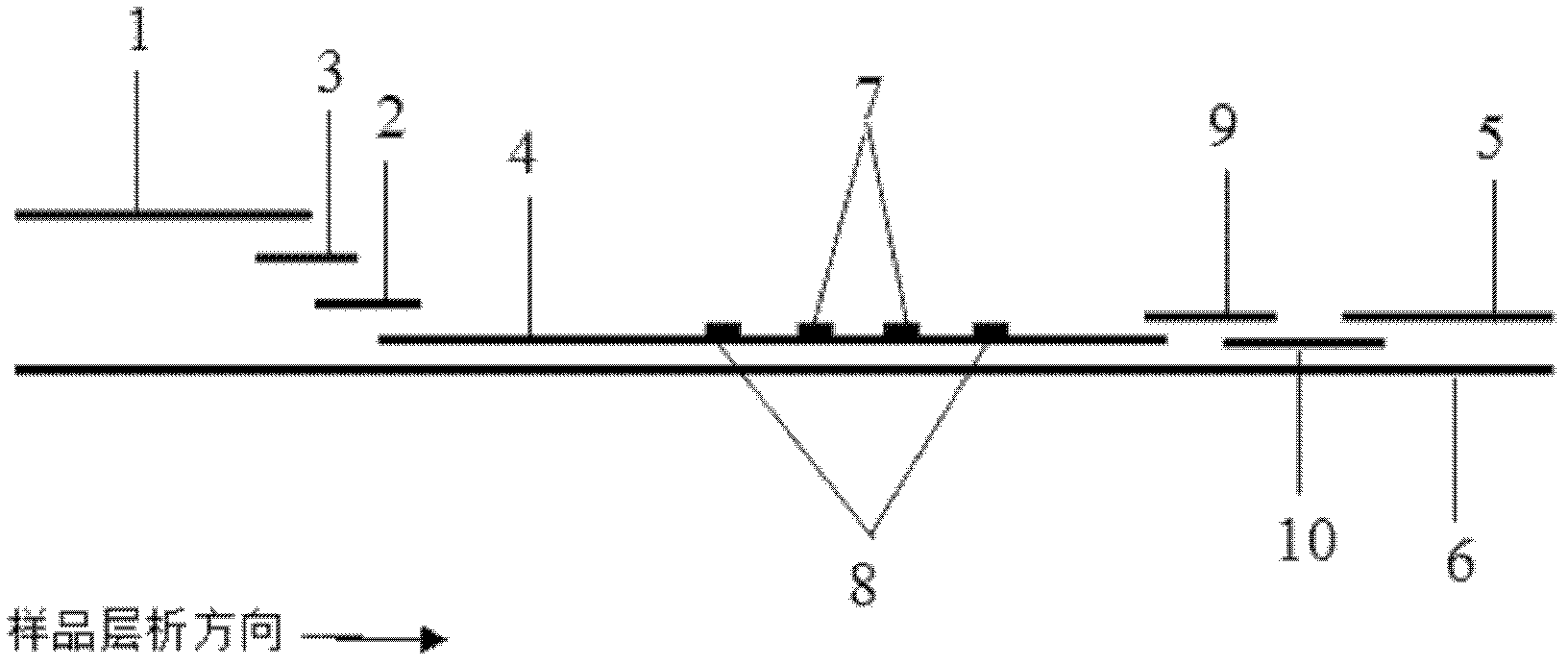
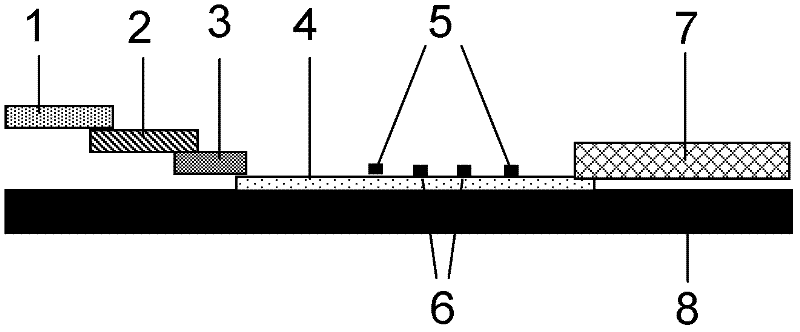
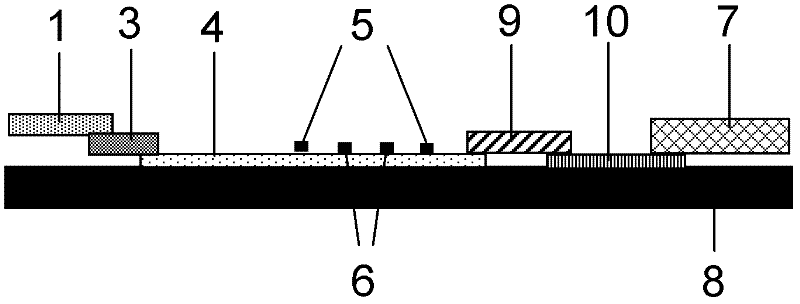
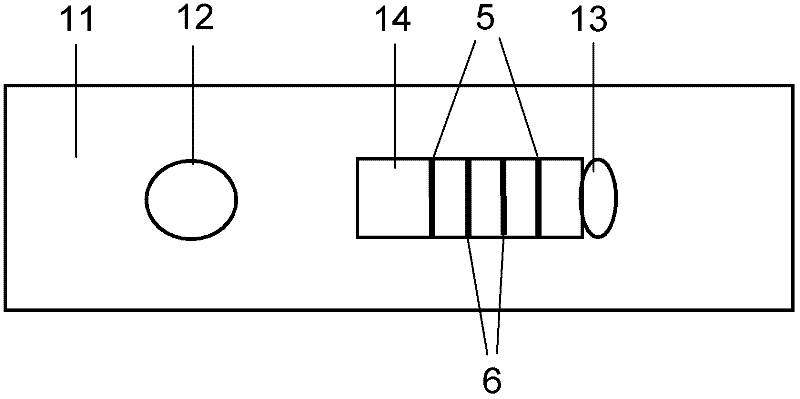
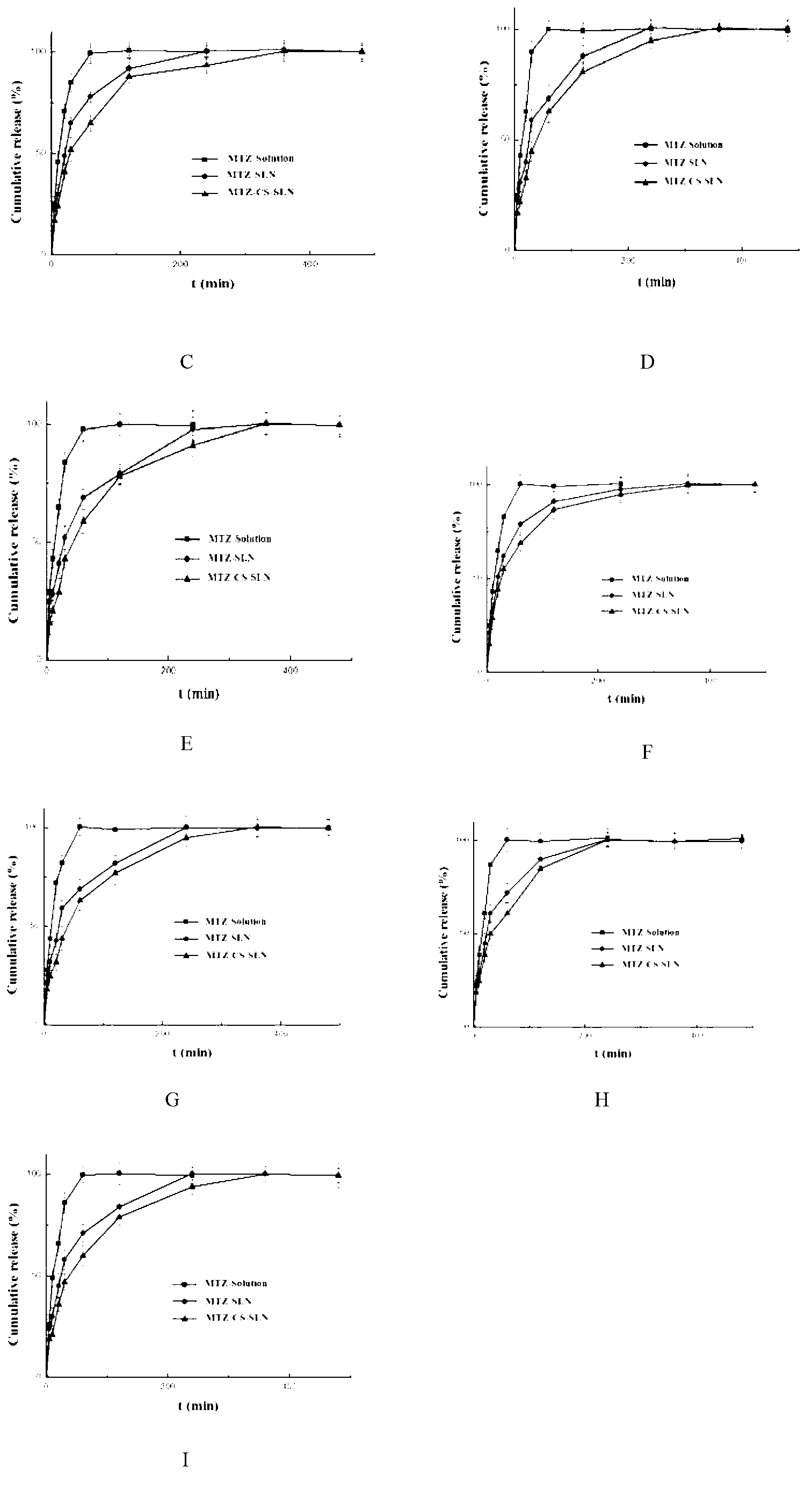Patents
Literature
56results about How to "Enhance specific binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
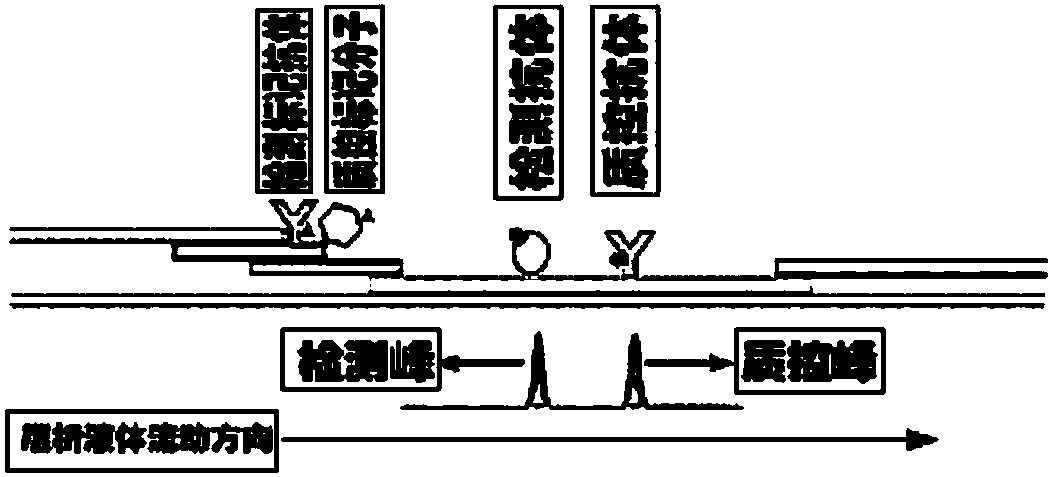
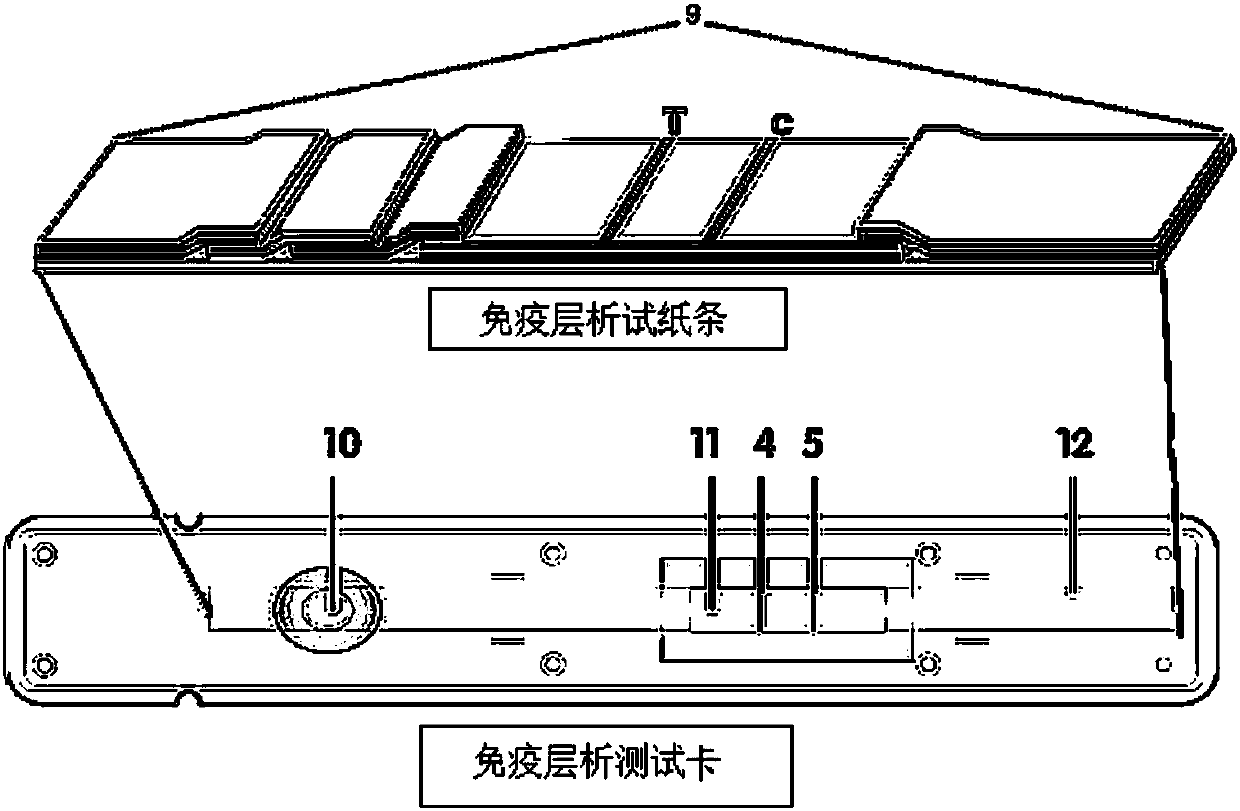
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
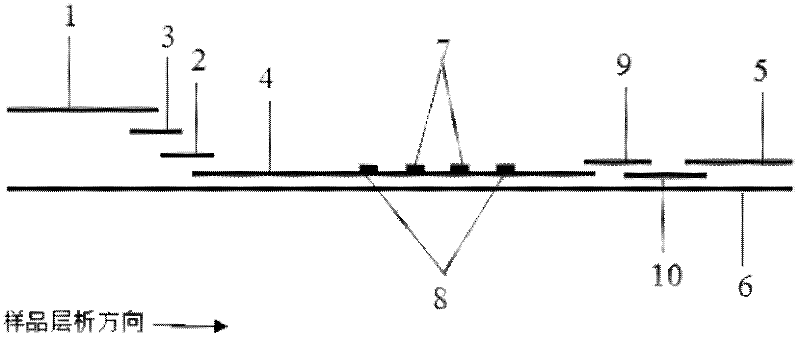
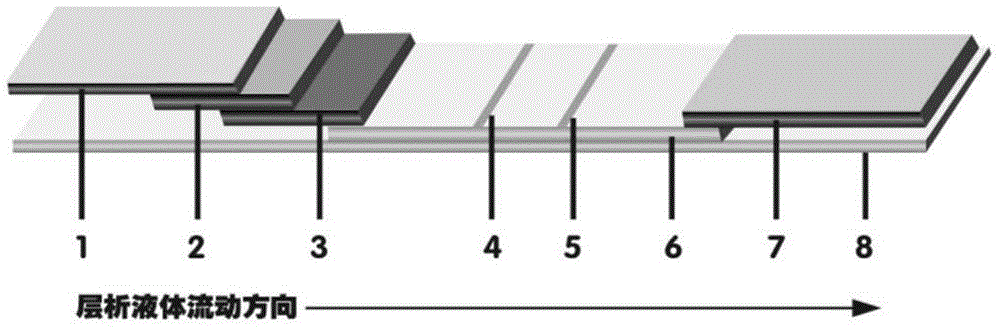
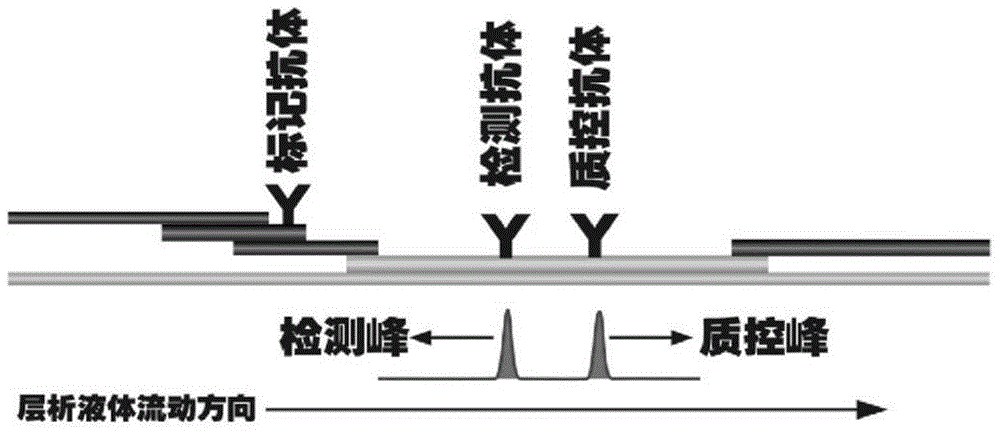
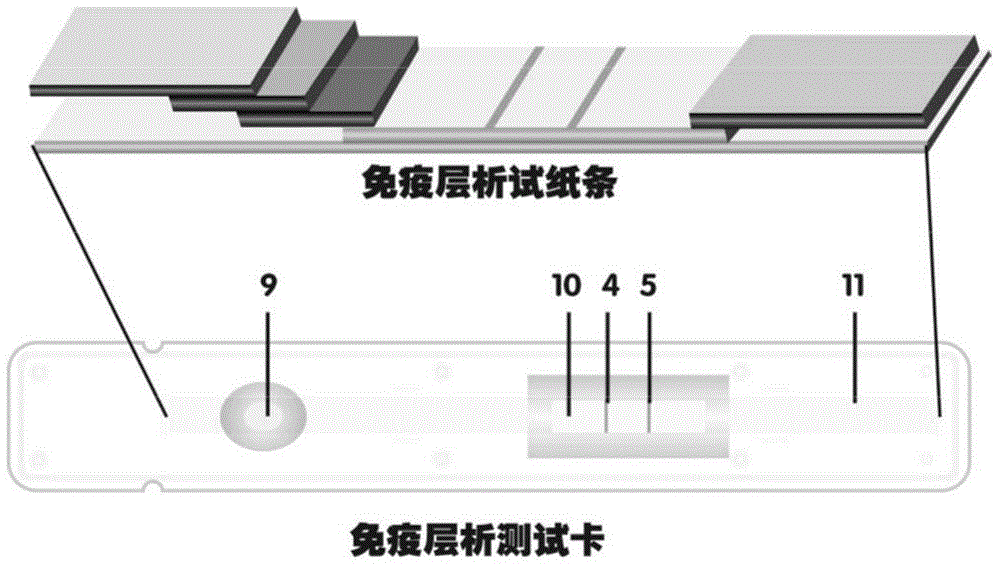
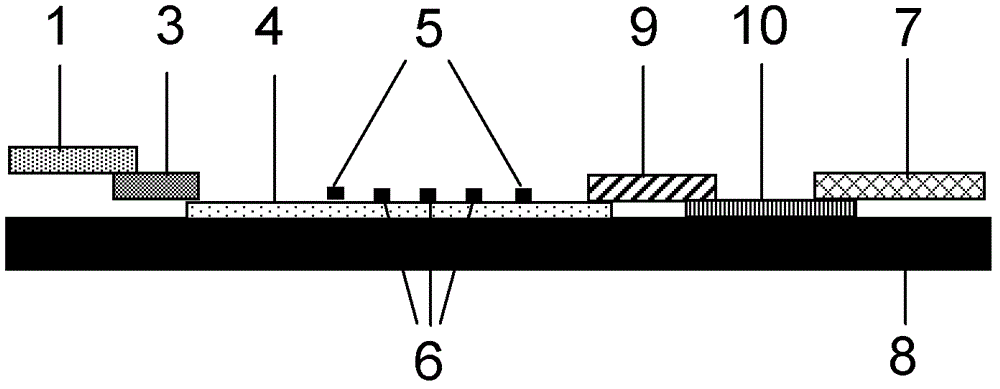
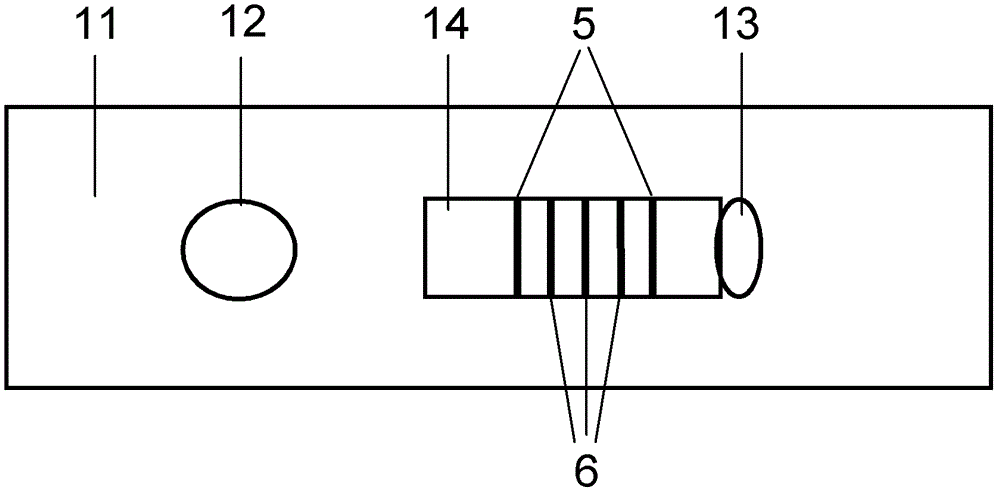
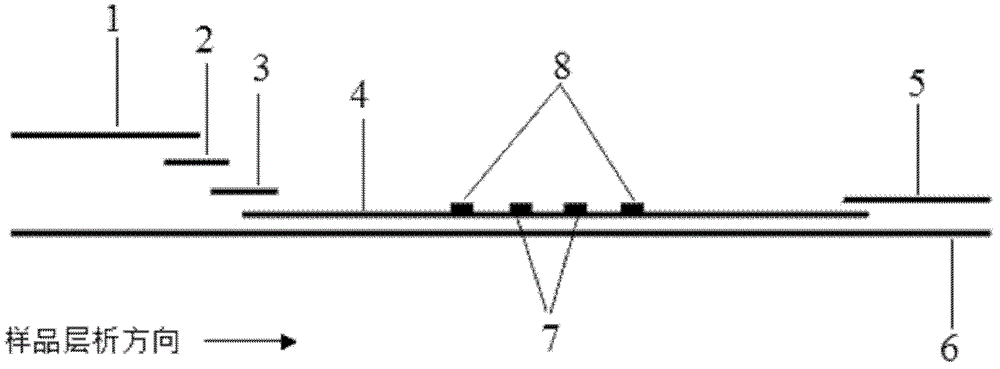
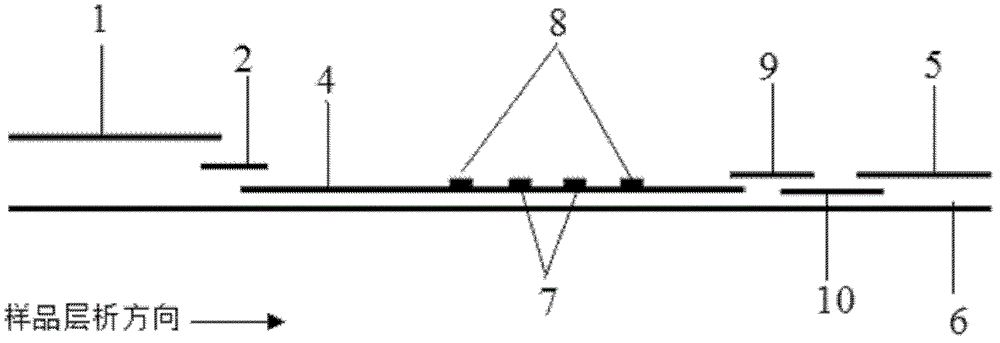
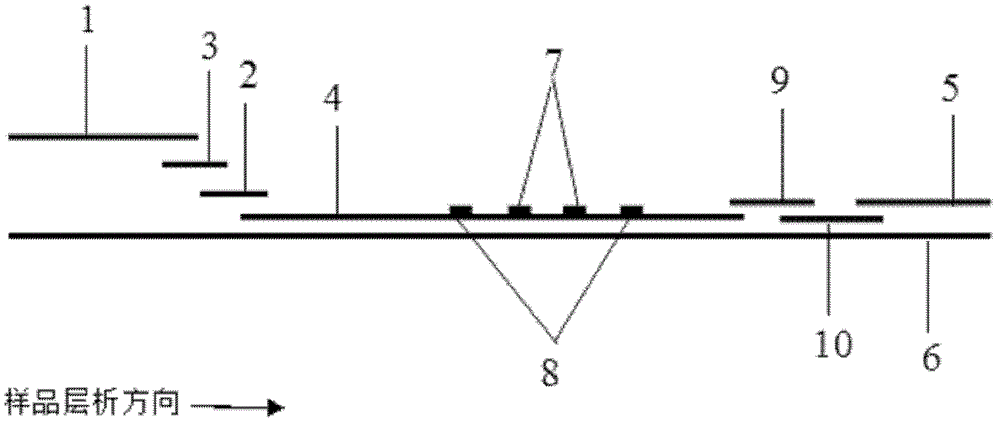
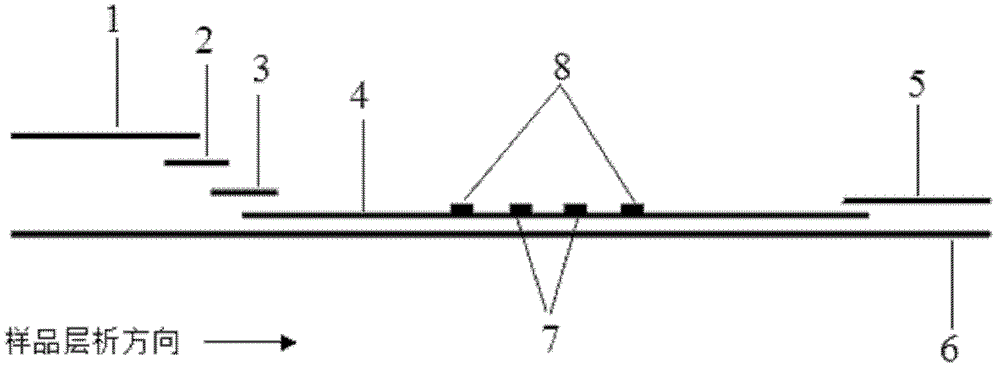
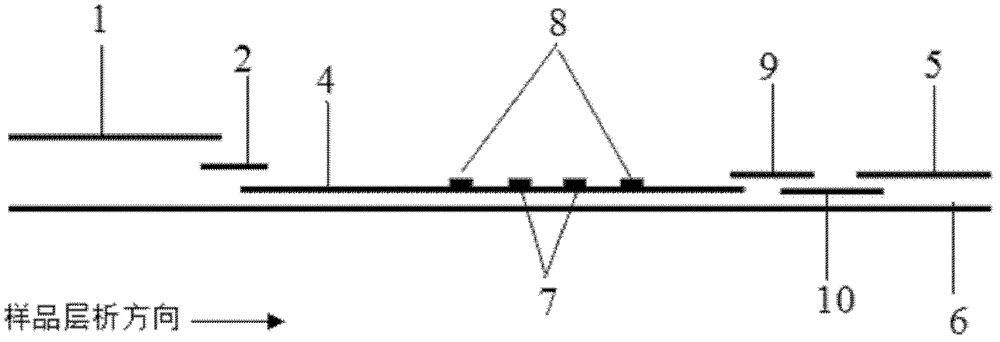
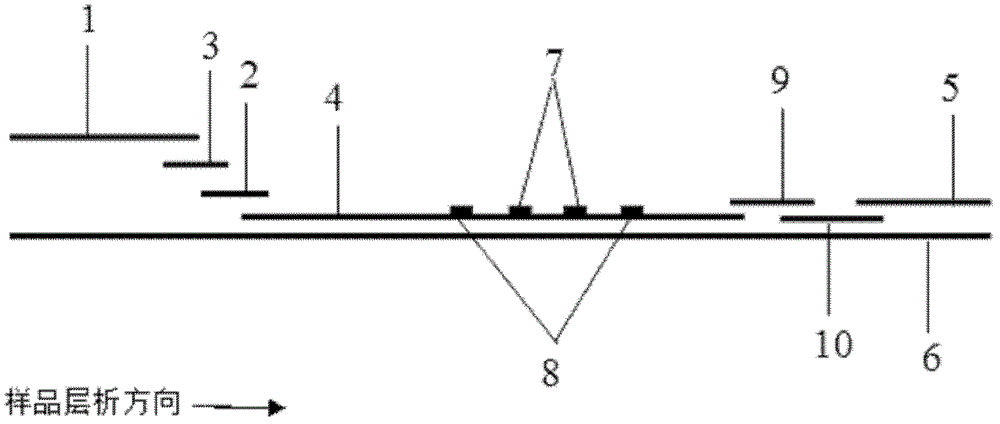
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Kit suitable for rapidly detecting AMH and INHB by using double-tagging time resolution fluorescence immunoassay method and use method of kit
InactiveCN104730247AReduce dosageReduce detection stepsBiological testingFluorescence/phosphorescenceFluorescence immunoassayFluorescence
The invention provides a kit suitable for rapidly detecting AMH and INHB by using a double-tagging time resolution fluorescence immunoassay method. The kit comprises (1) a solid phase carrier coated by an AMH coating antibody and an INHB coating antibody; (2) a mixed calibration product of AMH and INHB; (3) an AMH detection antibody marked by using a lanthanide element 1; (4) an INHB detection antibody marked by a lanthanide element 2; (5) an immunoreaction accelerant liquid; (6) a concentrated washing liquid; and (7) a reinforcing liquid. The invention further provides a use method of the kit for rapidly detecting AMH and INHB by using the double-tagging time resolution fluorescence immunoassay method. By adopting a double-tagging time resolution fluorescence immunoassay technique, the defect that conventional AMH and INHB respectively need a single kit, that is, one kit can be only used for detecting AMH or INHB can be overcome, the detection steps can be reduced, the sampling time can be shortened, and the use amount of a blood sample can be reduced.
