Stem cell freezing and storing medium and preparation method and freezing and storing method thereof
A cryopreservation method and stem cell technology, applied in the field of stem cell cryopreservation and its preparation, can solve the problems of inability to meet the clinical needs of stem cell therapy, serum contamination, etc., to prevent the risk of contamination and allergens, reduce negative effects, and be safe. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
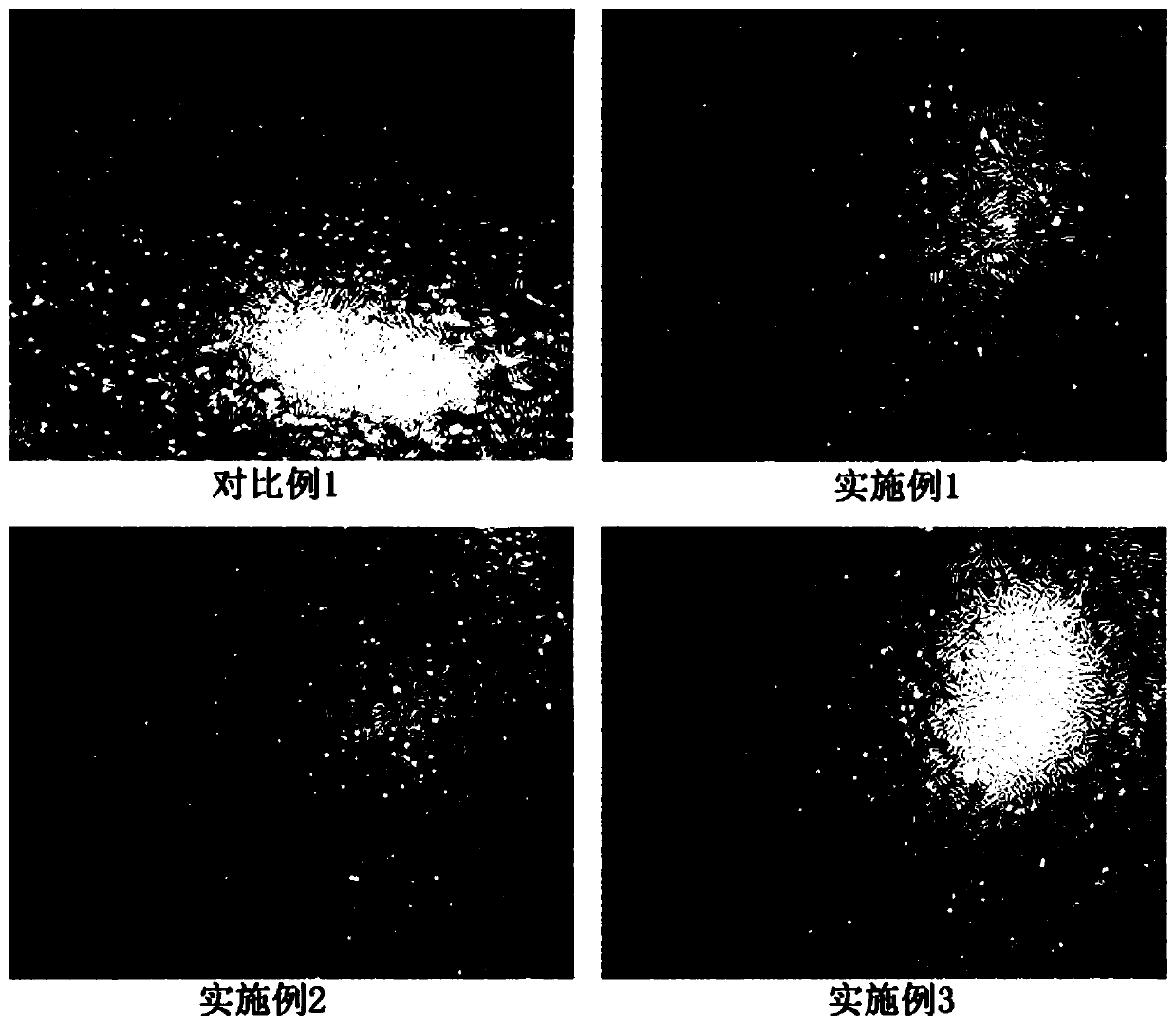
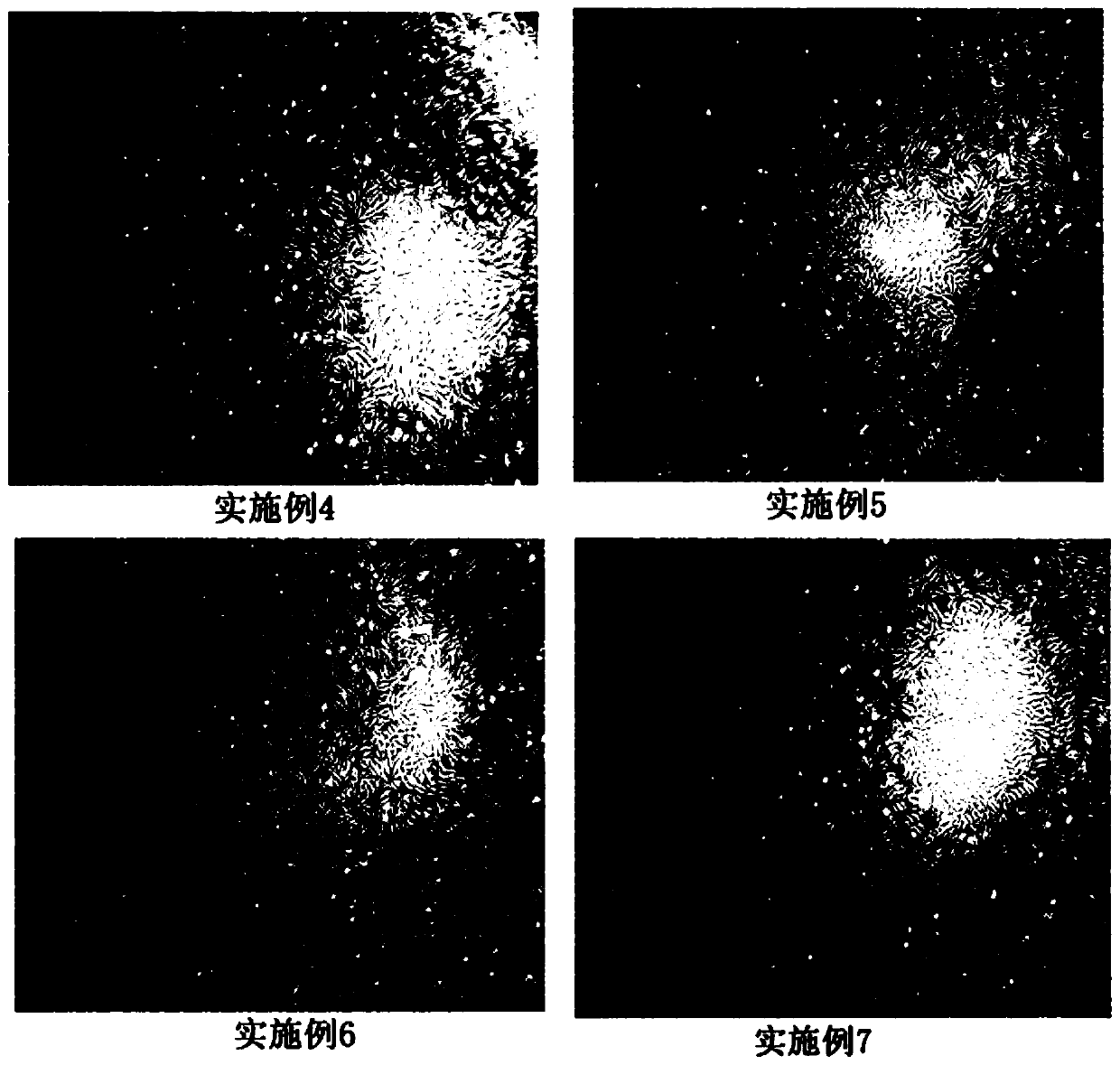
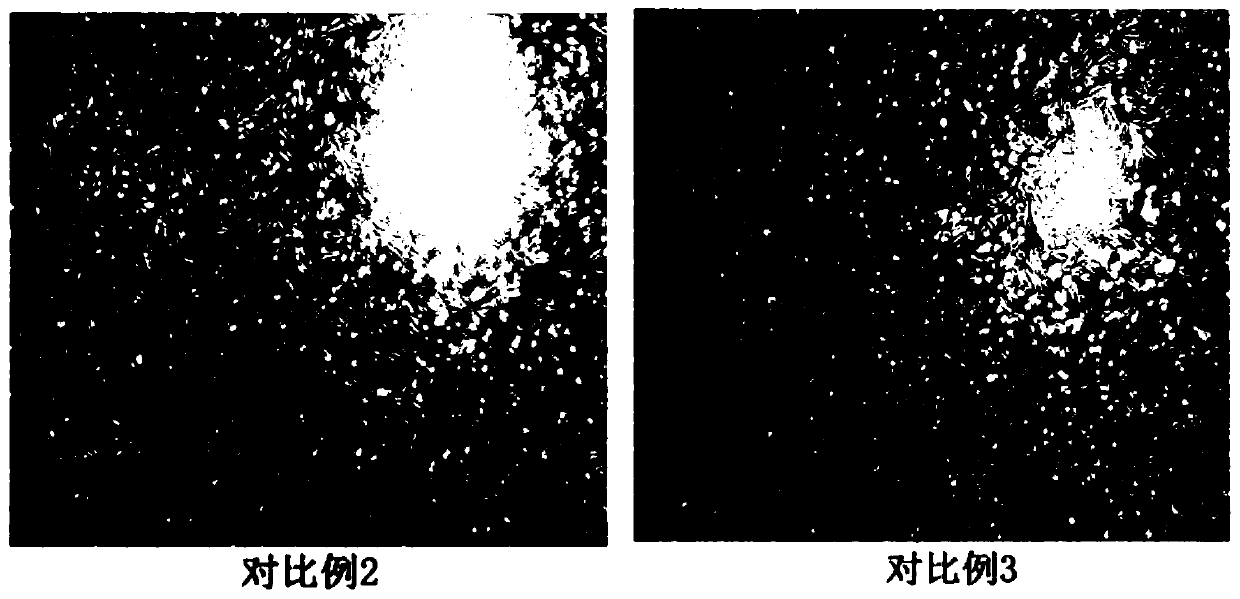
Image
Examples
preparation example Construction
[0042] The present invention also provides a preparation method of Plasma-Lyte A electrolyte injection, which comprises the following steps:
