Dual-RMCE-mediated (dual-recombinase mediated cassette exchange-mediated) TCR (T cell receptor) gene replacement system and method
A gene replacement, gene technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1TCR-RMCE replacement experiment
[0040] 1. Construction of the carrier
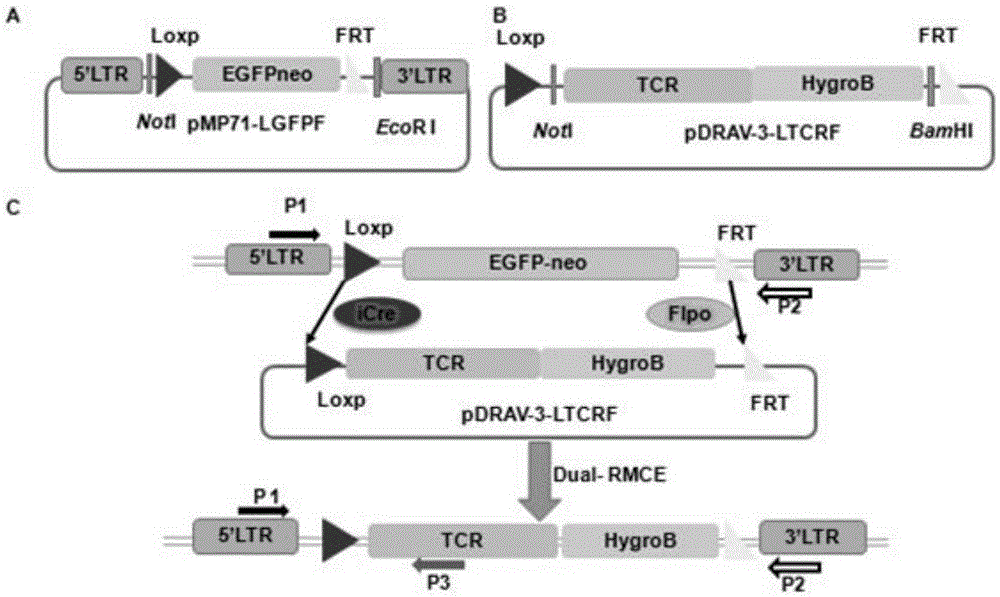
[0041] 1. Construction of the retroviral integration vector pMP71-LGFPF, which contains L oxp-E GFP neo- F RT gene replacement component, the construction method is: use not I-Loxp-F primer (5'-AATGCGGCCGCataacttcgtatagcatacattatacgaagttatcTACCGGGTAGGGGAGGCG-3') and FRT- Eco RI-R primer (5'-TGAATTCGAAGTTCCTATACTTTTCTAGAGAATAGGAACTTCTCCAGAAGAACTCGTCAAG-3'). Amplified 2.0 kb plasmid from pSR-GFP / Neo plasmid (VEC-PRT-0005 / 0006, purchased from Oligoengine Company) by PCR not I-Loxp-EFGPneo-FRT- Eco RI fragment. The PrimerStarMax PCR reaction system was used, and the reaction conditions were: 95°C, 2min; 95°C, 15s; 60°C, 30s; 72°C, 2min, 35 cycles; 72°C, 5min. After the PCR reaction, digest with NotI and EcoRI, and use glue to recover the PCR product, and connect it to the pMP71 vector.
[0042] 2. Construction of TCR replacement vector pDRAV-3-LTCRF: through NotI - TCR136...