Owner:GUANGZHOU FENGHUA BIOENG
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Reversibly crosslinked biodegradable polymersome with asymmetric membrane structure as well as preparation method and application thereof to nucleic acid medicines
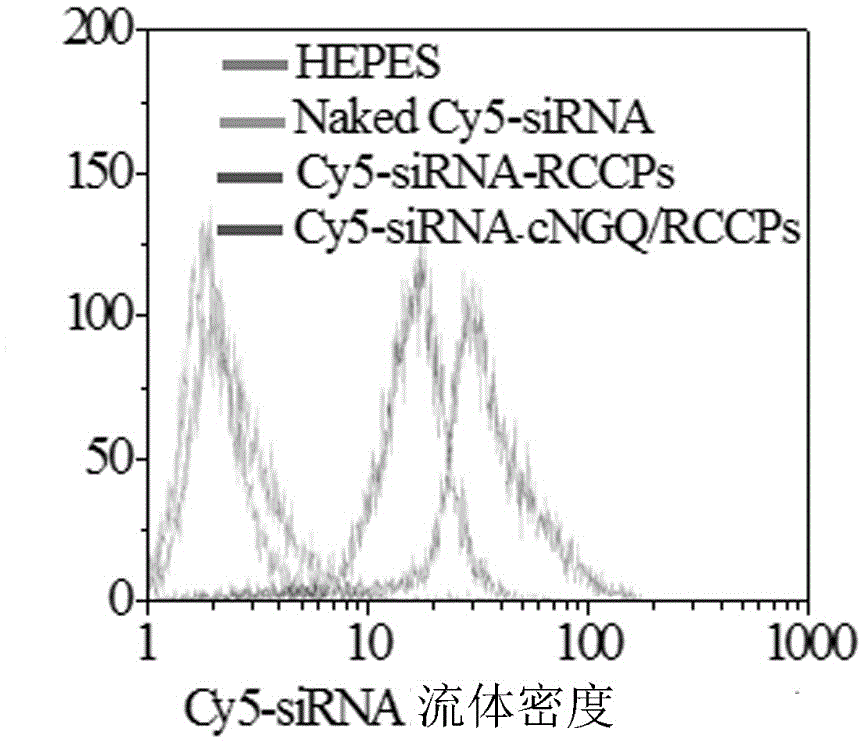
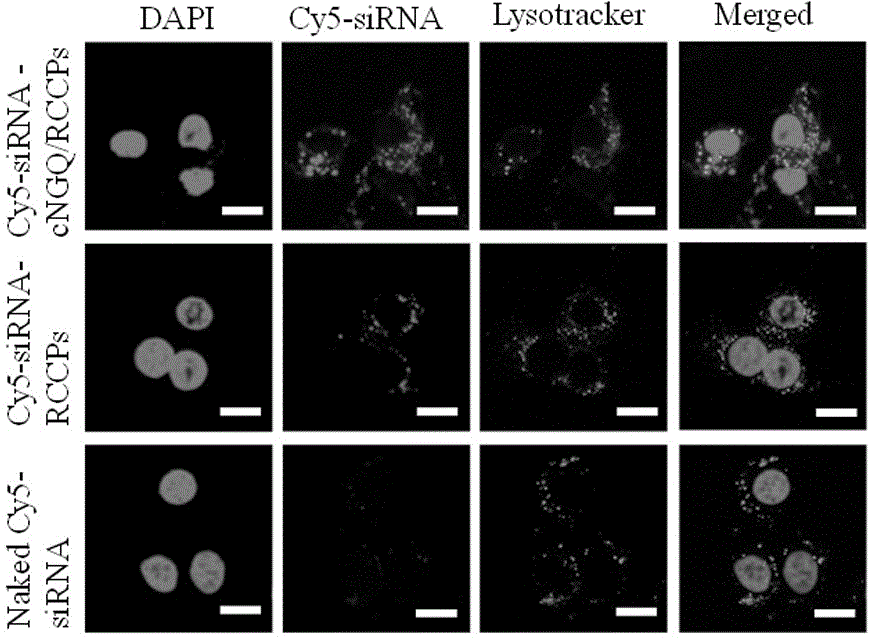
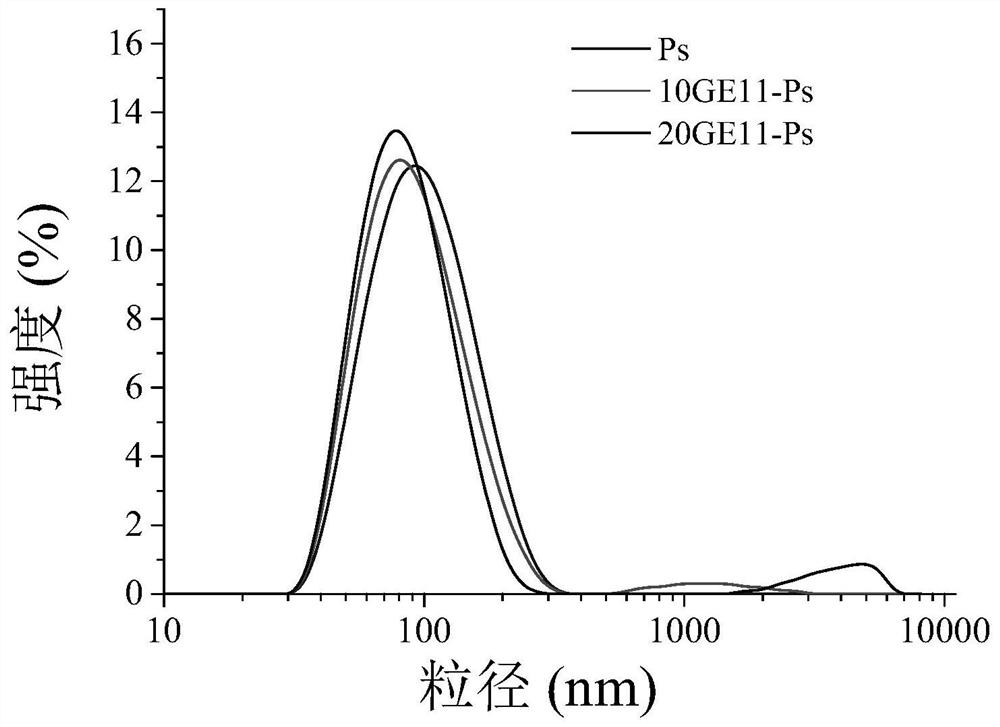
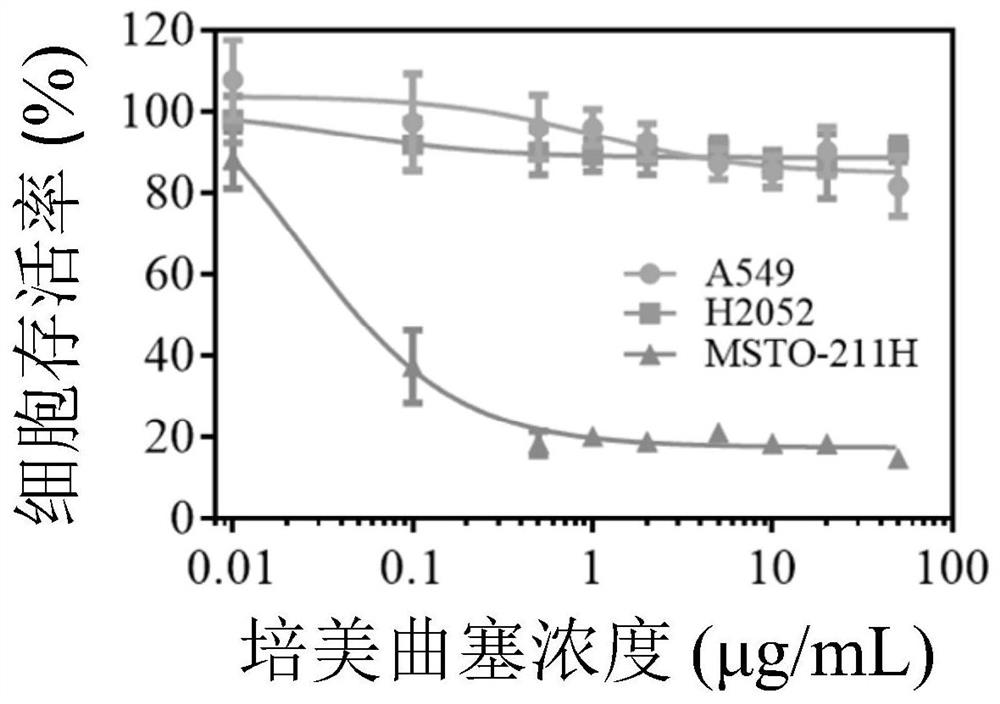
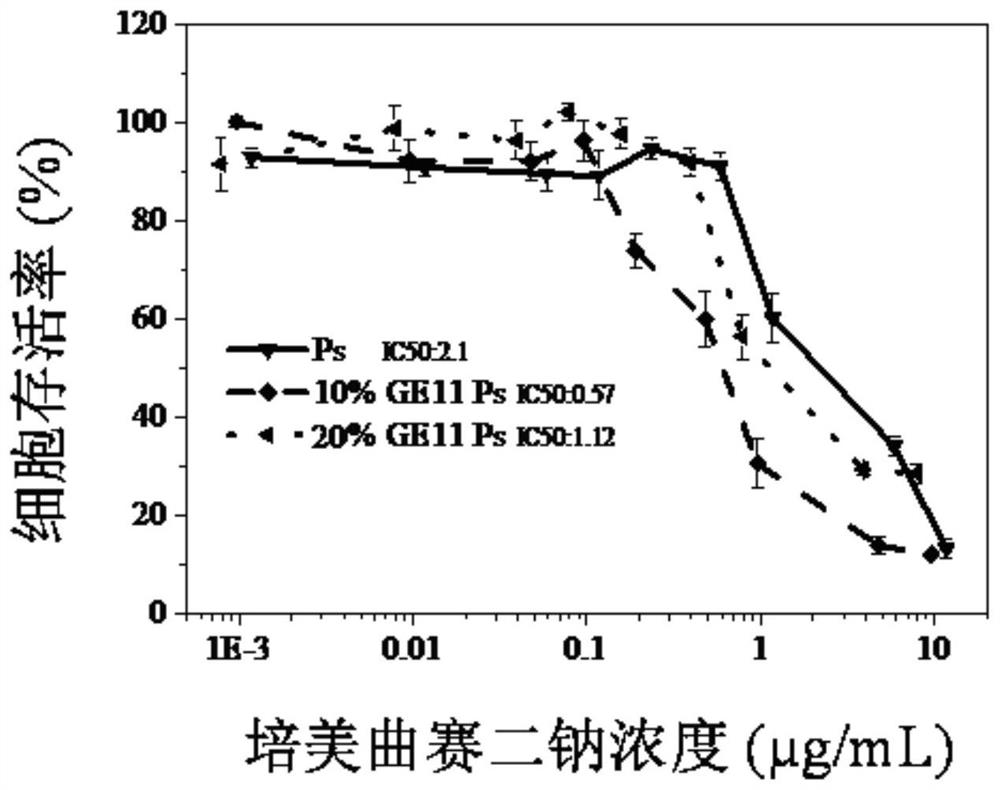
ActiveCN106177975AImprove loading performanceAvoid stabilityOrganic active ingredientsGenetic material ingredientsCancer cellSide effect
The invention discloses a reversibly crosslinked biodegradable polymersome with an asymmetric membrane structure as well as a preparation method and application thereof to nucleic acid medicines. The polymersome and the preparation method have the advantages that a triblock polymer PEG-P(TMC-DTC)-PEI or PEG-P(DLLA-DTC)-PEI is synthesized and is self-assembled and self-crosslinked to obtain the polymersome with the asymmetric membrane structure or the triblock polymer is linked with a targeting molecule and is self-assembled to obtain targeting RCCPs; the inner shell of the polymersome is PEI and is used for compounding and loading nucleic acid through electrostatic interaction; a membrane is reversibly crosslinked biodegradable polyester / polycarbonate with good biocompatibility; dithiolane of a side chain is similar to a human body natural antioxidant lipoic acid; the outer shell of the polymersome takes PEG as the background and can target cancer cells; by studying that the carrier is compounded with functional siRNA and studying the gene silencing effects in vitro and vivo, in vivo blood circulation and bio-distribution, the condition of treating lung cancer in situ bearing mice and toxic and side effects of the polymersome, the polymersome is expected to become a nanosystem platform integrating the advantages of simplicity, stability, multifunction, and the like and is used for carrying out efficient and active targeting delivery on siRNA to tumors in situ.
