Cell adhesion promoting polypeptide and preparation method thereof
A cell adhesion, a part of the technology, applied in the field of peptides and its preparation, can solve the problem that the affinity efficiency needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
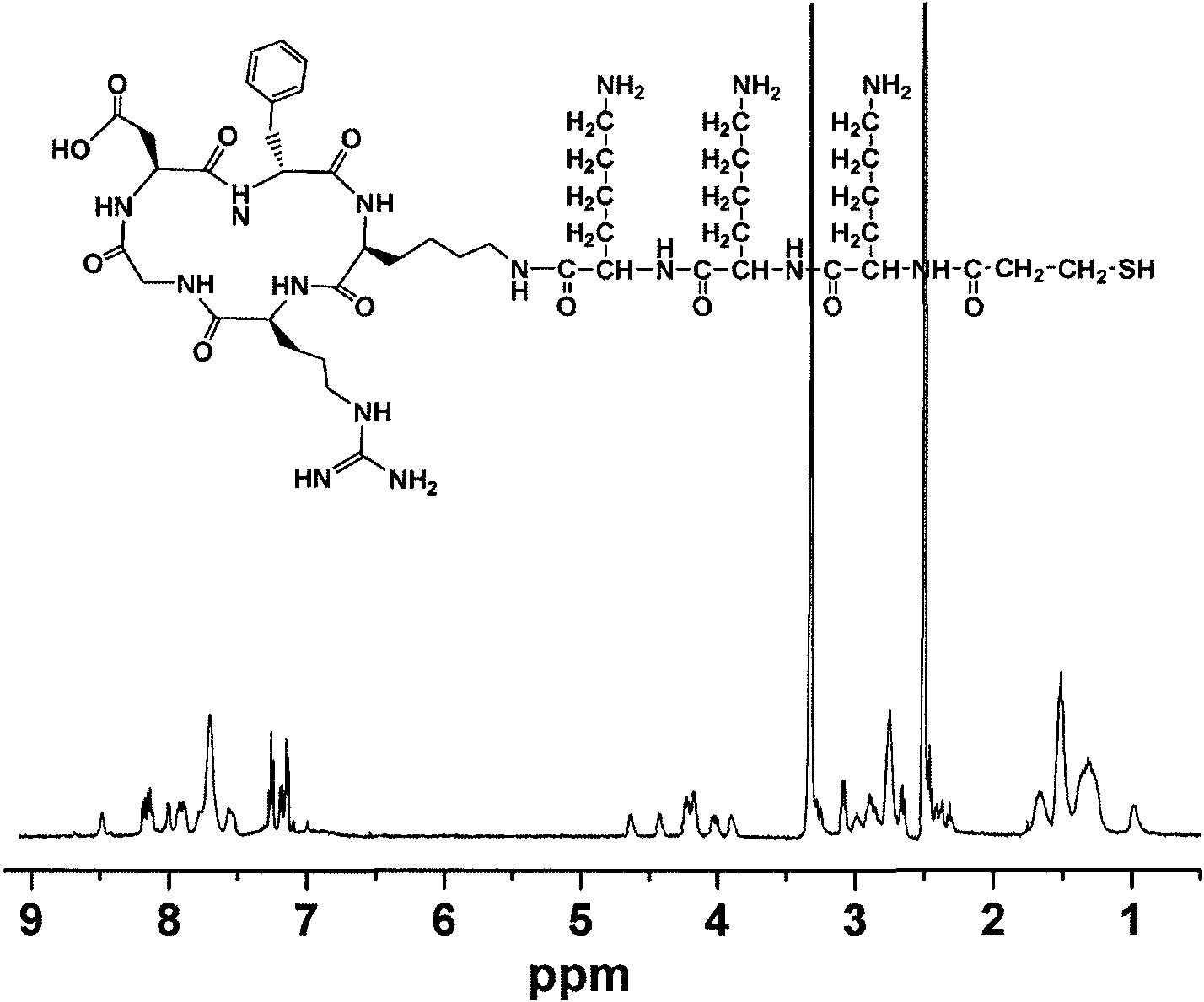
[0046] The detailed structural formula of the cyclic peptide / linear peptide hybrid polypeptide molecule (1) referred to as RGD-K can be found in figure 1 , after using the single character of amino acid residues, the structural formula can be expressed as:
[0047]
[0048] Synthetic steps include:
[0049] 1) Linear polypeptide