Embodiment 2
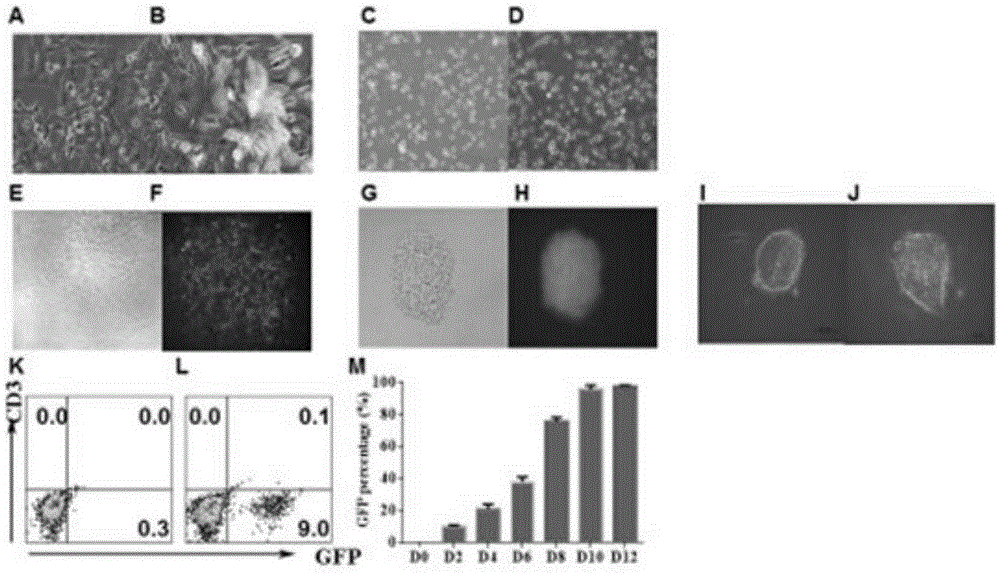
[0059] Example 2 Application of PCR to detect RMCE-mediated gene replacement
[0060] 1. The PCR process is as follows:
[0061] Collect 1×10 6 Add 500 μL digestion solution (NaCl100mM, Tris-HCl50mM, EDTA25mM, SDS0.9%), add 5μL, 20mg / mL proteinase K, digest at 55℃ for 3h, mix well, add 500μL phenol chloroform, invert up and down 20 times, Centrifuge at 13,000 rpm for 10 min; take the supernatant into a new tube, add isopropanol (about 450 μL) at a ratio of 1:1, invert and let stand for 5 min (white flocculent DNA can be seen). Centrifuge at 13000rpm for 15min, discard the supernatant, and suck up the liquid with a gun. Add 500 μL of 75% ethanol, centrifuge at 13,000 rpm for 5 minutes; pour off the supernatant, and suck up the liquid with a gun (be careful not to pour or suck off the white precipitate), and repeat the alcohol wash. Dry, add 100 μL ddH 2 O (or TE), 55 degrees, 20min to dissolve.
[0062] Select the LTR region of the retrovirus, select P1 and P2 and P3 of ...
Embodiment 3
[0065] Example 3 Teratoma formation and in vivo differentiation of ES cells
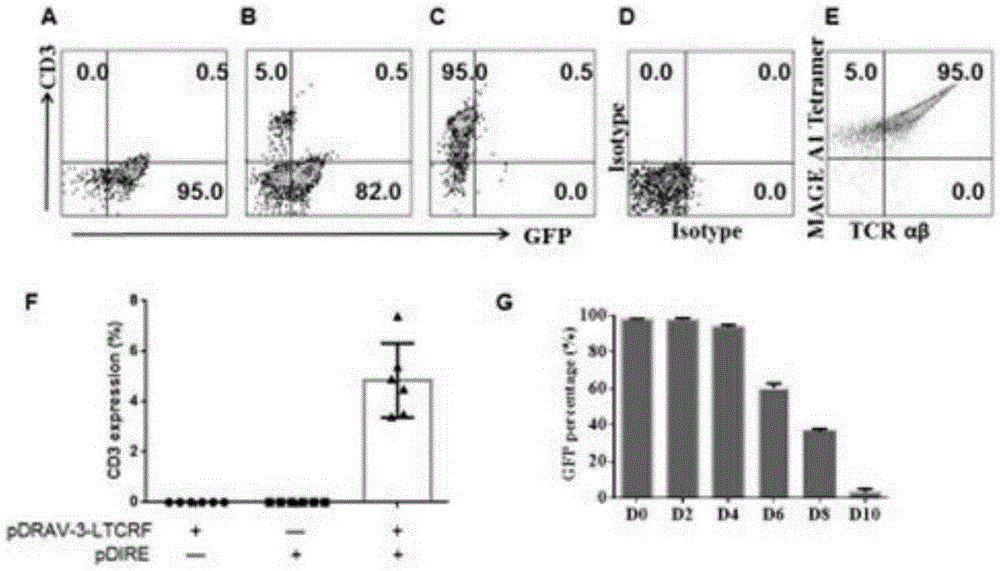
[0066] Mouse TCR-ES cells 5×10 6, subcutaneously injected into NOD-Scid immunodeficient mice, and divided into four groups: (1) TCR-ES cell group; (2) TCR-ES+OP9 cell group; (3) TCR-ES+OP9-DL1 group; (4) TCR-ES+OP9+OP9-DL1 group; (5) ES cell group. All groups were simultaneously injected with SCF (200ng) and TPO (200ng) (Pepro-Tech) at the time of injection, and after 3 weeks of differentiation, hFLT3 (200ng) and IL-7 (200ng) were injected subcutaneously around the teratoma. After 7 weeks, the mice were sacrificed to detect the cell phenotypes of teratoma, spleen, bone marrow, and peripheral blood, and the tetramer staining and cytokine secretion of T cells were detected at the same time.
[0067] Collect 10 from Teratomas 5 cells (differentiated from TCR-ES cells), add 10 cells loaded with KVLEYVIKV-MAGEA1 polypeptide 5 a T 2 cells (10 -5 M), choose anti-CD3 (0.2mg / L) + anti-CD28 (1mg / L) as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com