Owner:SUZHOU UNIV
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Oligonucleotides aptamer special for distinguishing fumonisin B1
ActiveCN103013999AHigh purityImprove efficiencyOrganic compound preparationMicrobiological testing/measurementChemical synthesisMagnetic bead
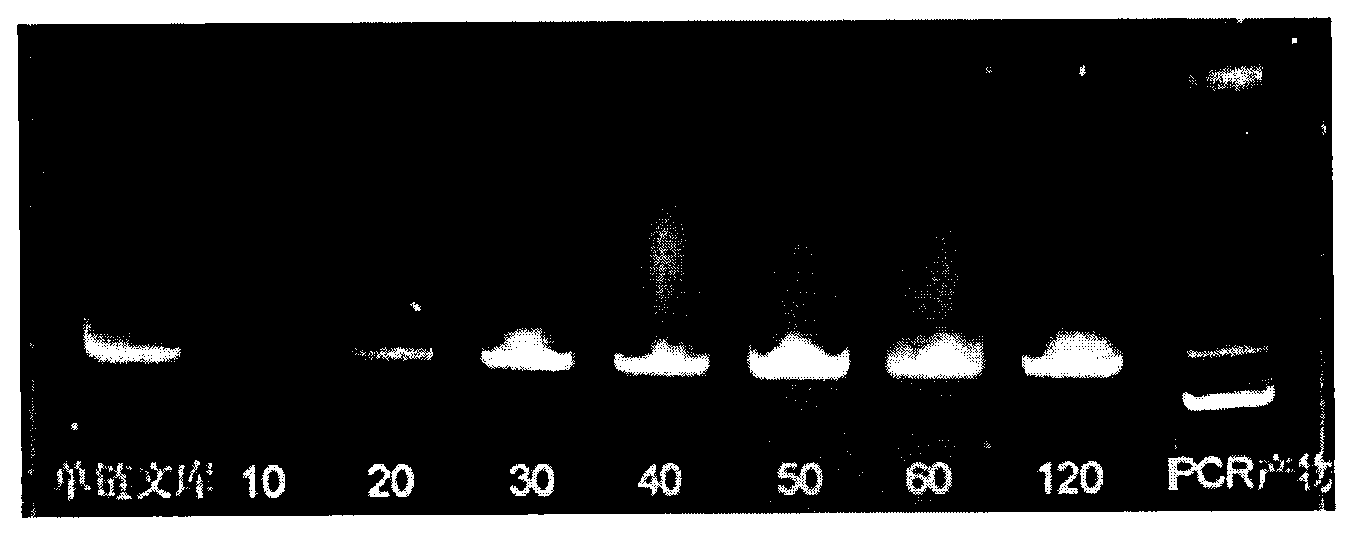
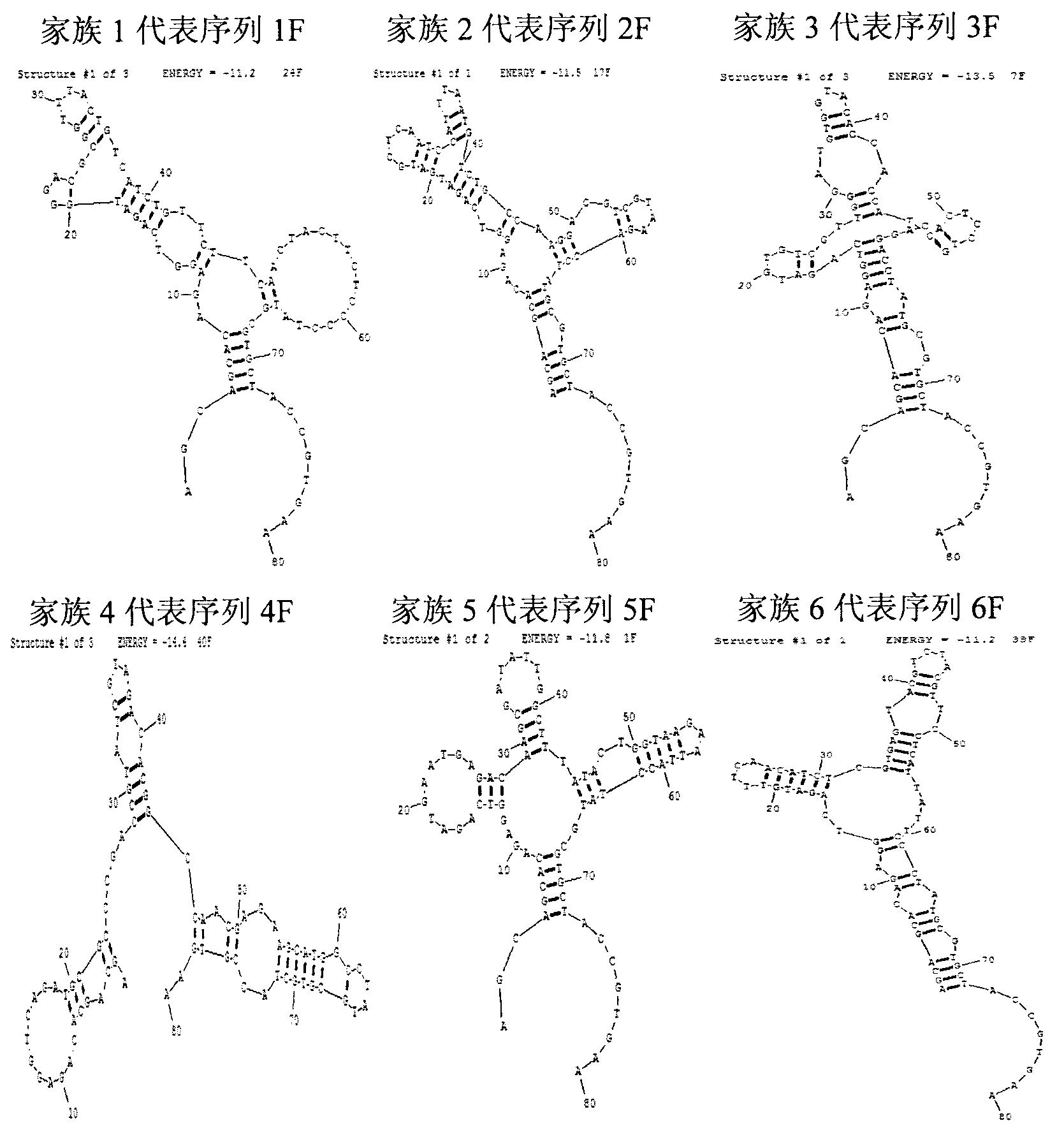
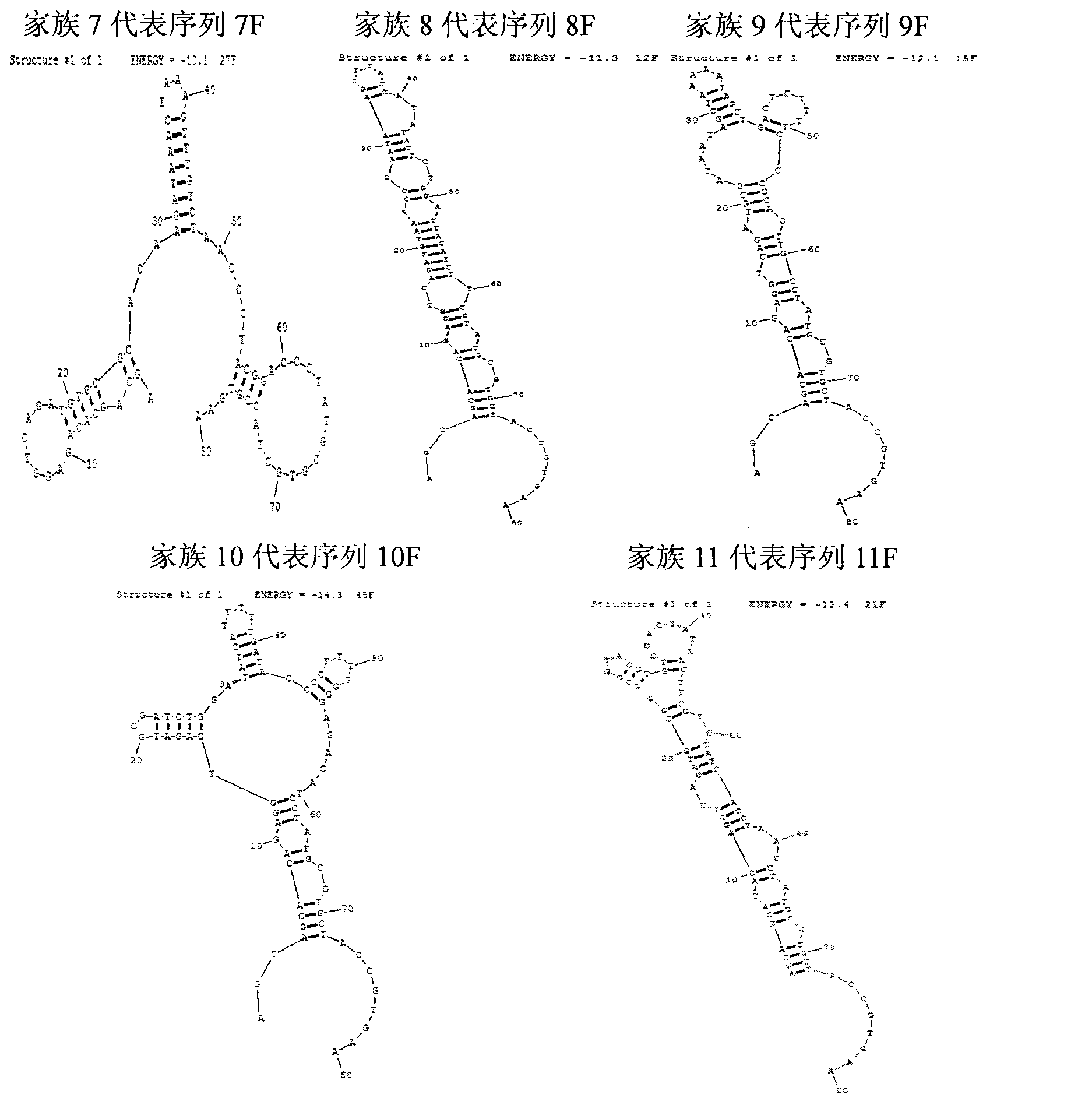
The invention provides a group of single-stranded DNA nucleic acid aptamers of fumonisin B1. Each aptamer is obtained by carrying out screening, amplification, sequencing, and analysis on affinity and specificity on a random original single-stranded DNA library with the combination of an SELEX (systematic evolution of ligands by exponential enrichment) technology and a magnetic bead with fumonisin B fixed on the surface. As a special efficient distinguishing aptamer of fumonisin B, the group of DNA aptamers provides a new choice for developing a method for replacing the existing methods which are used for detecting fumonisin B by depending on an antibody, and can be used for analyzing and detecting or separating the fumonisin B in the environment of enriched food. The nucleic acid aptamer is small in molecular weight, low in chemical synthesis cost, easy to mark, strong in affinity and specificity, reversible in degeneration renaturation, high in speed, and suitable for repeated use, room-temperature transportation and long-term storage.
Owner:JIANGNAN UNIV
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
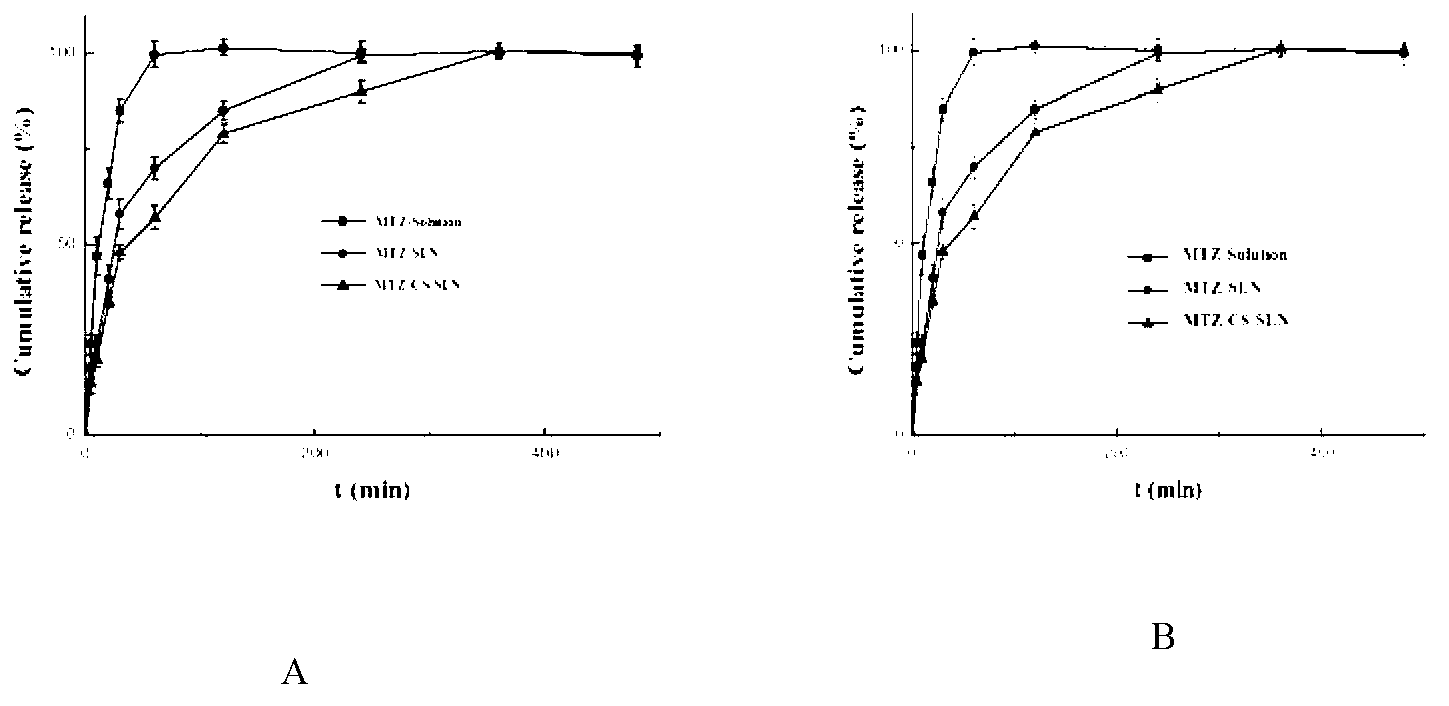
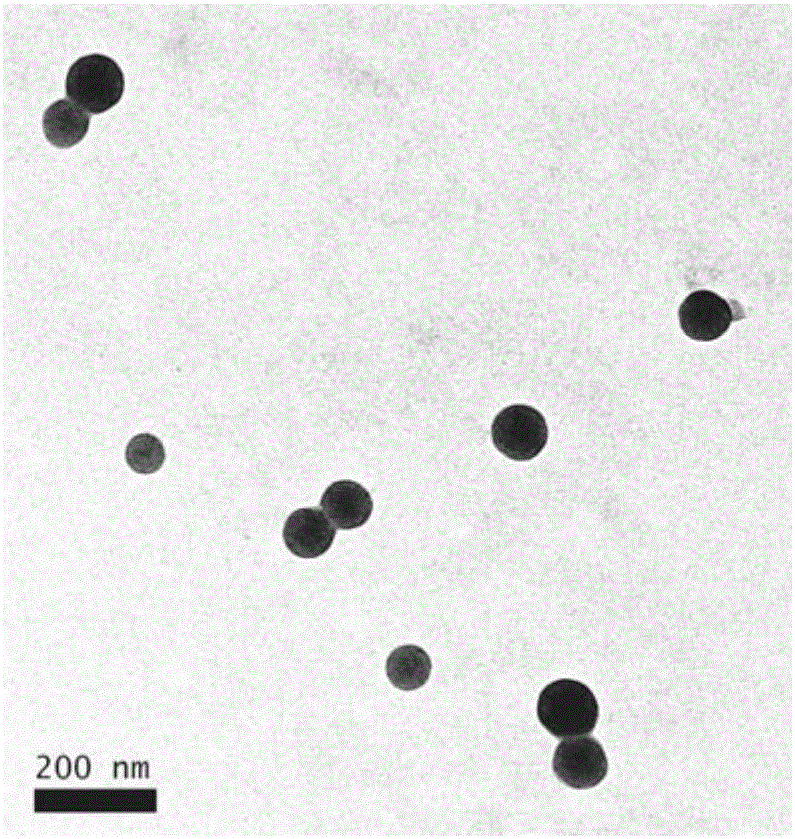
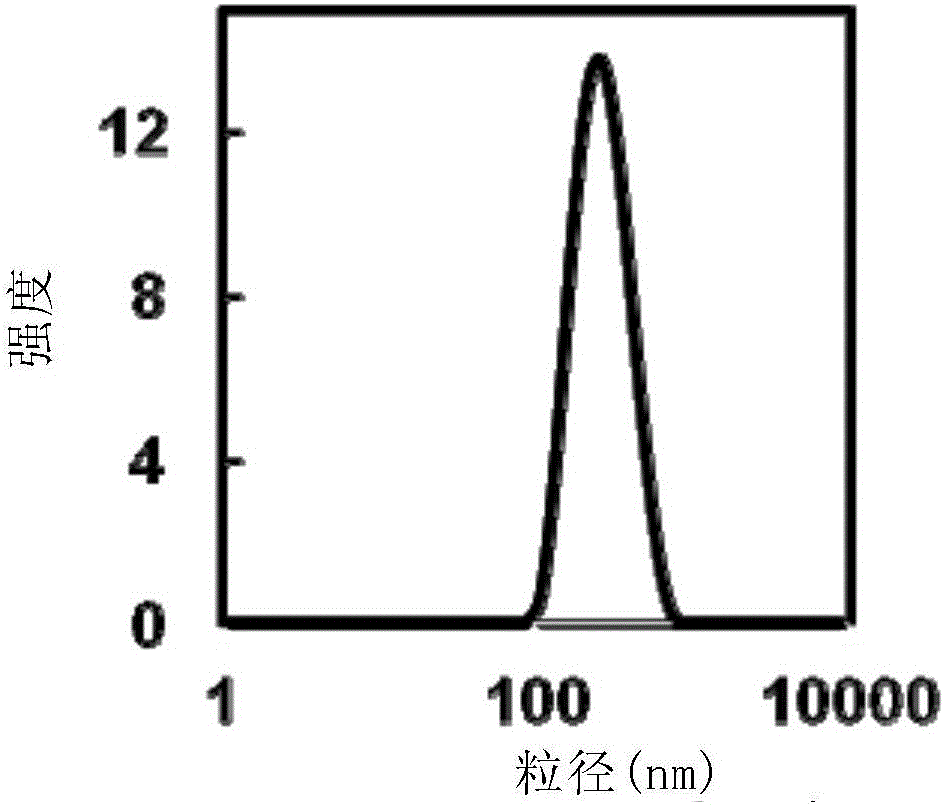
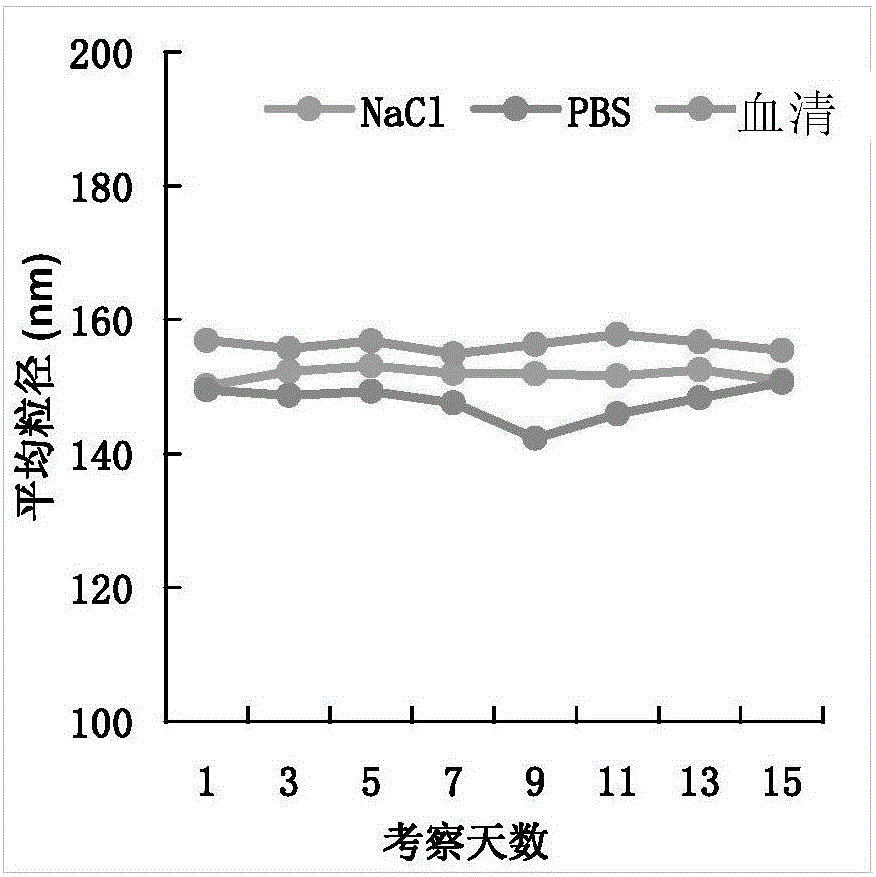
Chitosan-modified methazolamide solid lipid nanoparticles and preparation method thereof
InactiveCN102793672AEasy to stayIncrease uptakeOrganic active ingredientsPowder deliveryLipid formationCorneal permeability
The invention provides chitosan-modified methazolamide solid lipid nanoparticles and a preparation method thereof. The chitosan-modified methazolamide solid lipid nanoparticles can be used as eye drops. The preparation method is suitable for industrial production. The chitosan-modified methazolamide solid lipid nanoparticles are characterized in that based on 30ml of a nanoparticle-containing solution, the chitosan-modified methazolamide solid lipid nanoparticles comprise 5mg of methazolamide, 25 to 150mg of at least one lipid material, 25 to 150mg of phospholipid, 5 to 100mg of chitosan, 50 to 250mg of at least one non-phospholipid surfactant and 50 to 250mg of at least one assistant surfactant. The chitosan-modified methazolamide solid lipid nanoparticles have small particle sizes and high drug entrapment efficiency. Compared with solid lipid nanoparticles which are not modified by chitosan, the chitosan-modified methazolamide solid lipid nanoparticles have higher stability and better corneal permeability because of positive charges on the surfaces of the chitosan-modified methazolamide solid lipid nanoparticles so that drug bioavailability is improved. Therefore, the chitosan-modified methazolamide solid lipid nanoparticles have a large clinical application potential in glaucoma treatment.
