Combinations for t-cell immunotherapy and uses thereof
A technology of cells and uses, applied in the field of combinations and uses thereof for T cell immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0071] The present invention is illustrated in further detail by referring to the following experimental examples. The examples are provided for purposes of illustration only and are not intended to be limiting unless otherwise stated. Accordingly, the invention should in no way be construed as limited to the following examples, but rather should be understood to cover any and all variations which become apparent as a result of the teachings provided herein.
example 1
[0073] Isolation and identification of γ9δ2 T cells
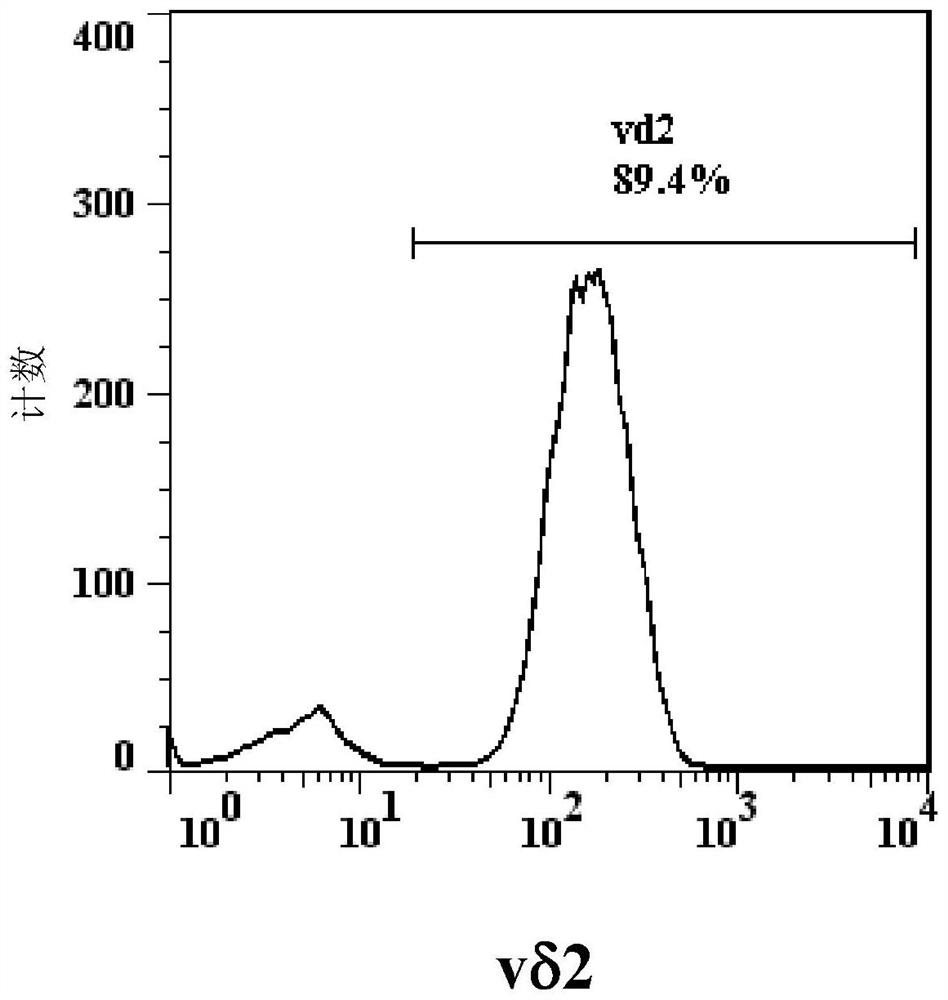
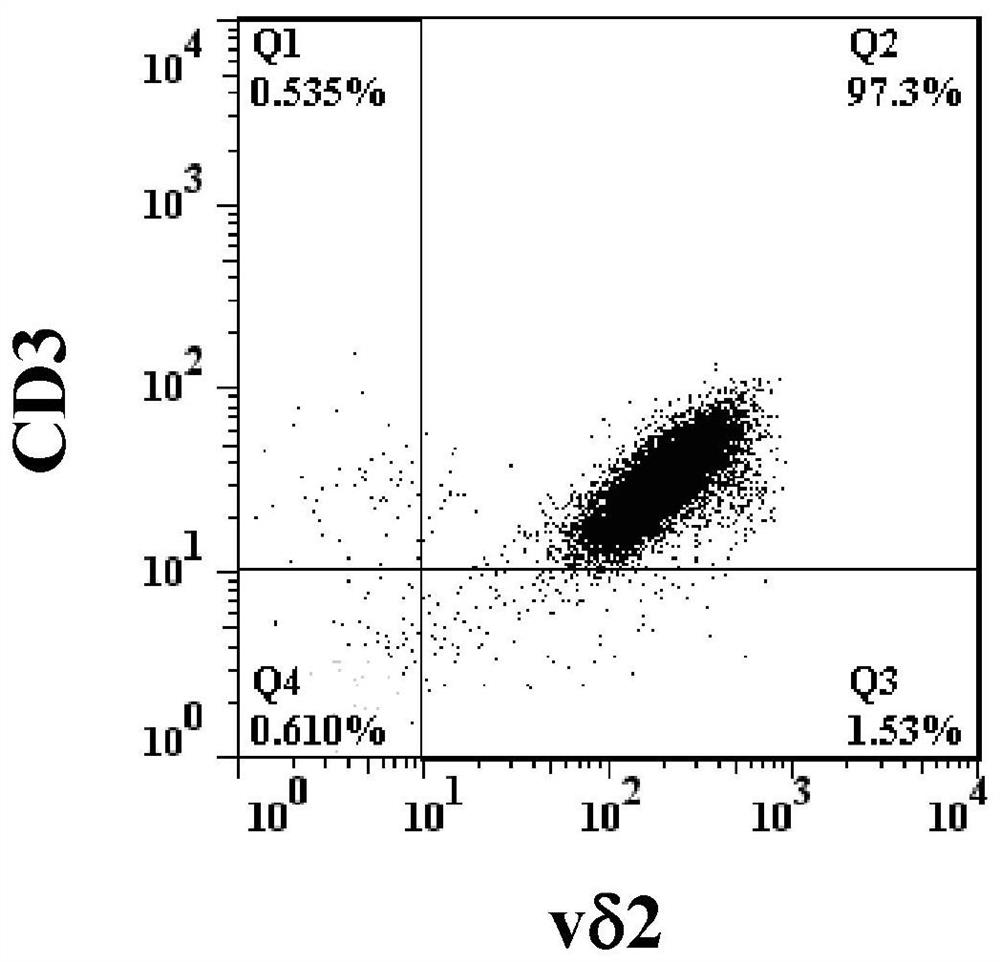
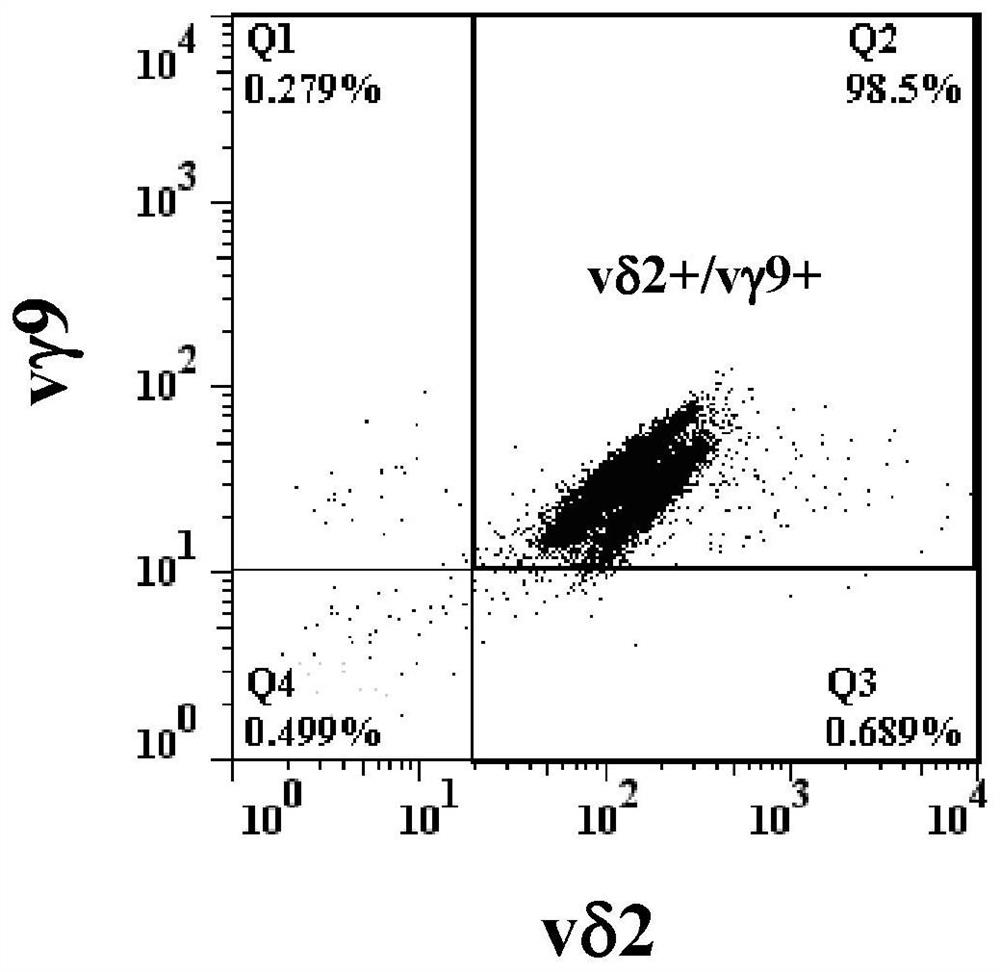
