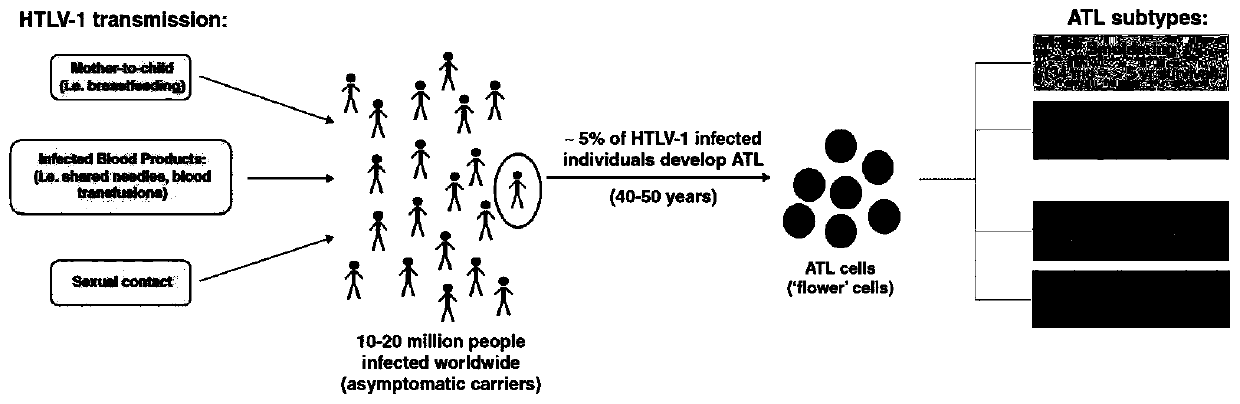
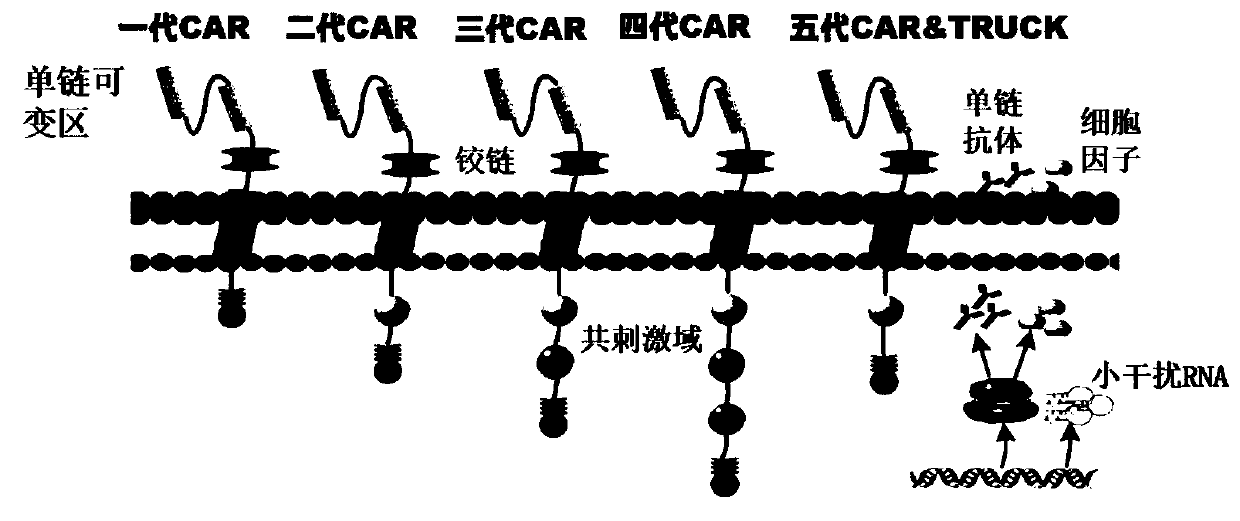
A car-t therapeutic vector for t lymphocytic leukemia and its construction method and application
A lymphocyte and leukemia technology, applied in the field of medical biology, can solve the problem of CAR-T cell therapy and other problems, and achieve the effect of flexible anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1 Construction of CAR-T cells
[0093] 1. Construction, purification and detection methods of recombinant lentiviral vectors lvCAR7-1-lvCAR7-7.