Owner:NANJING MEDICAL UNIV
Active targeting brain-tumor-resisting drug and preparation method thereof
ActiveCN105476975APowerful targetingEnhance specific bindingOrganic active ingredientsEmulsion deliveryBrain tumorBrain section
The invention relates to an active targeting brain-tumor-resisting drug and a preparation method thereof and specifically discloses a compound. The compound comprises a nano-carrier with the particle size less than 200nm and a target molecule, and the target molecule is coupled to the surface of the nano-carrier and has high affinity on a neuropeptide receptor Y1 on a blood brain barrier and a neuropeptide receptor Y2 on brain tumor cells. The invention further discloses a composition comprising the compound and a preparation method and application of the composition. The compound or the composition can effectively cross the blood brain barrier to get into the brain and can be combined with the brain tumor cells in a high specificity manner so as to deliver the antitumor drug into the tumor cells, drug concentration in the cells is heightened effectively, and the brain tumor cells are killed powerfully.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Filtering particles from blood or other media
InactiveCN103959037AMinimized mechanical stressPrevent pressure gradientsLaboratory glasswaresIndividual particle analysisVisual inspectionEngineering
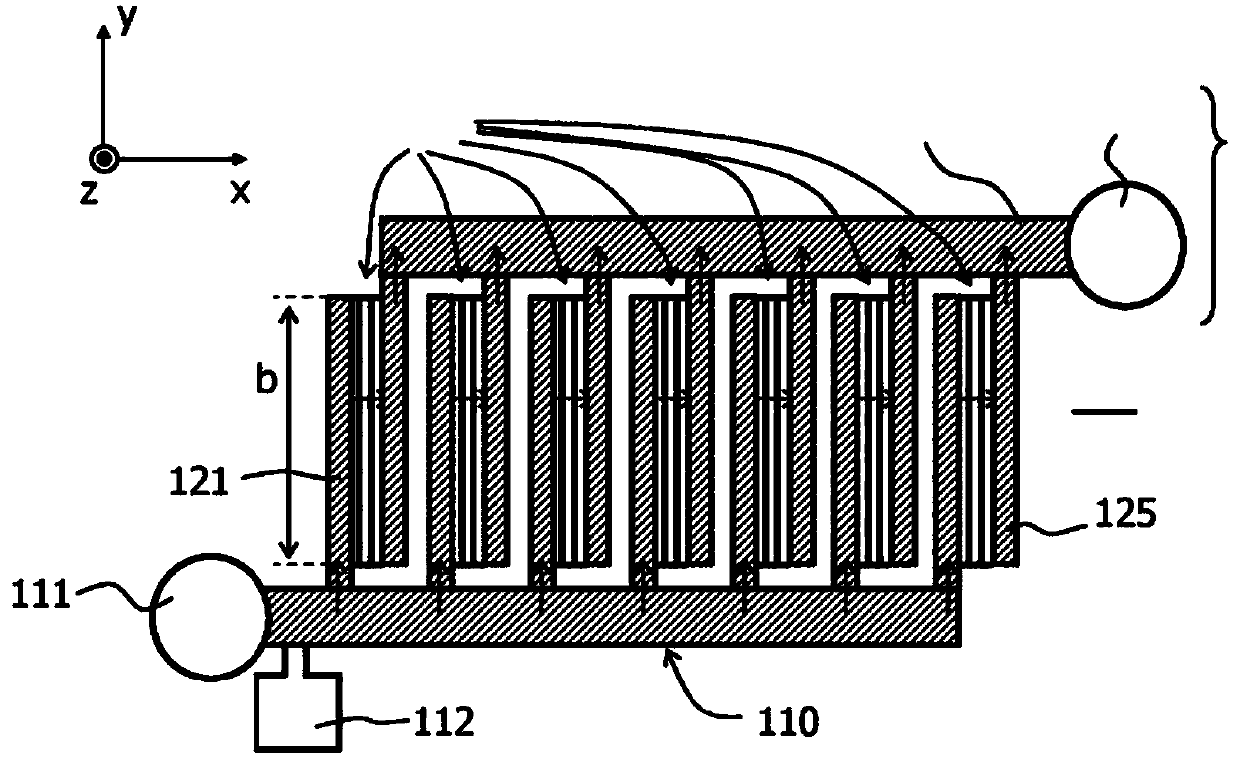
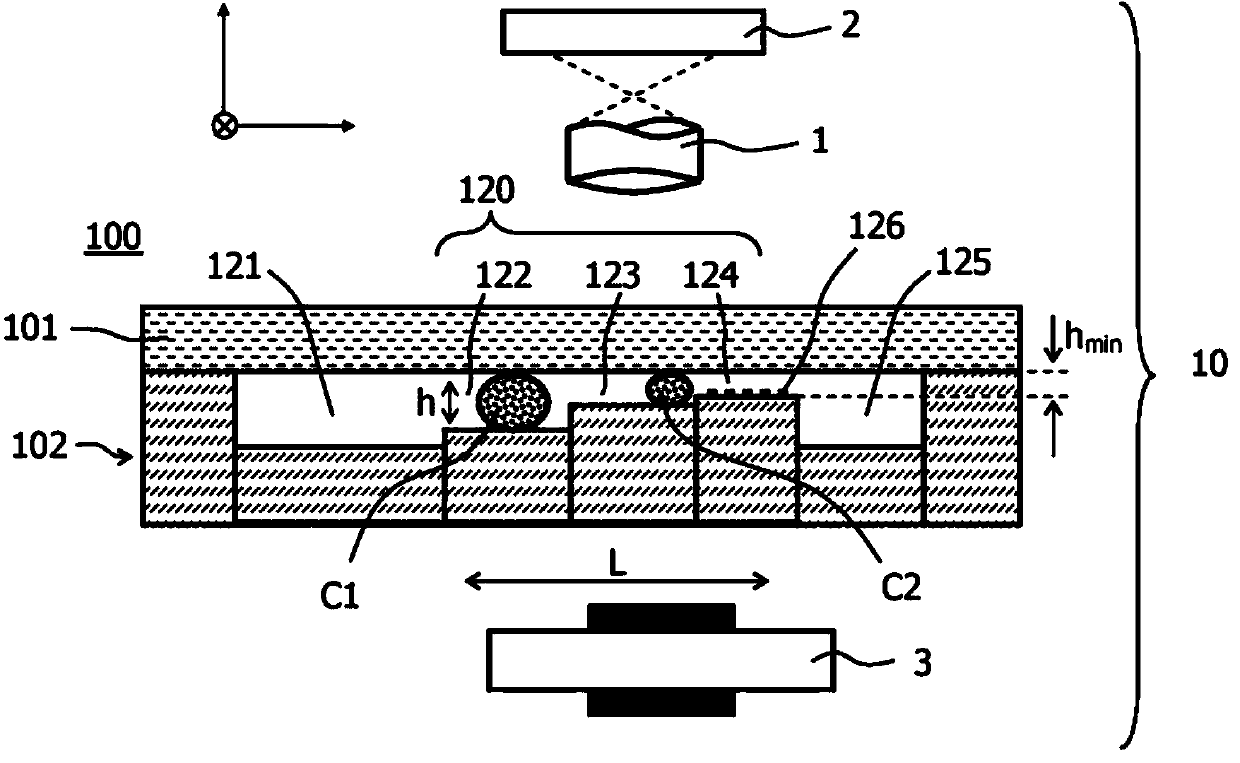
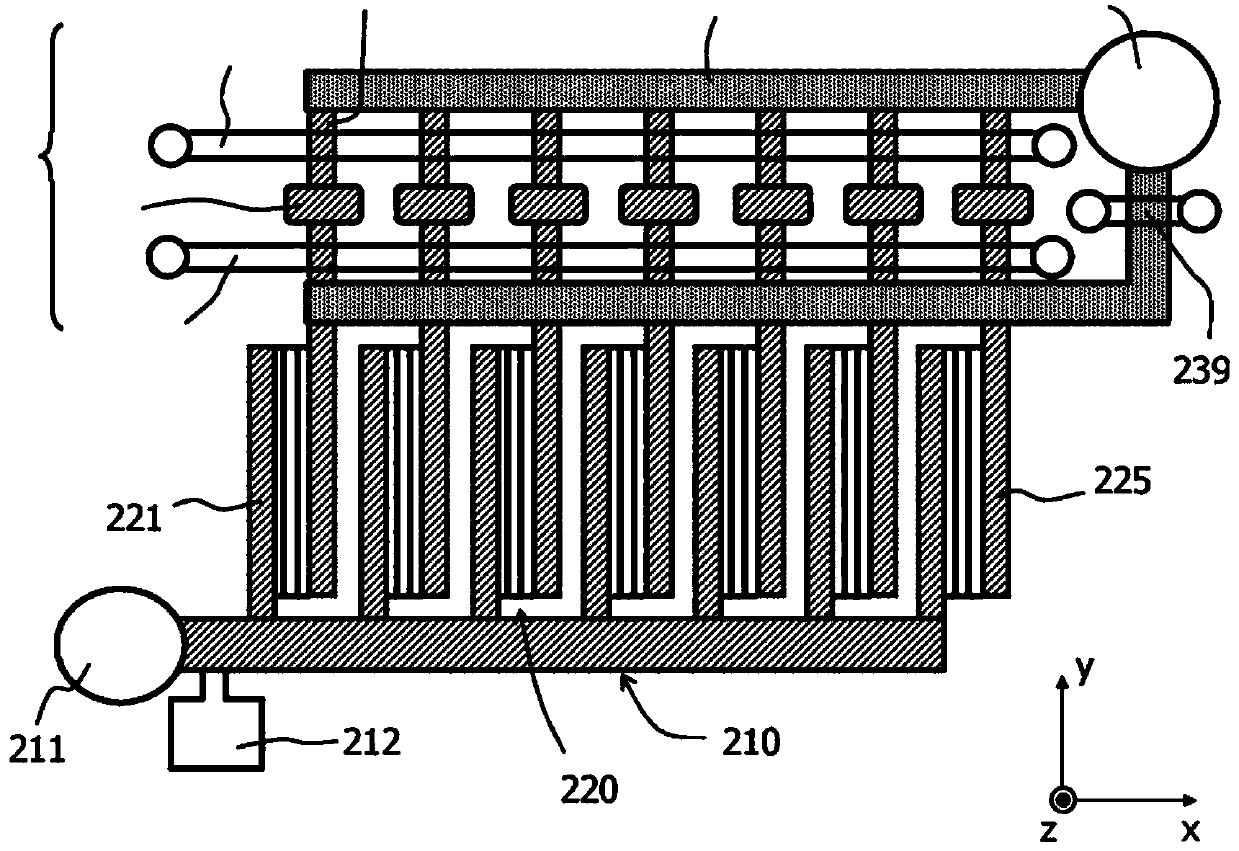
A filter element (200) and a method are disclosed for retaining particles of a medium, for example (rare) cells of blood. The filter element (200) comprises at least one opening (220) that (i) has an elongate cross section and / or (ii) has a cross section that decreases in flow direction (x) and / or (iii) is bordered by a transparent wall. Preferably, the filter element (100, 200, 300) is provided with a plurality of elongate openings (220) of stepwise decreasing cross section that are arranged on a common transparent slide. Thus high flow rates can be realized throughout the filtering process, and the retained particles are immediately ready for visual inspection without a need for a further transfer.
