Efficient synthesis method of novel fluorescent material 1,3-dihydroisobenzofuran compound
A compound and addition technology, applied in the field of spectral properties, can solve problems such as poor atom economy and difficult availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
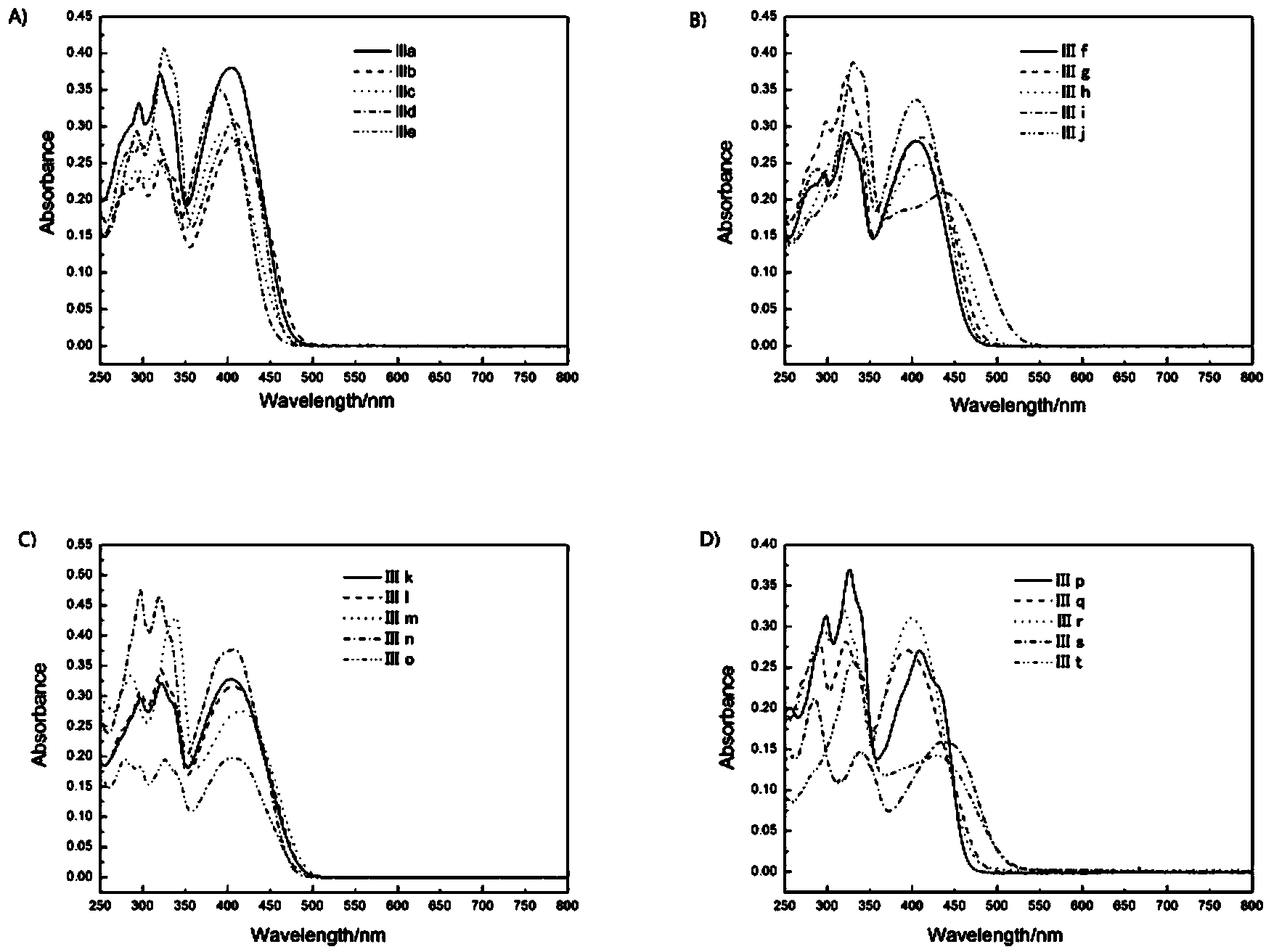
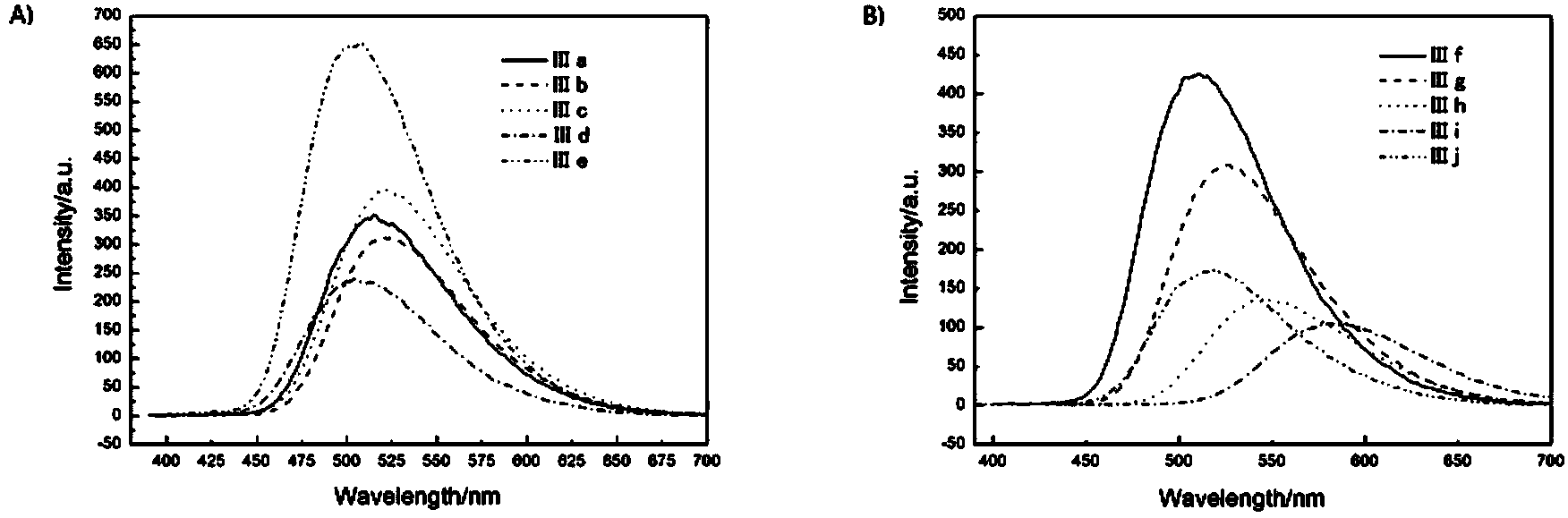
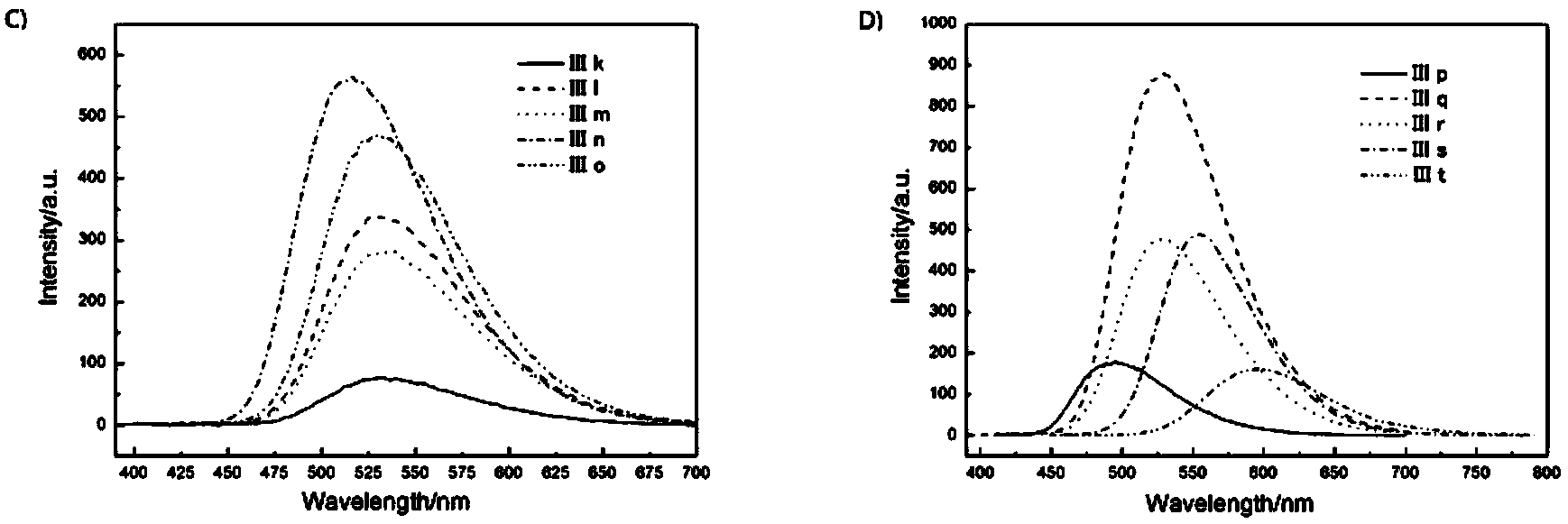
Image
Examples
Embodiment 1~20
[0018] Embodiment 1~20 is synthetic embodiment
Embodiment 1
[0020] The preparation of compound shown in formula III a:
[0021]
[0022] Under nitrogen protection, (Z)-1-benzylidene-1,3-dihydroisobenzofuran (62.5mg, 0.3mmol) and 4-dimethylaminobenzaldehyde (67.1mg, 0.45mmol) were dissolved In 1.0mL