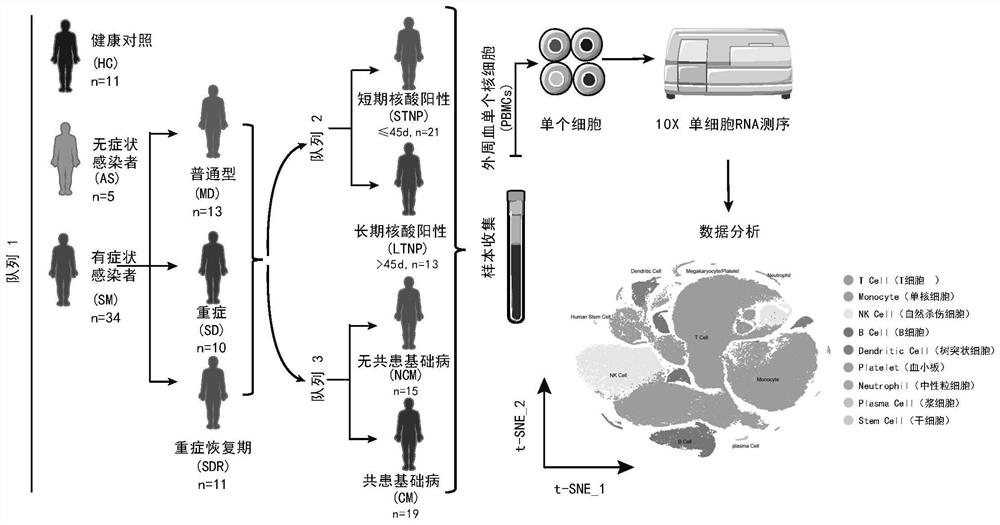
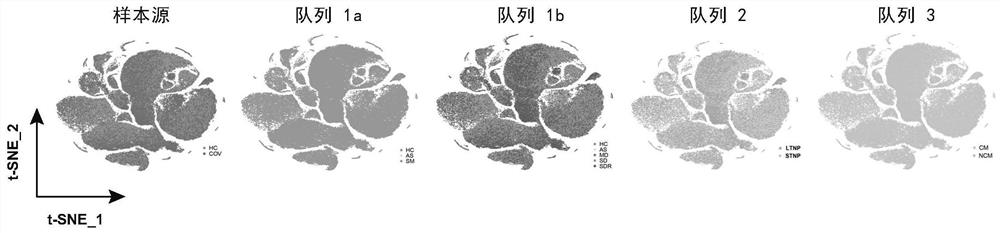
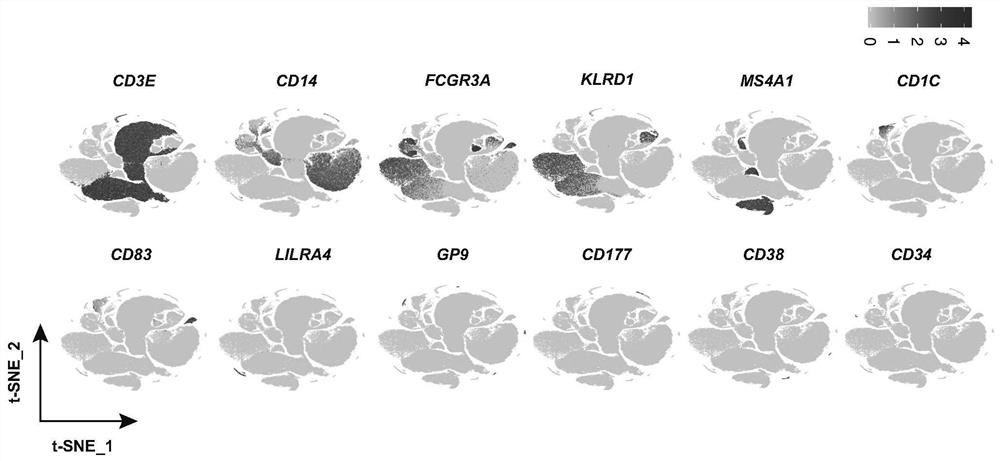
Identification of specific immune cell subtypes in new coronavirus infected peripheral blood and application of specific immune cell subtypes in new coronavirus infected peripheral blood
PendingCN112870226ARegulate immunitySpeed up the removal processMicrobiological testing/measurementEpidemiological alert systemsDiseaseSpecific immunity
The invention relates to identification of specific immune cell subtypes in new coronavirus infected peripheral blood and application of the specific immune cell subtypes, and provides immune cell populations and subpopulations which play an important role in occurrence and development of COVID-19 diseases. The first class can be used for T cell immunotherapy, DC cell immunotherapy and B cell immunotherapy of new coronal pneumonia and immunotherapy of old patients; the second class can be used for screening and designing a medicine for inhibiting cytokine storm and a medicine for inhibiting T cell reduction; the third class can be used for diagnosis of new coronal pneumonia.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
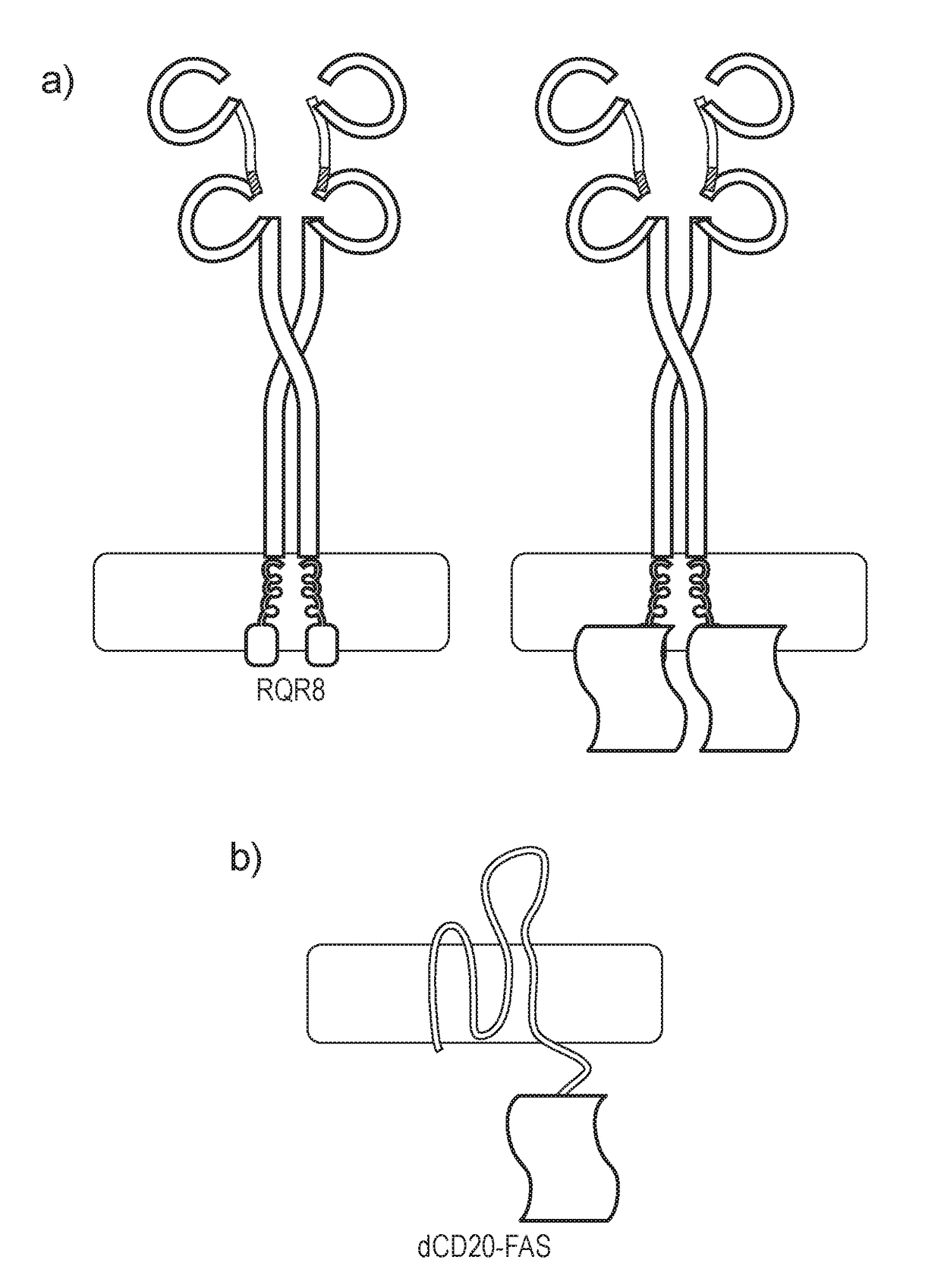