[0050] NH 2 Synthesis of -Asp(OtBu)-D-Phe-Lys[-Lys(Boc)-Lys(Boc)-Lys(Boc)-thiol]-Arg(Pbf)-Gly-OH
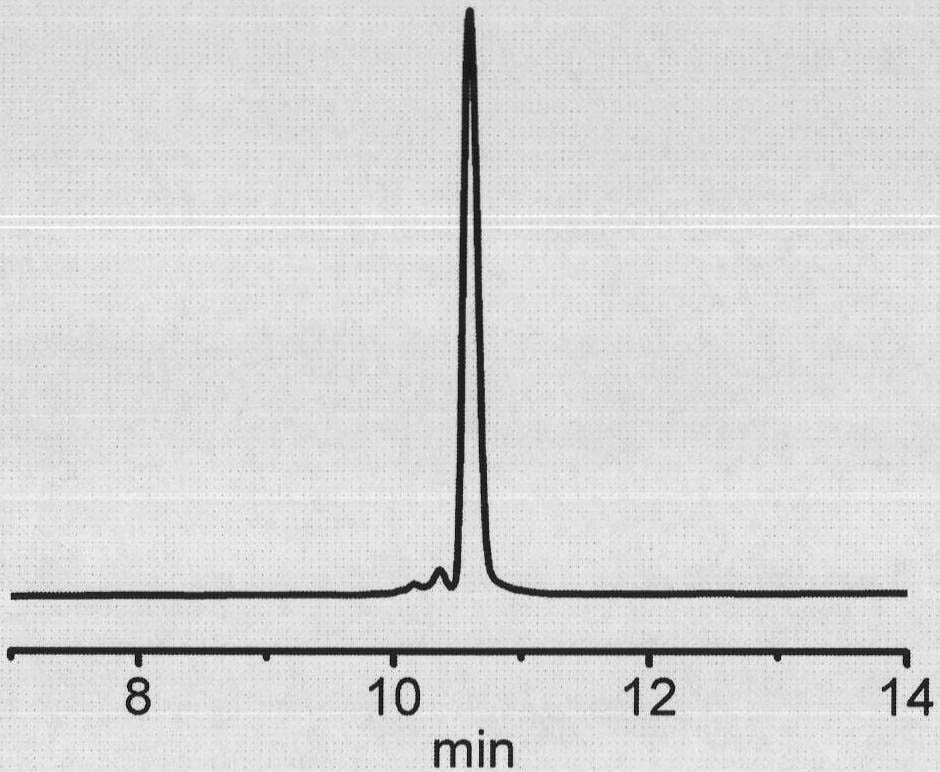
[0051] The synthesis of linear polypeptides is carried out on dichlorotrityl resin by the method of 9-fluorene-methoxyacyl (Fmoc). First soak a certain amount of resin with anhydrous dichloromethane for 20 minutes, add Fmoc protected glycine pre-dissolved in anhydrous dichloromethane, and N, N-diisopropylethylamine (DIPEA), and react at room temperature 2 hours. The unreacted active sites on the resin were subsequently reacted with alcohol for 15 minutes to perform capping. After completion of the reaction, clean the resin with dimethyl sulfoxi...