Owner:KONINKLJIJKE PHILIPS NV
Fluorescence immunochromatographic assay and kit for quantitative detection of creatine kinase isoenzyme (CK-MB)
ActiveCN102520173ASolve the backgroundSolve the signal indistinguishableMaterial analysisDiseaseCreatine kinase
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of acute myocardial infarction marker-creatine kinase isoenzyme (CK-MB). The fluorescence immunochromatographic assay for quantitative detection of the CK-MB realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the CK-MB, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
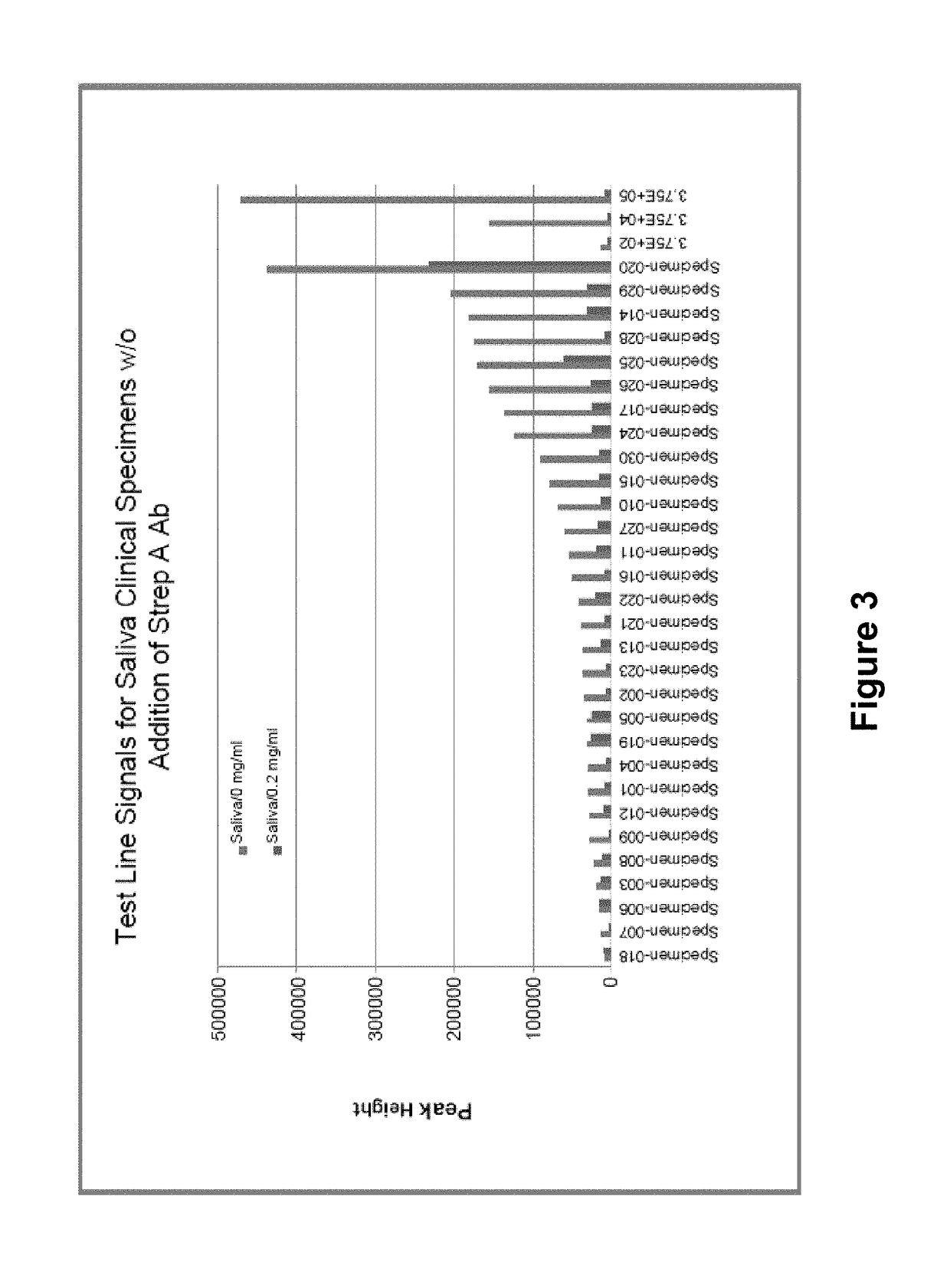
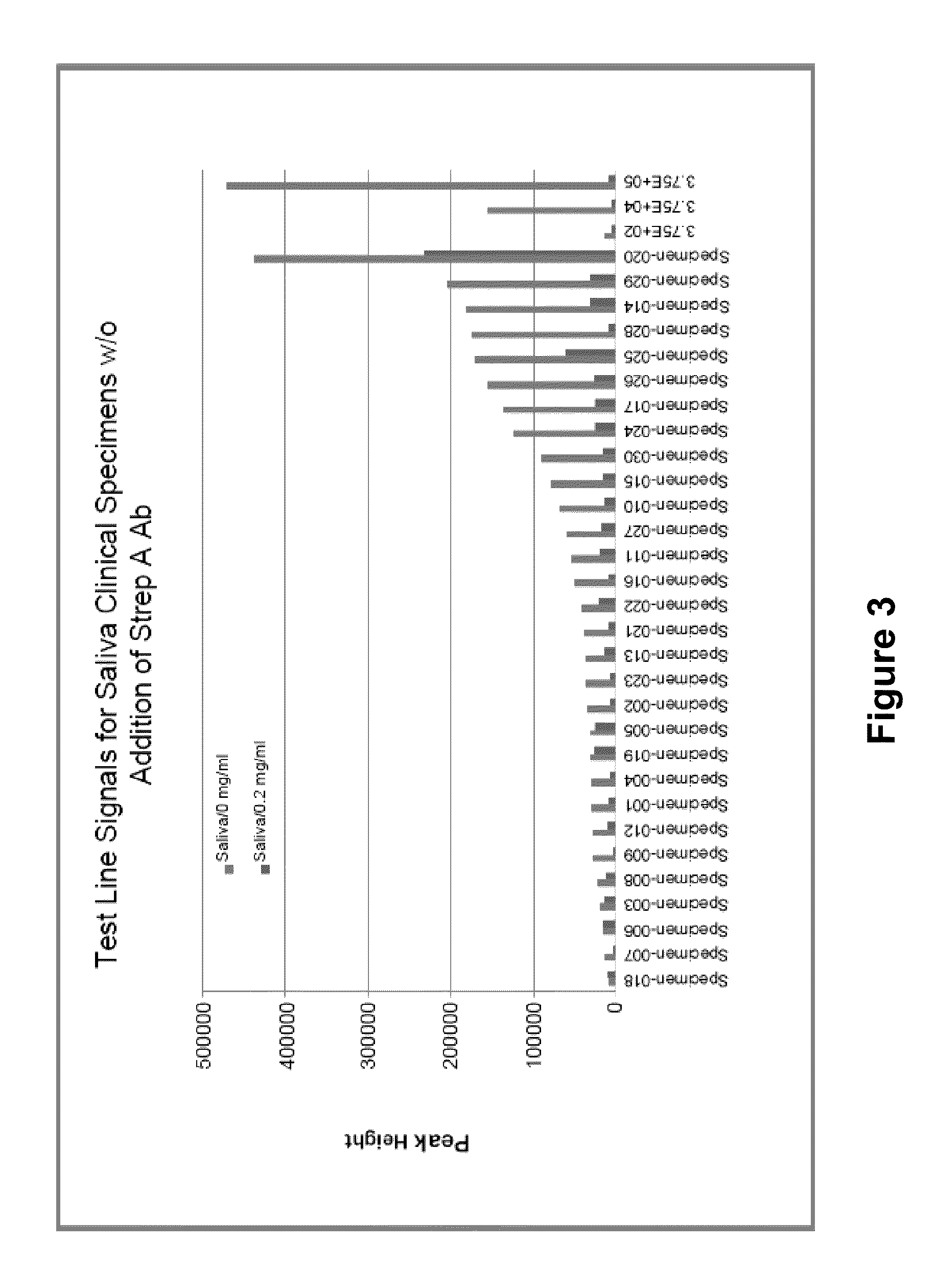
N-acetyl-D-glucosamine for enhanced specificity of Strep A immunoassay
ActiveUS10168329B2Reduce probabilityReduce false alarm rateMaterial analysisN Acetyl D GlucosamineStreptococcus mitis
Methods, compositions and kits for detecting Group A streptococcus in a biological sample are described. More particularly, the present disclosure provides an immunoassay in which the specificity of detection of Group A streptococcus is enhanced by addition of N-acetyl-D-glucosamine. These methods, compositions and kits are useful in convenient, reliable and early diagnosis of streptococcal infection in a human subject.
Owner:QUIDEL
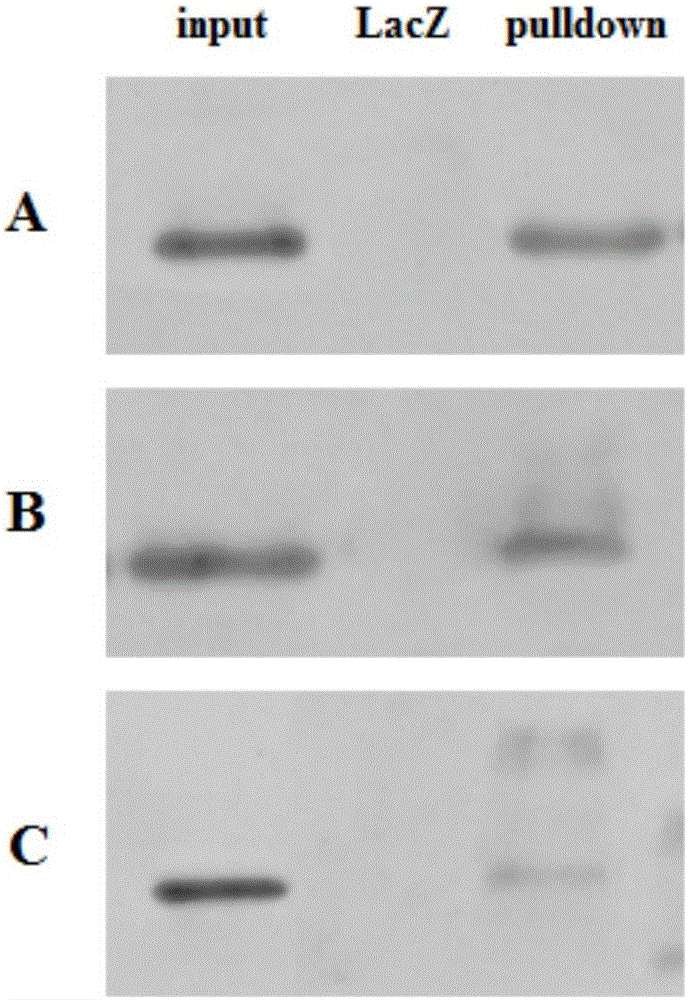
DNA pulldown method and kit
ActiveCN106093437AReduce non-specific bindingImprove non-specificityBiological testingMagnetic beadGenomic DNA
The invention relates to a DNA pulldown method and a kit. The method includes the following steps of pretreating magnetic beads, wherein the magnetic beads marked by BSA(bovine serum albumin) and oligonucleotide random primer closed streptavidin are used; preparing a probe-magnetic bead compound; extracting and pretreating cell nucleus protein, wherein deoxyribonuclease is added into an extracting nucleoprotein sample for incubation, the magnetic beads are added for incubation and then removed, and supernatant is collected; carrying out pulldown; collecting the protein sample. The DNA pulldown method has the following advantages that BSA and an oligonucleotide random primer are used for closing the magnetic beads in advance, and nonspecific binding between the magnetic beads, protein and genomic DNA is reduced. Nuclease contamination is prevented through systemic nuclease protection, stability and reliability of experiments are improved, repeatability is high, and the experiment process is simplified.
Owner:GUANGZHOU BIOSENSE BIOSCI
Polymerase chain reaction (PCR) amplification buffer solution for high-GC-content genes, and amplification method and application of PCR amplification buffer solution
InactiveCN105861491AEnhance specific bindingCompatible with broad spectrumMicrobiological testing/measurementFermentationBetaineApplications of PCR
The invention discloses a polymerase chain reaction (PCR) amplification buffer solution for high-GC-content genes. The PCR amplification buffer solution for the high-GC-content genes is prepared from the following components: 150m mol / L of Tris-HCl, 40 m mol / L of (NH4)2SO4, 2.6 mol / L of betaine monohydrate, 0.02% of emulsifying agent Tween 20 and 20% of PCR reaction enhancer; after the components are collocated, a product is filtered to remove bacteria and then is stored at the temperature of 20 DEG C below zero. The invention also discloses an amplification method and application based on the PCR amplification buffer solution for the high-GC-content genes. The PCR amplification buffer solution uses the (NH4)2SO4 to replace KCL, thus improving the specificity of combination of a primer and a template and being capable of being compatible with a broader spectrum of Mg<2+> range.
Owner:马琪
Two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH) and preparation method of kit
InactiveCN107621540ALong fluorescence lifetimeHigh luminous intensityMaterial analysisQuantitative accuracyBlood plasma
The invention discloses a two-photon fluorescence immunochromatography kit for quantitative determination of anti-Mullerian hormone (AMH), which utilizes fluorescent dye as a maker. The two-photon fluorescence immunochromatography kit, realizing fluorescence immunochromatography quantitative determination, has the advantages of good stability, wide linear range, good specificity, high sensitivity,high quantitative accuracy and easy and quick operation, can be applied to detection of whole blood samples, serum samples and plasma samples simultaneously, and is applicable to medical treatment ofhospital at different levels and family practice.
Owner:DEMAIJI BIOTECH BEIJING
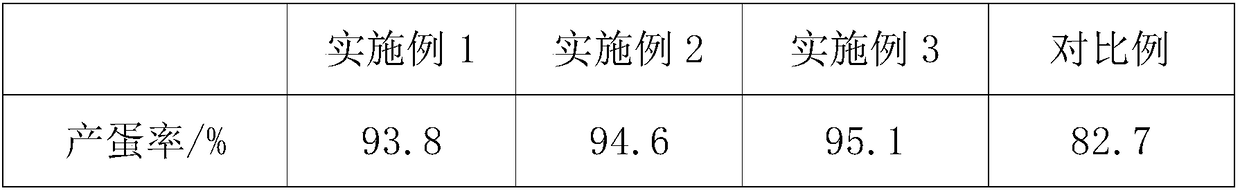
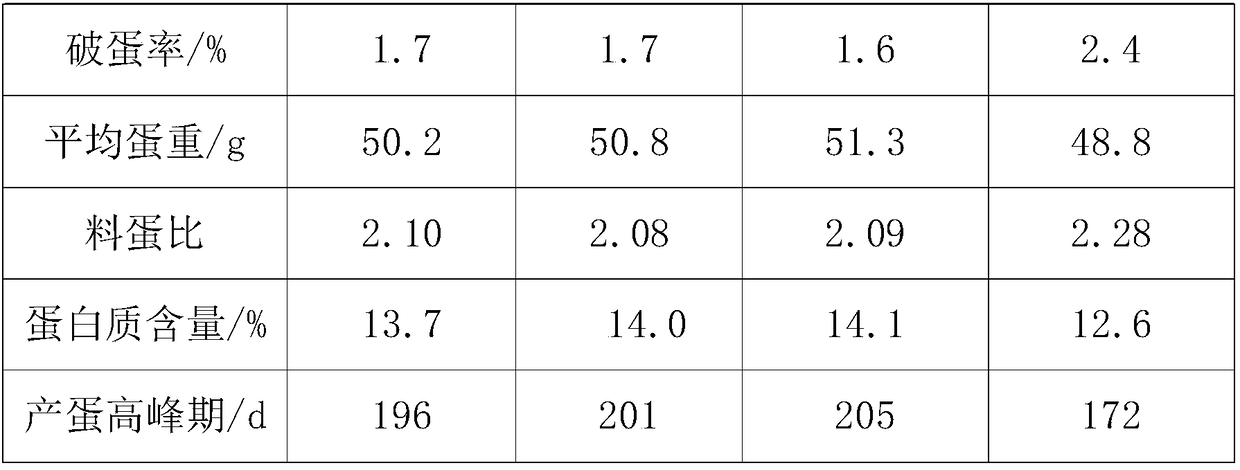
Feed for improving egg yield of laying hens, preparation process and feeding method of feed
InactiveCN108783072AMaintain continuous high productionEasy to synthesizeFood processingAnimal feeding stuffBeta-CaroteneTryptophan
The present invention discloses a feed for improving the egg yield of laying hens, a preparation process and a feeding method of the feed. The feed consists of 90-93% of a basic feed, 5-7% of dried traditional Chinese medicine residue powder and 2-3% of a functional additive. The functional additive comprises the following components in parts by weight: 0.02-0.03 part of choline chloride, 0.5-0.7part of resveratrol, 0.06-0.1 part of dihydropyridine, 2-3 parts of soybean isoflavones, 3-5 parts of bee pollen, 1.4-2.6 parts of glycyrrhizin, 3-4 parts of beta-carotene, 6-8 parts of linoleic acid,0.8-1.2 parts of iron methionine, 0.8-1 part of lysine and 1-2 parts of tryptophan. The feeding method is as follows: 1-4-week chicks are fed 6-8 times every day, and every time the feeding amount is4-5g per chick; 5-6-week chicks are fed 4-6 times every day, and every time the feeding amount is 13-15g per chick; and 8-20-week growing chickens are fed 3 times every day, and every time the feeding amount is 60-80g per chickens. The feed not only provides abundant nutrient substances for laying hens in egg-producing period, but also stimulates the secretion and expression of estrogen in the body of laying hens, performs exogenous supplement of natural plant estrogens, and prolongs the egg-production peak.