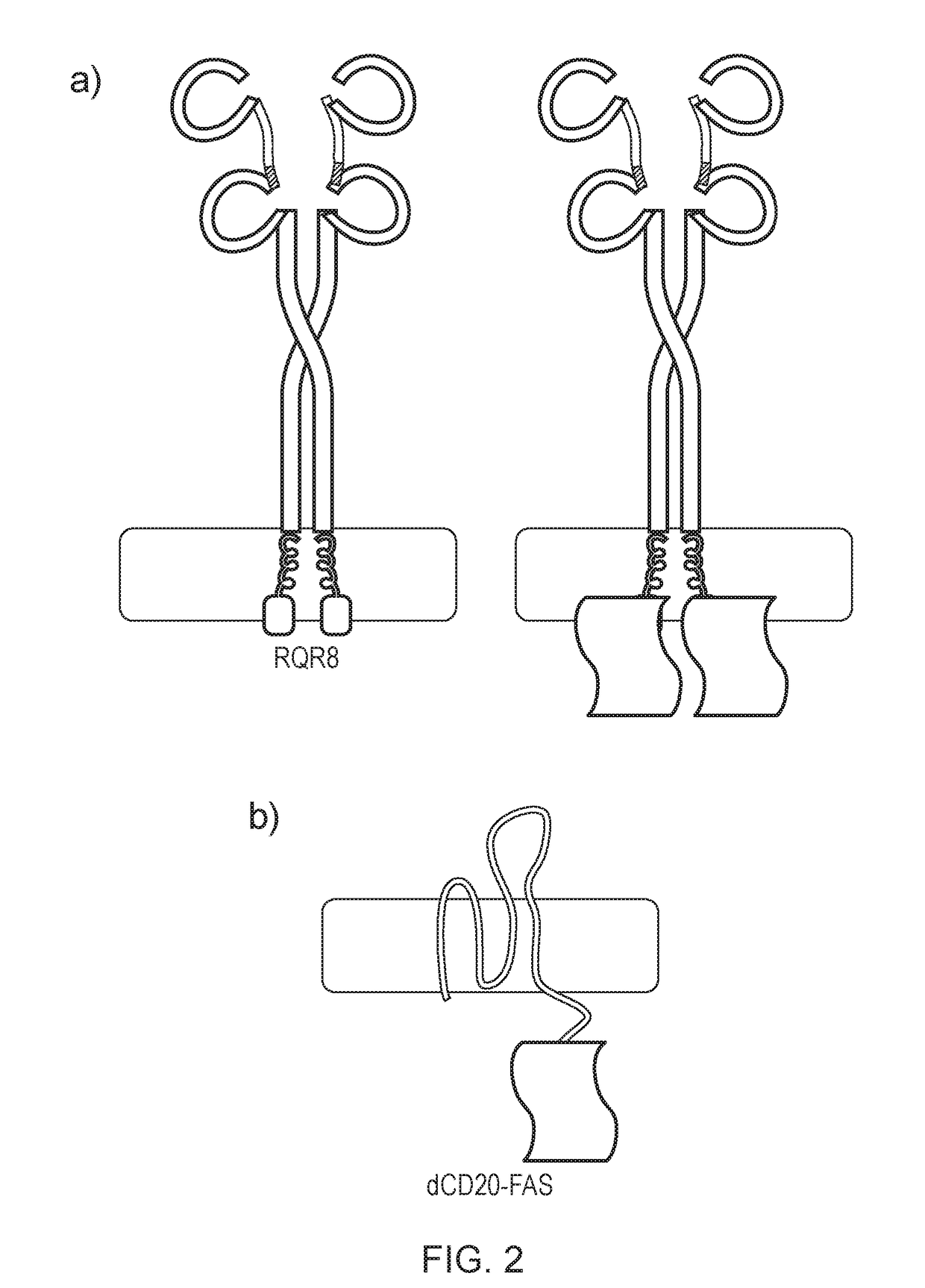
Chimeric protein
ActiveUS20180169189A1Low basal toxicityHigh induced toxicityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyBiological activationT cell immunity
The present invention provides a chimeric protein which comprises a multi-spanning transmembrane protein fused to a FAS endodomain, wherein the multi-spanning transmembrane protein binds an extracellular ligand, leading to activation of the FAS endodomain. The chimeric protein is useful as a suicide gene. The invention also provides a cell, such as a T cell comprising such a chimeric protein, which is useful in adoptive T cell immunotherapy approaches.
Owner:AUTOLUS LIMIED
Methods of t cell expansion and activation
The present disclosure relates to methods, cells, and compositions for preparing T cell populations and compositions for adoptive cell therapy. In particular, provided herein are methods for efficiently expanding and activating T cell populations for genetic engineering and adoptive T cell immunotherapies. Also provided are cells and compositions produced by the methods and methods of their use.
Owner:WISCONSIN ALUMNI RES FOUND
Method for the treatment of a tumor patient with adoptive t cell immunotherapy