[0043] (1) Use an electronic balance to accurately weigh 5.26g of USP grade sodium chloride (NaCl), 0.37g of USP grade potassium chloride (KCl), and 0.37g of USP grade magnesium chloride hexahydrate (MgCl). 2 ·6H 2 O) 0.3g, pharmacopoeia grade sodium gluconate (C 6 h 11 NaO 7 )5.02g, pharmacopoeia grade sodium acetate trihydrate (C 2 h 3 NaO 2 ·3H 2 O) 3.68g;
[0044] (2) Add the auxiliary materials weighed in step (1) into a 1L beaker in turn, add 900mL of ultrapure water into the beaker, fully dissolve, and configure it into an aqueous solution;
[0045] (3) Measure the pH of the aqueous solution obtained in step (2) with a pH meter, and adjust the pH of the aqueous solution to 7.2 to 7.4, and finally set the volume to 1 L;
[0046] (4) Divide the aqueous solution obtained in step (3) into several 500mL blue cap reagent bottl...
Embodiment 1
[0056] A stem cell cryopreservation liquid, which is composed of the following components by weight: 1 part of trehalose, 0.2 parts of dextran 40, 3 parts of human serum albumin, 3 parts of dimethyl sulfoxide, 5 parts of hydroxyethyl starch and Maili A injection 87.8 parts.
Embodiment 2
[0058] A stem cell cryopreservation liquid, which is composed of the following components by weight: 1 part of trehalose, 0.5 parts of dextran 40, 5 parts of human albumin, 5 parts of dimethyl sulfoxide, 6 parts of hydroxyethyl starch and Maili A injection 82.5 parts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com