Owner:安徽鲜森绿色食品有限公司
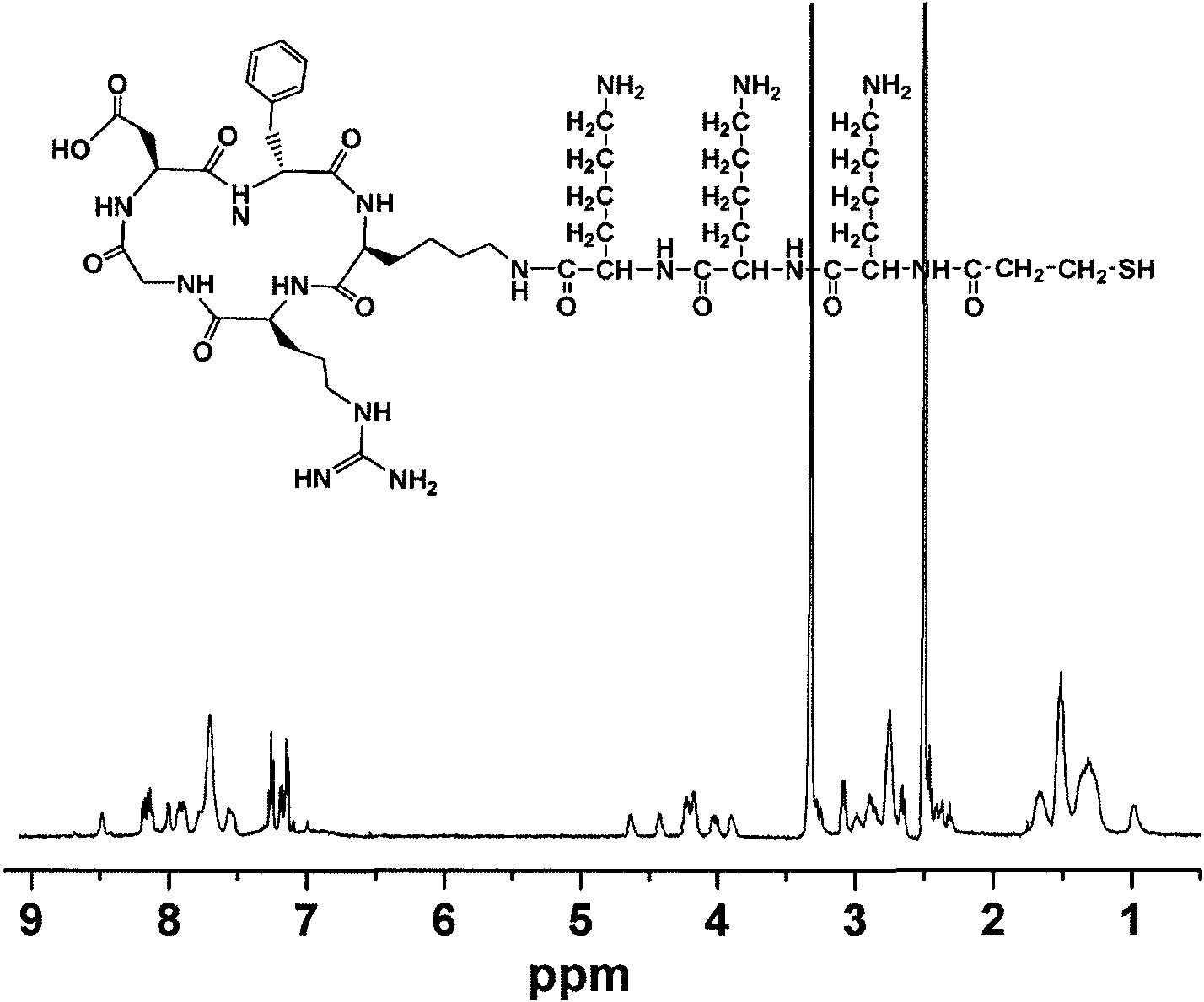
Cell adhesion promoting polypeptide and preparation method thereof
InactiveCN101817873AImprove adhesionEnhance specific bindingPeptide preparation methodsCarrier-bound/immobilised peptidesNon specificChemistry
The invention belongs to the technical field of biological materials, regenerative medicine and pharmacy and particularly relates to a polypeptide capable of obviously inducing and synchronously promoting the specific and nonspecific adhesion of cells, preparation method thereof and use thereof. The polypeptide of the invention comprises two structures. One end of the polypeptide is a circular peptide chain which has affinity with integrin, is favorable for the specific adhesion of the cells and has an arginine-glycine-aspartic acid sequence; and the other end of the polypeptide is a linear peptide chain which can react with cell envelopes to promote the non-specific adhesion of the cells. Meanwhile, the end of the branch chain of the polypeptide has a group which can be modified for functionalization, so that the polypeptide can be immobilized on the surfaces of biomaterials such as gold and polylactic acid-polyglycolide. Thus, the polypeptide can improve biocompatibility considerably and is favorable for improving cell adhesion. The polypeptide can be used for modifying biomaterials and used in targeted medicaments.
Owner:FUDAN UNIV
N-acetyl-d-glucosamine for enhanced specificity of strep a immunoassay
ActiveUS20130196337A1Reduce probabilityReduce false alarm rateBioreactor/fermenter combinationsBiological substance pretreatmentsN Acetyl D GlucosamineStreptococcus mitis
Methods, compositions and kits for detecting Group A streptococcus in a biological sample are described. More particularly, the present disclosure provides an immunoassay in which the specificity of detection of Group A streptococcus is enhanced by addition of N-acetyl-D-glucosamine. These methods, compositions and kits are useful in convenient, reliable and early diagnosis of streptococcal infection in a human subject.
Owner:QUIDEL
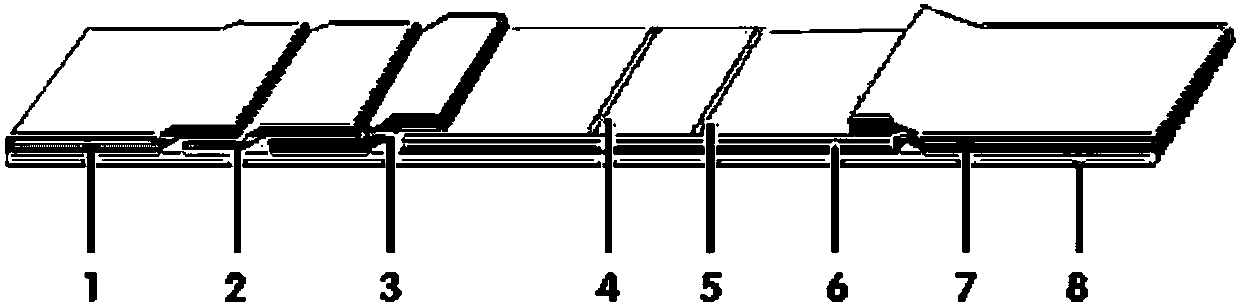
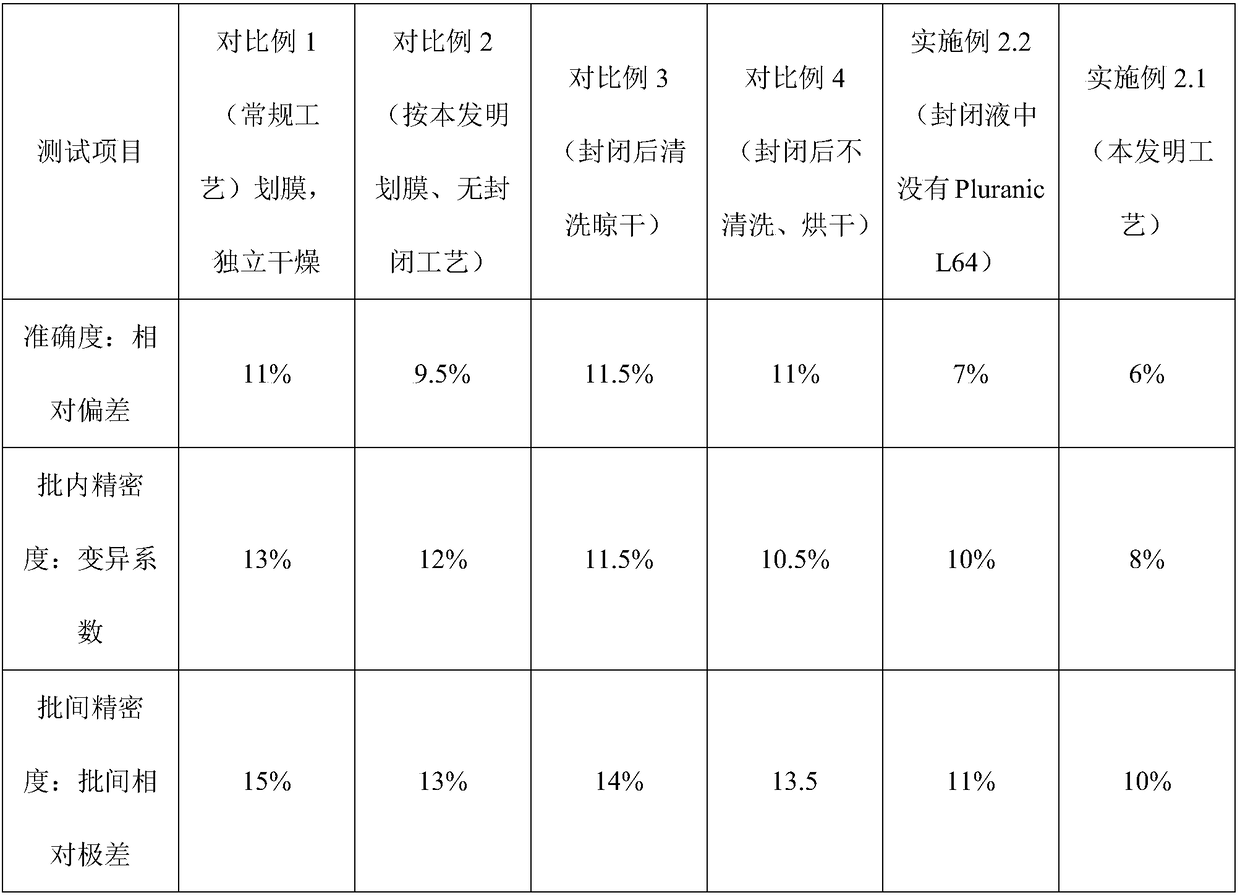
NC (Nitrocellulose Filter) membrane and production technology for preparing NC membrane by lineation
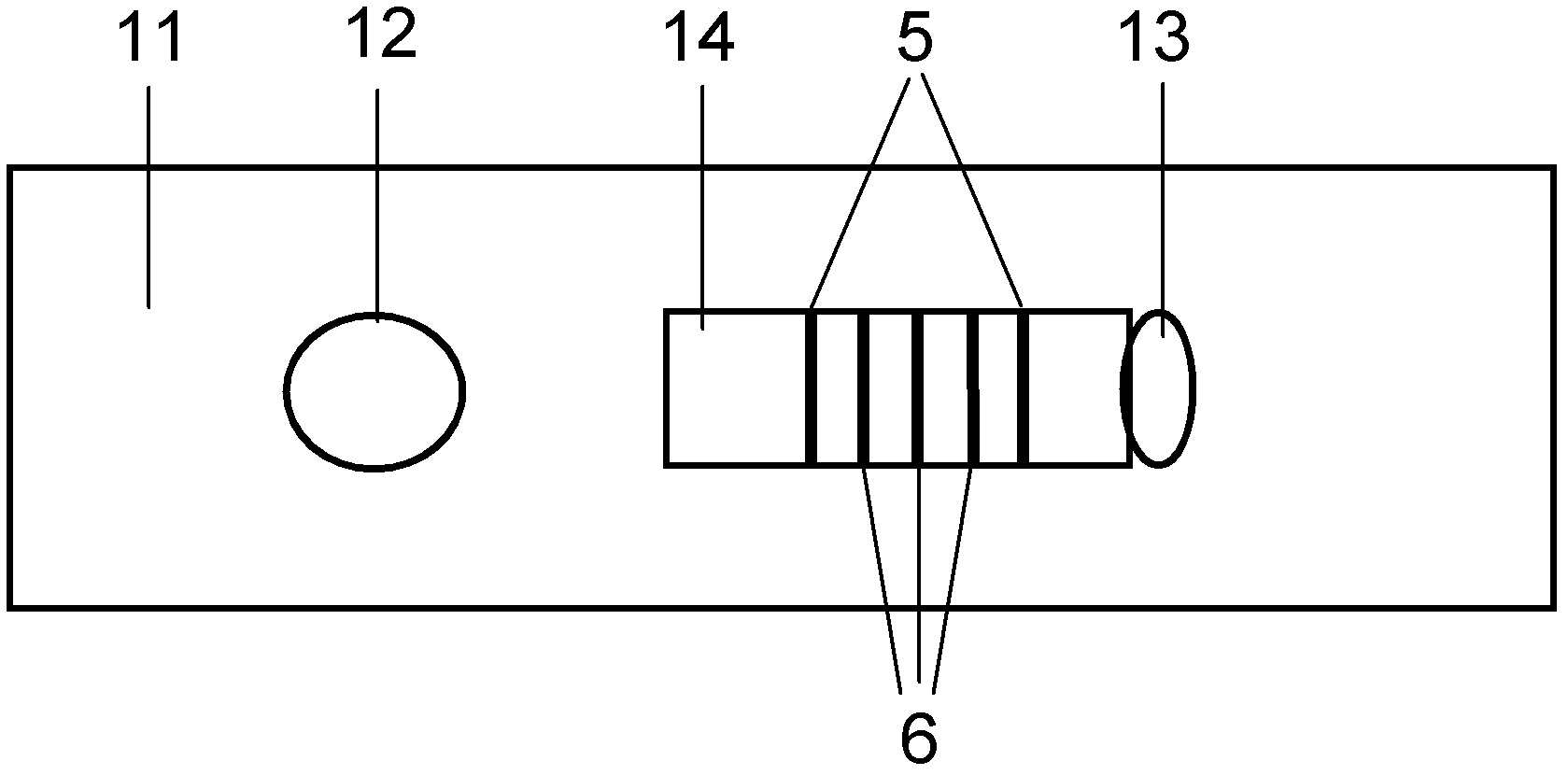
The invention discloses a production technology for preparing an NC (Nitrocellulose Filter) membrane by lineation. The production technology comprises the following working procedures: operating membrane lineation in a continuous state, and drying after membrane lineation; after the working procedure of drying after membrane lineation is carried out, sealing a membrane which operates under a continuous state, and drying after sealing, wherein the operation time of the working procedure of drying after membrane lineation is 2-4 min, and the operation time of membrane sealing and working procedure of drying after sealing is 3-6 min. The NC membrane obtained by the technical scheme of the invention can be used for effectively avoiding the diffusion and the widening of an antibody line, the detection fluorescence of the antibody line is centralized, meanwhile, the specificity combination of the antibody line is improved so as to obviously lower the intra-batch differences and the inter-batch differences of production, and the accuracy and the repeatability of a detection result are improved.