PendingUS20210169937A1Reduce riskIncrease the number of cellsPeptide/protein ingredientsMammal material medical ingredientsDiseaseOncology
Owner:NORDWEST POLYBIOCEPT BIOSCI GMBH
A car-t therapeutic vector for t lymphocytic leukemia and its construction method and application
ActiveCN108018312BGood killing efficiencyExtended half-lifeMammal material medical ingredientsFermentationSequence signalSingle-Chain Antibodies
The invention discloses a CAR-T (Chimeric antigen receptor T-cell immunotherapy) therapeutic vector for T lymphocytic leukemia. The therapeutic vector comprises a lentivirus skeleton plasmid, a humanEF1 alpha promoter and a CAR chimeric receptor domain, wherein the CAR chimeric receptor domain contains CD8 leader chimeric acceptor signal peptide, a CD7 single-chain antibody light chain VL, a CD7single-chain antibody heavy chain VH, an antibody inner hinge UCLinker, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane region, a TCR chimeric receptor T cellactivation domain and a chimeric receptor co-stimulatory factor region. The invention further discloses a construction method of the vector and an application of the vector in preparation of a drug for treating T lymphocytic leukemia.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Blockade of tumor-derived ILT4 in adoptive T-cell therapy
ActiveCN110917356BHigh benefitIncrease lethalityImmunological disordersAntineoplastic agentsMelanomaOncology
The invention provides an application of blocking tumor-derived ILT4 in adoptive T cell therapy. Researches find that the tumor-derived ILT4 has an induction effect on microenvironment T cell aging, molecular and metabolic mechanisms of the tumor-derived ILT4 are explored in detail, and it is proved from a preclinical model that the targeted ILT4 can reverse the immunosuppressive microenvironmentof tumors and participate in targeted therapy of malignant tumors, and a B6 mouse malignant melanoma adoptive T cell immunotherapy model is further constructed, and the feasibility of ILT4 blocking combined ACT therapy is studied. An effective means is provided for overcoming ACT treatment drug resistance.
Owner:JINAN CENTER HOSPITAL
Combinations for t-cell immunotherapy and uses thereof
ActiveCN109589336BMammal material medical ingredientsAntibody ingredientsAntigenTransmissible disease
Owner:MANYSMART THERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com