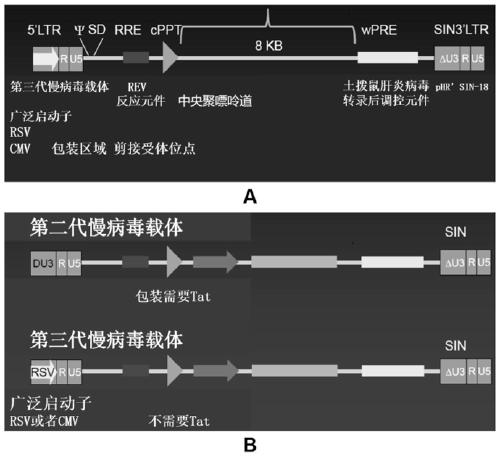
[0094] see image 3 , the construction method of the recombinant lentiviral vector of the present invention is as follows:
[0095] 1. The human EF1α promoter and CAR structure [CAR7-1~CAR7-7] were cloned into the lentiviral backbone plasmid pLenti-3G basic to obtain recombinant lentiviral plasmids pCAR7-1~pCAR7-7 respectively. The sequence and number of components are shown in Table 1;
[0096] Table 1
[0097]
[0098] (1) The lentiviral backbone plasmid pLenti-3G basic was double-digested with Cla I and EcoR I restriction endonucleases, and the product was subjected to 1.5% agarose gel electrophoresis to confirm the 5823bp fragment V1, which was recovered by tapping and placed in In the Eppendorf tube, recover the corresponding fragments (see Table 2) with the agarose gel recovery kit of MN Company, and m...
Embodiment 2
[0210] CAR-T cell pathogen detection and expression detection.
[0211] 1. Endotoxin detection;
[0212] (1), endotoxin working standard is 15EU / branch;
[0213] (2), Limulus reagent sensitivity λ=0.25EU / ml, 0.5ml / tube
[0214] (3) Dilution of endotoxin standard substance: Take one endotoxin standard substance, dilute it with BET water in proportion to dissolve into 4λ and 2λ respectively, seal with parafilm, shake and dissolve for 15min; each step of dilution should be mixed in the vortex Mix on the mixer for 30s;
[0215] (4) Adding samples: Take several LAL reagents, add 0.5 ml of BET water to each tube to dissolve, and distribute to several endotoxin-free test tubes, each tube has 0.1 ml. Two of them are negative control tubes, add 0.1ml of BET water;
[0216] Two are positive control tubes, add 0.1ml of endotoxin working standard solution with 2λ concentration;
[0217] 2 tubes are sample positive control tubes, add 0.1ml sample solution containing 2λ endotoxin stand...
Embodiment 3
[0247] Example 3 Functional testing of CAR-T cells.
[0248] 1. Evaluation of target cell killing effect.
[0249] (1) Culture target cells separately [CD7 + K562, K562 cells] and effector cells [CAR-T cells];
[0250] (2) Collect target cells 4x10 5 cells and CAR-T cells 2.8x10 6 cells, 800g, centrifuge for 6min, discard the supernatant;
[0251] (3) Resuspend the target cells and effector cells in 1ml D-PBS(-) solution, centrifuge at 800g for 6min, discard the supernatant;
[0252] (4) Repeat step 3 once;
[0253] (5) Resuspend effector cells with 700ul medium (AIM-V medium + 1-10% FBS), and resuspend target cells with 2ml medium (AIM-V medium + 1-10% FBS);
[0254] (6) Set up experimental wells with effect-to-target ratios of 1:1, 5:1, and 10:1, and the grouping conditions are as follows Figure 11 As shown, and set up the control group (K562 cells), each group has 3 duplicate wells;
[0255] (7) 250g, 5min plate centrifugation;
[0256] (8) Cultivate for 4 hours i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com