Probe for a biological specimen and labelling method and screening method using the probe
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of Staining Compound (1)
[0150]To a solution obtained by dissolving 3.0 g (11.4 mmol) of an aldehyde derivative (A-2) in 20 mL of acetic acid, added were 2.2 g (11.5 mmol) of a compound (B-20) and 0.9 g of ammonium acetate, followed by stirring under reflux for 2 hours. After completion of the reaction, 50 mL of water were slowly added dropwise thereto while cooling, and the mixture was cooled to room temperature. A solid precipitate was filtrated, washed with 100 mL of water twice, and further washed with 50 mL of 2-propanol, to thereby afford 3.2 g (yield: 64.4%) of a target product (1).
Analysis Result of Compound (1)
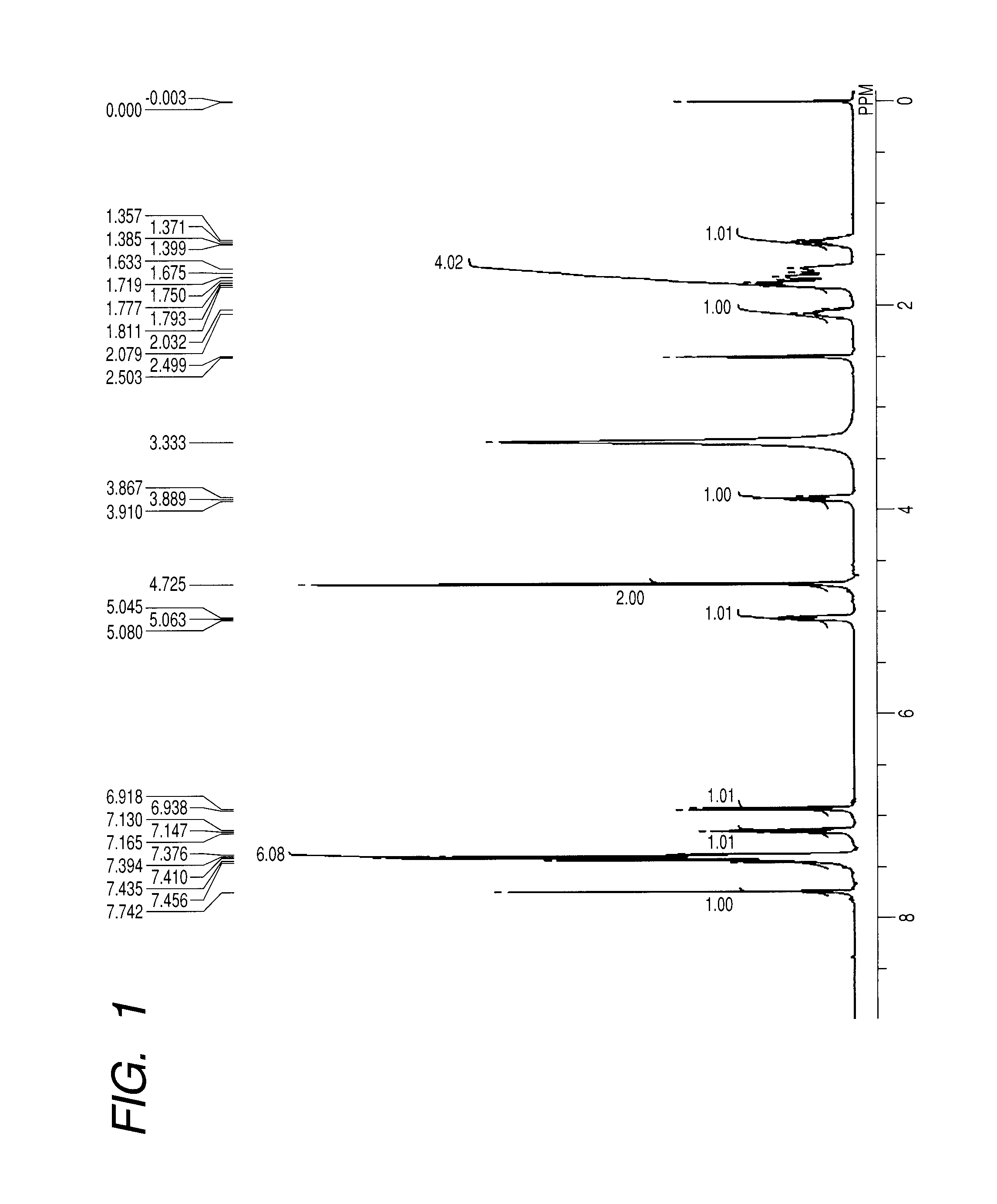
[0151][1]1H NMR (400 MHz, DMSO-d6, room temperature): δ [ppm]=1.36-1.40 (m, 1H), 1.63-1.81 (m, 4H), 2.04-2.08 (m, 1H), 3.89 (t, 1H, J=8.47 Hz), 4.73 (s, 2H), 5.06 (t, 1H, J=7.10 Hz), 6.93 (d, 1H, J=8.24 Hz), 7.15 (t, 1H, J=7.10 Hz), 7.38-7.46 (m, 6H), 7.74 (s, 1H).