Owner:HEBEI TEWENTE BIOTECH DEV CO LTD
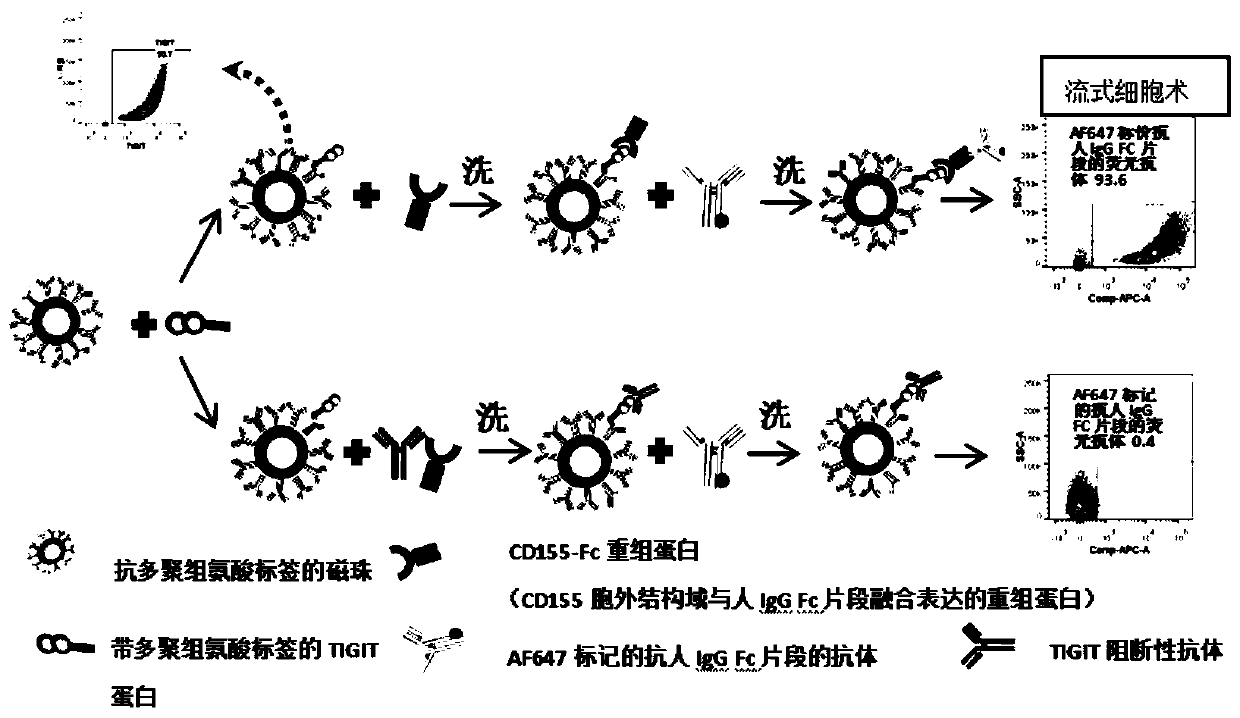
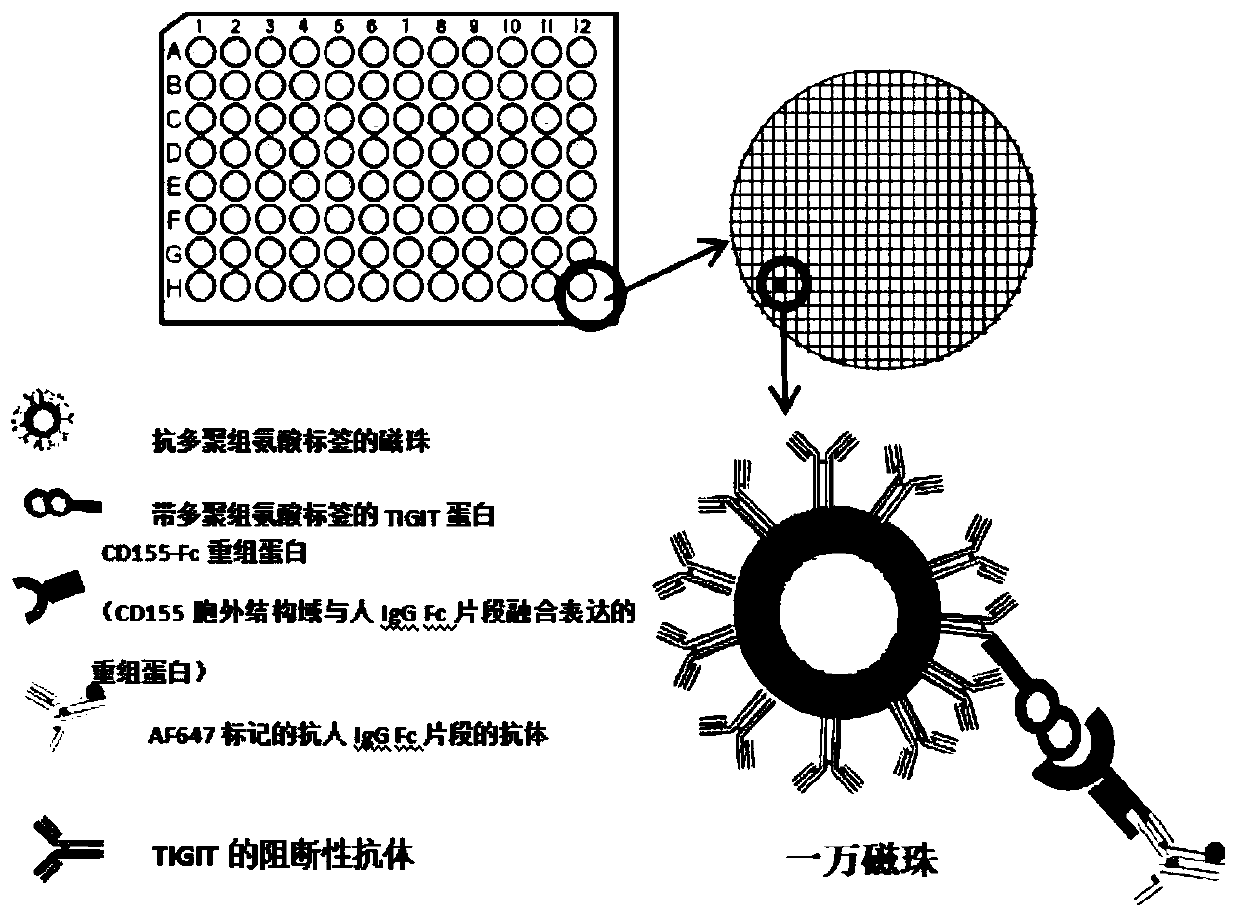
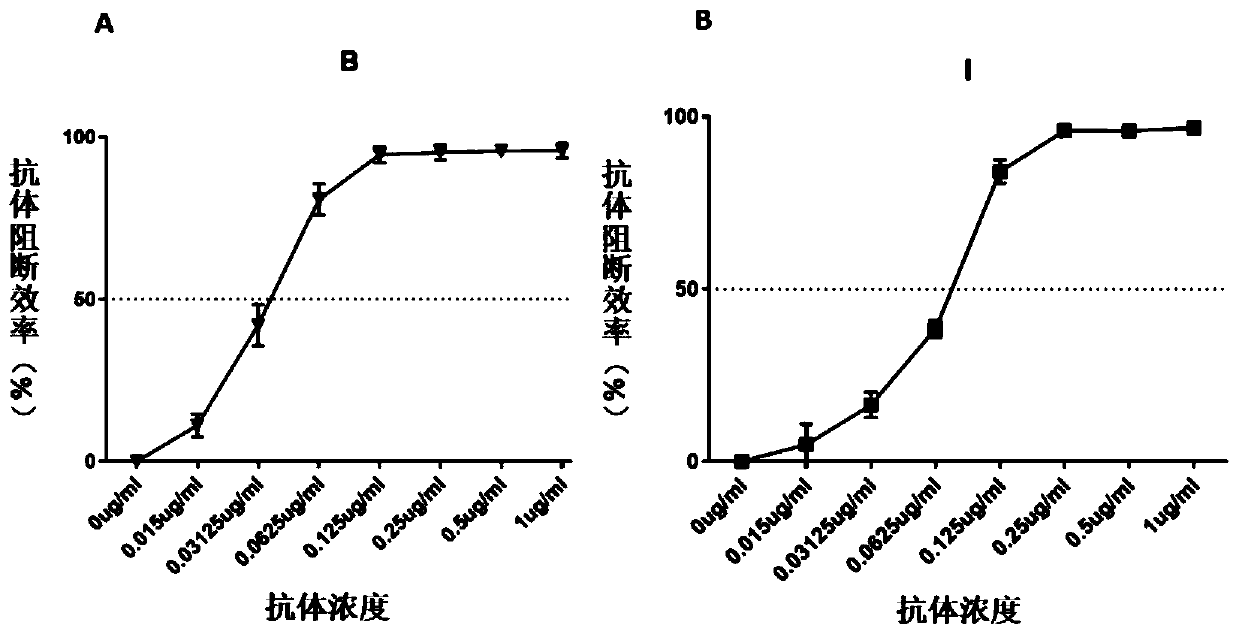
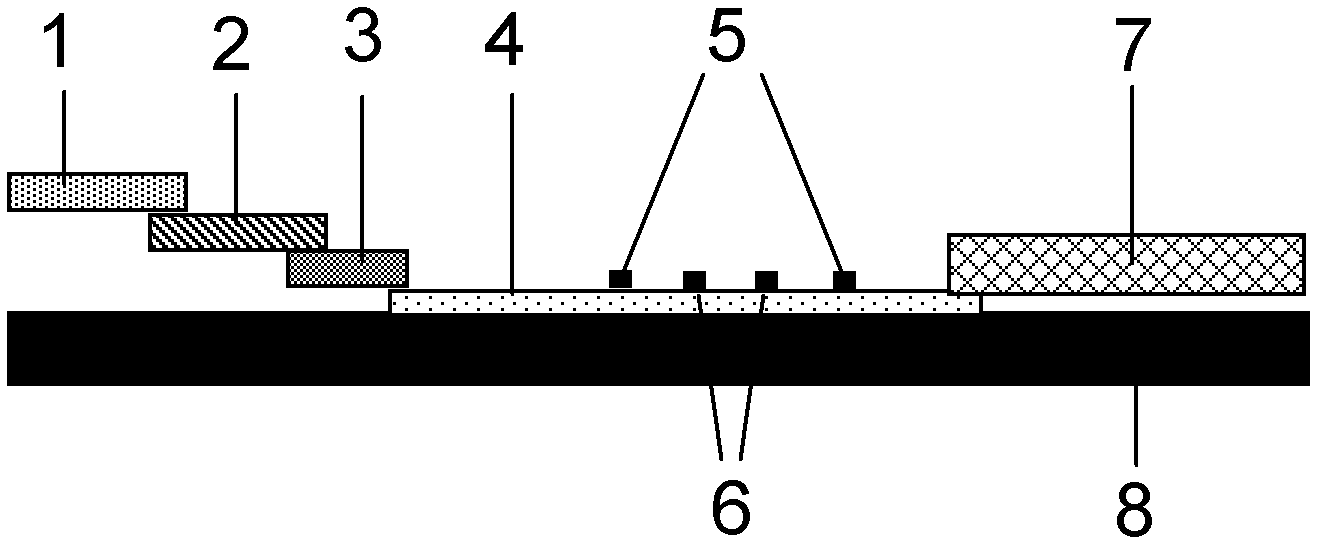
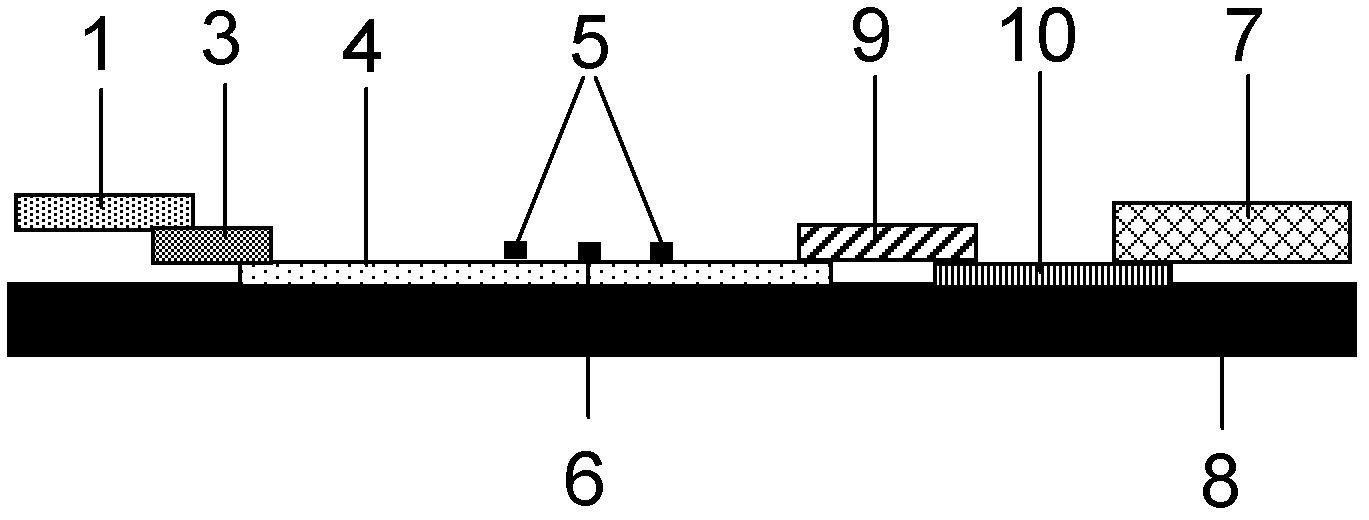
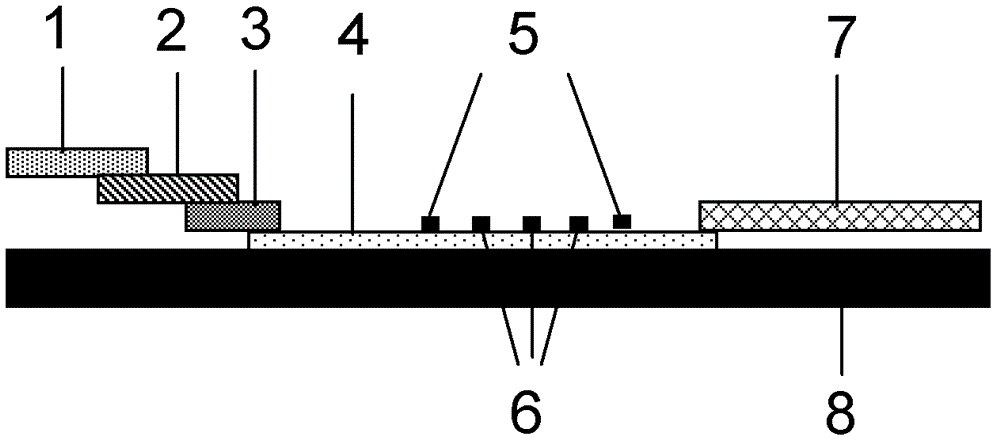
Method for evaluating blocking activity of immune checkpoint molecular blocking antibody by magnetic beads
The invention belongs to the field of immunological detection and particularly relates to a method for evaluating the blocking activity of an immune checkpoint molecular blocking antibody by magneticbeads. Magnetic beads with solid-phase anti-tag molecular antibodies and immune checkpoint molecular protein expressing label molecules (label I) subjected to solid-phase on the surfaces of the magnetic beads are used; the magnetic beads can be specifically bound with a ligand of an immune checkpoint molecule with another label molecule (label II), and then a label II specific binding antibody labeled by a biologic substance (fluorescent or visible light) is used for quantitatively detecting the immune checkpoint molecular protein / ligand molecular protein compound on the surface of the magnetic bead. When the blocking antibody of the immune checkpoint molecule, the magnetic beads with the immune checkpoint molecule protein in the solid phase and the ligand protein coexist, the blocking activity of the antibody is inversely proportional to the final luminous intensity of the magnetic beads, so that quantitative analysis on the blocking activity of the antibody is realized through lightintensity detection. The magnetic bead method is simple to operate, quick in reaction, economical and time-saving.
Owner:SHANXI UNIV
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165BHigh luminous intensityWide excitation spectrumMaterial analysisCritical illnessUrine sample
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192BHigh luminous intensityWide excitation spectrumBiological testingCreatine kinaseFluorescence
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceQuantum dot
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay kit for quantitatively detecting heart fatty acid binding protein
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Carbonic ester polymer vesicle for micromolecule-carrying medicine, and preparation method and application of carbonic ester polymer vesicle
ActiveCN111714457ASmall side effectsStrong enrichmentOrganic active ingredientsPharmaceutical non-active ingredientsPolyesterPolymer science
The invention discloses a carbonic ester polymer vesicle for a micromolecule-carrying medicine, and a preparation method and application of the carbonic ester polymer vesicle. The medicine is a hydrophilic negatively-charged micromolecule medicine; a reversible crosslinking biodegradable polymer vesicle with an asymmetric membrane structure is obtained in a way that a polymer is subjected to crosslinking after the polymer is self-assembled; the molecular chain of the polymer includes a hydrophilic chain segment and a hydrophobic chain segment, which are connected in sequence; the hydrophobic chain segment comprises a polycarbonate chain segment and / or polyester chain segment; the vesicle loads the medicine through the compounding of intracavity calcium acetate and medicine, i.e., pemetrexed in an alkaline buffer solution; a membrane is reversible crosslinking biodegradable polycarbonate with good biocompatibility; the dithiolane of a side chain is similar to a human body natural antioxidant, i.e., thioctic acid; and a shell takes PEG (polyethylene glycol) as a background and simultaneously contains a targeting molecule capable of carrying out targeting on cancer cells. The carbonic ester polymer is expected to become a nanometer medicine system which combines the advantages of being simple, stable and multifunctional with the advantage that the hydrophilic micromolecule medicine can be favorably subjected to entrapment into a whole. Under the method, the nanometer medicine system can be used for effectively coating a great quantity of negatively-charged micromolecule medicines.
Owner:SUZHOU UNIV
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422BHigh luminous intensityWide excitation spectrumBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com