Embodiment 2
[0065] The synthesis of polypeptide molecule (2), structural formula is as follows:
[0066]
[0067] Linear Polypeptide NH 2 -Asp(OtBu)-D-Phe-Lys[-[Lys(Boc)] 20The synthesis of -thiol(Trt)]-Arg(Pbf)-Gly-OH is similar to the synthesis steps of the linear polypeptide in Example 1, except that during the reaction of the side chain, the sequence of the added amino acid becomes, Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Aloc-Lys(Fmoc)-OH, repeat the polycondensation reaction of Fmoc-Lys(Boc)-OH 20 times, 3-Tritylmercapto-Propionic acid, Fmoc-D-Phe-OH, Fmoc- Asp(OtBu)-OH. The linear polypeptide with protective group, the cyclic peptide / linear peptide hybrid polypeptide after cyclization and removal of the protective group are purified by preparative HPLC to obtain the final white pure product with a purity of 98%. MALDI-TOF MS: m / z=2949.89 for [M+H] + .
Embodiment 3
[0069] The synthetic of polypeptide molecule (3), structural formula is as follows:
[0070]
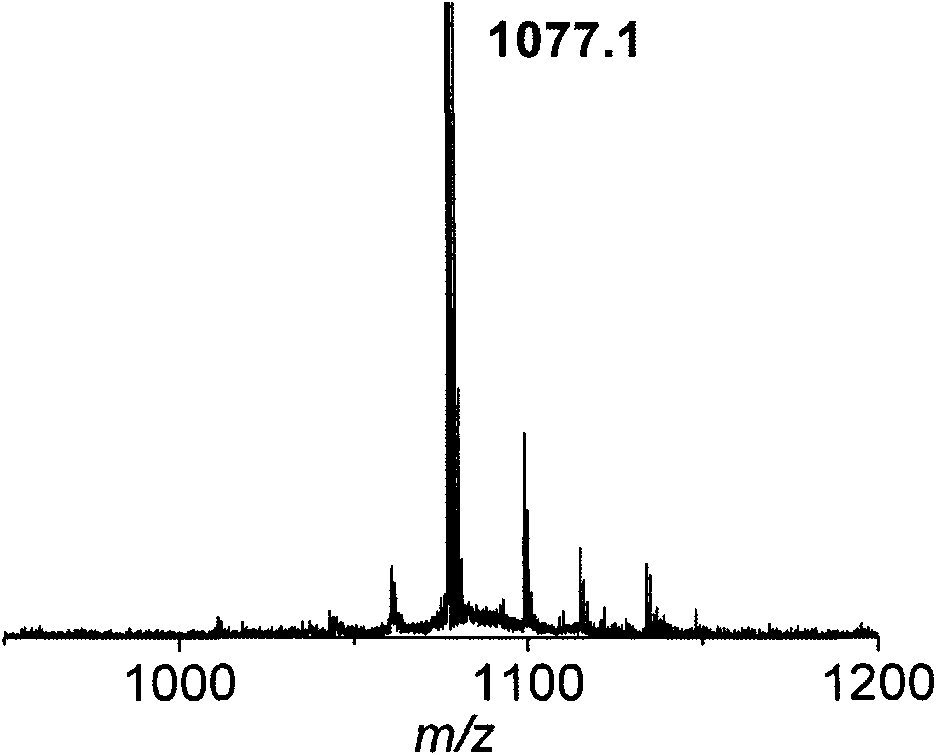
[0071] Linear Polypeptide NH 2 -Asp(OtBu)-D-Tyr-Lys[-Arg(Pbf)-Arg(Pbf)-Arg(Pbf)-thiol(Trt)]-Arg(Pbf)-Gly-OH is synthesized and linear in Example 1 The synthesis steps of the polypeptide are similar, except that when the side chain is reacted, the sequence of the added amino acid becomes, Fmoc-Gly-OH, Fmoc-Arg(Pbf)-OH, Aloc-Lys(Fmoc)-OH, Fmoc-Arg(Pbf )-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Arg(Pbf)-OH 3-Tritylmercapto-Propionic acid, Fmoc-D-Tyr-OH, Fmoc-Asp(OtBu)-OH. The straight-chain polypeptide with protective group, the cyclic peptide after cyclization and removal of the protective group were purified by preparative HPLC to obtain the final white pure product with a purity of 98%. MALDI-TOF MS: m / z=1177.07 for [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com