DMF, potassium tert-butoxide (0.06mmol, 1M in THF) and 18-crown-6 (23.8mg, 0.09mmol) were added under stirring at room temperature, and the reaction mixture was stirred at 110°C for 2 hours and cooled to After room temperature, add saturated ammonium chloride aqueous solution to the mixture to quench the reaction, transfer to a separatory funnel, extract with dichloromethane, combine the extracts, wash with saturated brine, add anhydrous sodium sulfate to dry, filter, spin dry The solvent was separated and purified on a silica gel column to obtain 100.4 mg of a yellow solid (the title compound, compound III a), with a yield of 99%.
[0023] Melting point: 145.6-148.2°C; 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.92(d,J=7.84Hz,2H),7.82(d,J=8.72Hz,...
Embodiment 2
[0025] The preparation of compound shown in formula III b:
[0026]
[0027] According to the method shown in Example 1, only the substrate aromatic aldehyde is changed. The yield of the title compound was 91%.
[0028] Melting point: 195.8-199.2°C; 1 H NMR (400MHz, CDCl 3 ,25℃)δ7.92(d,J=7.44Hz,2H),7.82(d,J=8.72Hz,2H),7.59-7.65(m,2H),7.33-7.46(m,4H),7.21- 7.26(m,1H),6.64(d,J=8.36Hz,2H),6.15(s,1H),6.14(s,1H),3.38(br s,4H),2.04(t,J=6.52Hz, 4H); 13 C NMR (100.6MHz, CDCl 3 ,25℃)δ152.4,148.8,146.8,135.6,134.6,133.2,129.9,129.1,128.6,128.3,128.2,126.1,122.4,119.9,119.3,111.8,100.9,98.3,47.7,25.6, TOHRMS( calcd for C 26 h 24 NO + [M+H] + :366.1852,found:366.1860.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com