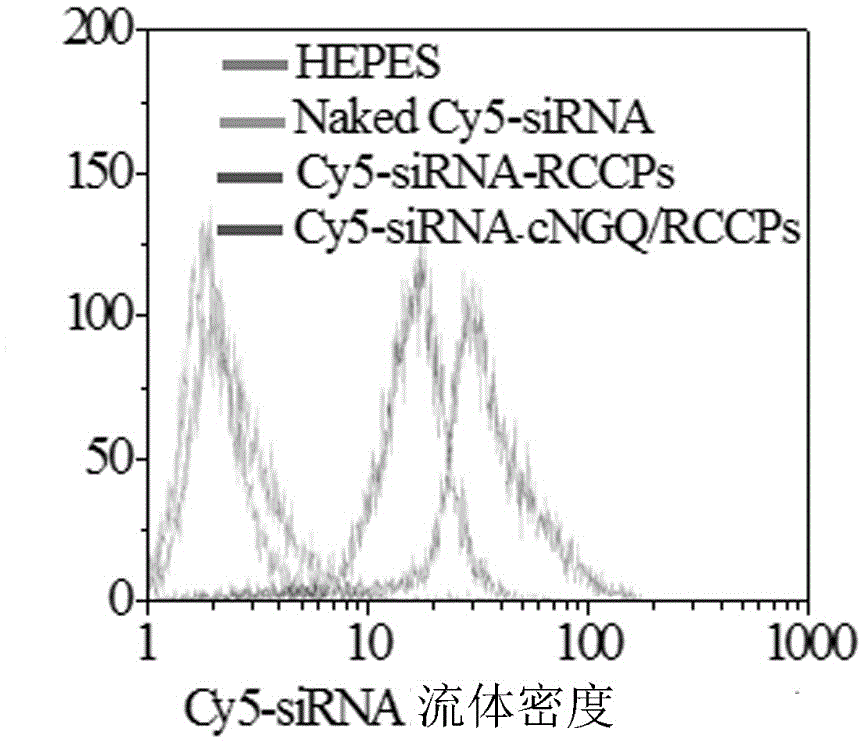
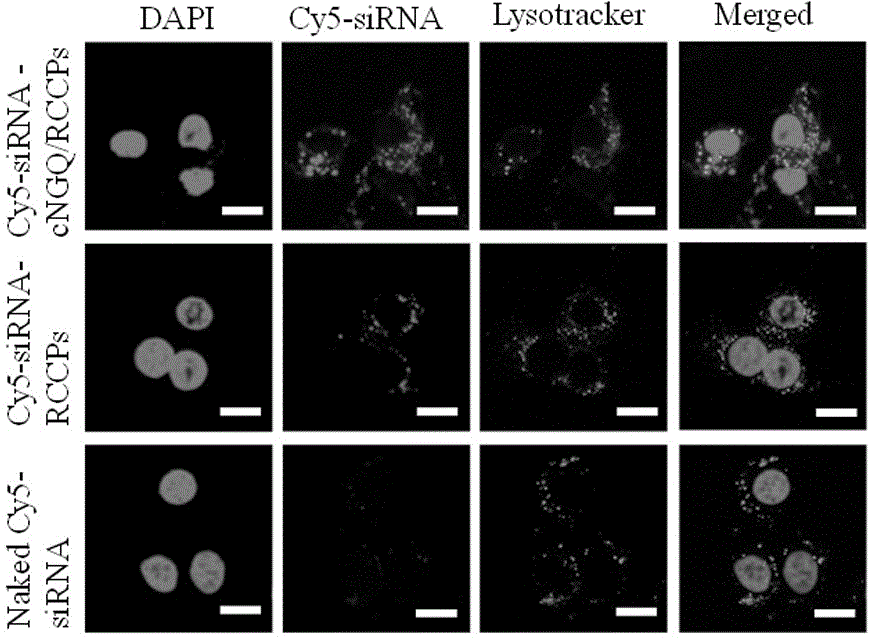
Reversibly crosslinked biodegradable polymersome with asymmetric membrane structure as well as preparation method and application thereof to nucleic acid medicines
A technology for degrading polymers and membrane structures, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, etc. It can solve the problems of instability in the body, poor targeting, and high cytotoxicity. Achieve the effect of avoiding losses and toxic side effects, solving disease problems, and efficient migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Synthesis of PEG5k-P(DTC4.4k-TMC19.8k)-bPEI1.8k block copolymer
[0084] The synthesis of PEG5k-P(DTC4.4k-TMC19.8k)-bPEI is divided into two steps, the first is ring-opening polymerization to prepare PEG5k-P(DTC4.4k-TMC19.8k) diblock copolymer, the specific operation is as follows, in In the nitrogen glove box, weigh MeO-PEG-OH ( M n = 5.0 kg / mol, 0.50 g, 100 μmol), TMC (2.0 g, 19.2 mmol) and DTC (0.50 g, 2.60 mmol) were dissolved in dichloromethane (DCM, 7.0 mL), and the ring-opening polymerization catalyst bis Zinc (bistrimethylsilyl)amine (29 mg, 75 μmol). The airtight reactor was sealed and placed under magnetic stirring in an oil bath at 40 °C for 2 days. After terminating the reaction with glacial acetic acid, precipitate twice in glacial ether, filter with suction, and dry under vacuum at room temperature to obtain PEG5k-P (DTC4.4k-TMC19.8k).
[0085] Next, PEG5k-P (DTC4.4k-TMC19.8k) was activated by p-nitrophenyl hydroxychloroformate NPC, and then...
Embodiment 2
[0087] Example 2 Synthesis of Mal-PEG6k-P (DTC4.8k-TMC19.2k)-lPEI1.2k block copolymer
