Freeze-dried rabies vaccine for human use and preparation method thereof
A rabies vaccine and freeze-drying technology, which is applied in the direction of freeze-drying transportation, medical preparations containing active ingredients, antiviral agents, etc., can solve the problems of unstable product quality and short validity period of liquid dosage forms, and achieve thermal stability and Effect of Potency, Good Thermal Stability and Potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
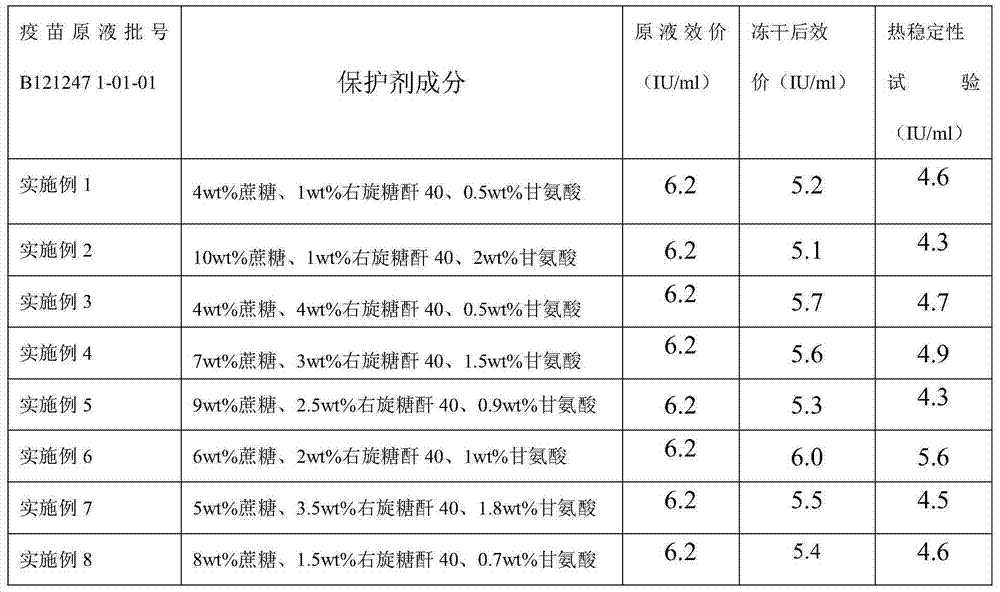
Embodiment 1
[0038] a. Preparation of lyoprotectant
[0039] Add sucrose and dextran 40 to water for injection, stir until completely dissolved, and steam sterilize at 115°C at 0.09 MPa for 45 minutes; add glycine to water for injection at 30°C, stir to dissolve completely, and use a 0.22 μm microporous filter Membrane sterilization filtration;
[0040] b. Preparation of semi-finished products
[0041] According to the determined protein content or antigen content of the pre-vaccine stock solution, the total protein content is not higher than 80 μg / dose for preparation, adding sucrose, dextran 40, and glycine, which is a semi-finished product;
[0042] c. Preparation of finished product
[0043] 3.1 Divide the semi-finished product and put it into the freeze-dryer box, set the freeze-drying parameters, and carry out freeze-drying;
[0044] 3.2 Pre-freezing stage: cool down until the sample temperature is below -45°C, and keep warm for 2 to 4 hours;
[0045] 3.3 Vacuum pumping stage: wh...
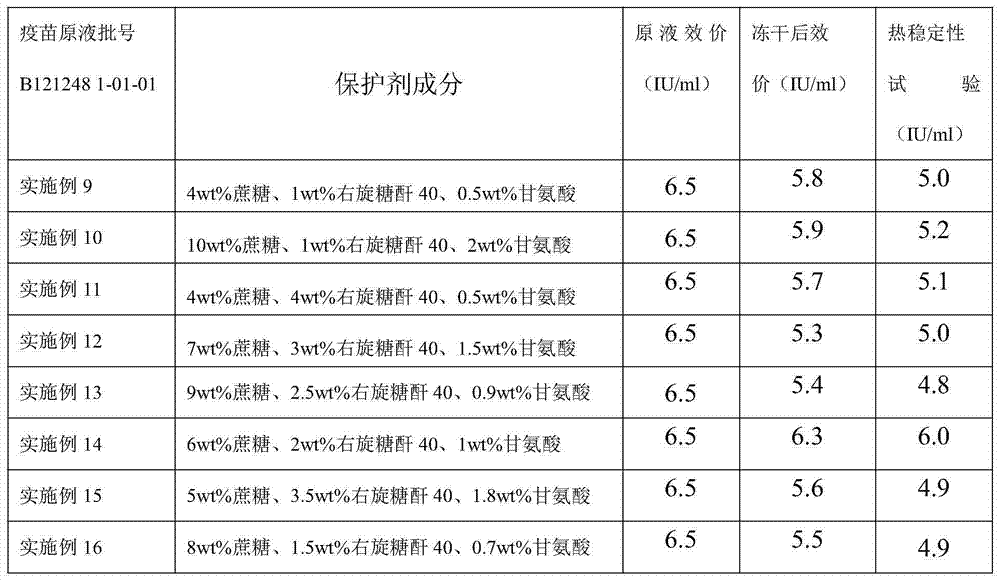
Embodiment 9
[0052] a. Preparation of lyoprotectant
[0053] Add sucrose and dextran 40 to water for injection, stir until completely dissolved, steam sterilize at 115°C at 0.09-0.10MPa for 45min; add glycine to water for injection at 15-30°C, stir to dissolve completely, use 0.22μm Microporous membrane sterilization filtration;
[0054] b. Preparation of semi-finished products
[0055] According to the determined protein content or antigen content of the pre-vaccine stock solution, the total protein content is not higher than 80 μg / dose for preparation, adding sucrose, dextran 40, and glycine, which is a semi-finished product;
[0056] c. Preparation of finished product
[0057] 3.1 Divide the semi-finished product and put it into the freeze-dryer box, set the freeze-drying parameters, and carry out freeze-drying;
[0058] 3.2 Pre-freezing stage: cool down until the sample temperature is below -45°C, and keep warm for 2 to 4 hours;
[0059] 3.3 Vacuum pumping stage: when the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com