[0074] According to the objective of the present invention, it is first necessary to cultivate effector memory type γ9δ2 T cells capable of killing blood cancer cells. γ9δ2 T cells can be identified by flow cytometry using anti-CD3 antibody, anti-γ9 antibody and anti-δ2 antibody at the same time. One of the characteristics of effector memory γ9δ2 T cells is that they do not have CD27 and CD45RA molecules on the cell surface.
[0075] According to the literature published by Kondo et al., Cytotherapy, 10, 842-56 (2008), peripheral blood mononuclear cells were treated with interleukin-2 (IL-2, 1000 U / ml) and zoledronic acid (Zoledronic acid) ) (1 μM / ml) stimulated culture for 14 days, the proportion of δ2 cells in the cultured cells was analyzed by flow cytometry using anti-δ2 antibody. Then, the δ2 cells were purified with MiltenyiBiotec's reagent set (TCRγ / δ+T cell isolation Kit / human), and the purified cells were analy...
example 2
[0078] γ9δ2 T cells and In vitro test of (BiTE) toxicity against blood cancer cells
[0079] This experiment was carried out with reference to the method disclosed by Sheehy et al. (J Immunol Methods, 249, 99-110 (2001)).
[0080] In this experiment, Raji, VAL and Daudi blood cancer cells that express CD19 molecules are used as target cells, among which Raji and Daudi cell lines are CD19 + Burkett lymphoma cells, and the VAL cell line is CD19 + ALL cells. RPMI-8226 cell line is CD19 - Multiple myeloma cells.
[0081] Raji, RPMI-8226, VAL and Daudi blood cancer cell lines were stained with 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) After staining, inoculate in each well of the culture plate (5×10 4 each / hole). The γ9δ2 T cells obtained from Example 1 (1×10 6 each / hole) and (15ng / hole) were added independently or jointly to each hole containing different cells, and after 6 hours of culture, the CFSE+ cells were analyzed by flow cytometry to analyze th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com