[0088] The synthesis of Mal-PEG6k-P(DTC4.8k-TMC19.2k)-lPEI1.2k is similar to embodiment one, also is divided into two steps, just the initiator MeO-PEG-OH in the first step wherein is changed to Maleimide-functionalized Mal-PEG6k-OH, ring-opening polymerization of TMC and DTC to obtain Mal-PEG6k-P (DTC4.8k-TMC19.2k), whose terminal hydroxyl was activated by NPC, and then combined with linear PEI (lPEI) primary amine ( M n =1.2 kg / mol) reaction. The specific operation was similar to that of Example 1, the product was precipitated twice in glacial ether, filtered with suction and dried under vacuum at room temperature to obtain the product. Yield: 93.2%. 1 H NMR (400 MHz, DTCl 3 ): PEG: δ3.38, 3.65; TMC: δ 4.24, 2.05; DTC: δ 4.32, 3.02, PEI: δ 2.56-2.98. The number average molecular weight of the polymer is calculated as 6.0-(4.8-19.2)-1.2 kg / mol through the integral ratio of the charac...
Embodiment 3
[0089] Example 3 Synthesis of cNGQ-PEG7k-P (DTC4.8k-TMC19.2k) diblock copolymer
[0090]The synthesis of cNGQ-PEG7k-P (DTC2.8k-TMC14.2k) is similar to Example 1, and it is also divided into two steps. The initiator MeO-PEG-OH in the first step is replaced by N-hydroxysuccinimide Functionalized NHS-PEG-OH, ring-opening polymerization of TMC and DTC to obtain NHS-PEG7k-P (DTC4.8k-TMC19.2k); secondly, peptide C(NGQGEQ) (cNGQ) with free primary amines combined with NHS-PEG7k -P(DTC4.8k-TMC19.2k) is bonded by amidation reaction. Briefly, NHS-PEG7k-P(DTC4.8k-TMC19.2k) (0.5 g, 0.017 mmol) and cNGQ (20 mg, 0.033 mmol) were successively dissolved in 5 mL DMF, reacted at room temperature for 2 days, and dissolved in distilled water Dialyzed for two days (MWCO 3500), freeze-dried to obtain the product cNGQ-PEG7k-P (DTC4.8k-TMC19.2k). Yield: 81.2%. 1 H NMR (400 MHz, DMSO- d 6 ): PEG: δ 3.51; TMC: δ4.23, 1.94; DTC: δ 4.13, 2.99; cNGQ: δ 6.84–7.61. The grafting rate of cNGQ measured b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com