[0152]FIG. 1 illustrates a spectrum thereof.
[0153][2] Mass spectrometry (ESI-TOF): m / z=435.0859 ...
synthesis example 2
Synthesis of Staining Compound (7)
[0154]To a solution obtained by dissolving 3.43 g (10.0 mmol) of an aldehyde derivative (A-16) in 20 mL of toluene, added were 1.13 g (10.0 mmol) of a compound (B-7) and 2.5 g (30.0 mmol) of piperidine, followed by stirring under reflux for 2.5 hours. After completion of the reaction, the mixture was cooled to room temperature, diluted with 100 mL of toluene, supplemented with 100 mL of water, and stirred. After the whole had been left to stand still, an organic layer was separated and dried over anhydrous sodium sulfate. After filtration, toluene was distilled off under reduced pressure, and recrystallization of the residue from ethanol afforded 1.3 g (yield: 31.7%) of a target product (7)
[Analysis Result of Compound (7)]
[0155][1]1H NMR (400 MHz, DMSO-d6, room temperature): δ [ppm]=1.36-1.47 (m, 1H), 1.64-1.81 (m, 4H), 2.04-2.13 (m, 1H), 3.90 (t, 4H, J=8.93 Hz), 5.18 (s, 1H), 6.89 (d, 1H, J=8.24 Hz), 7.19 (dd, 1H, J=2.52, 8.93 Hz), 7.34 (d, 1H, J=2...
example 1
In vivo Labelling with Probe for Biological Specimen
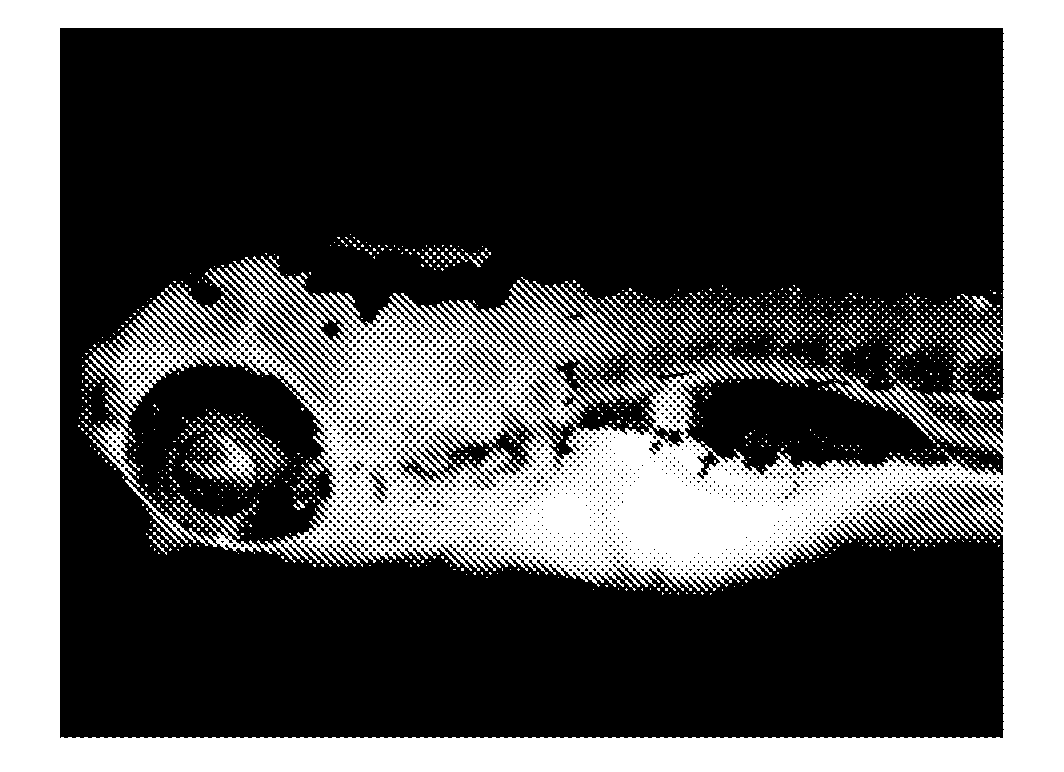
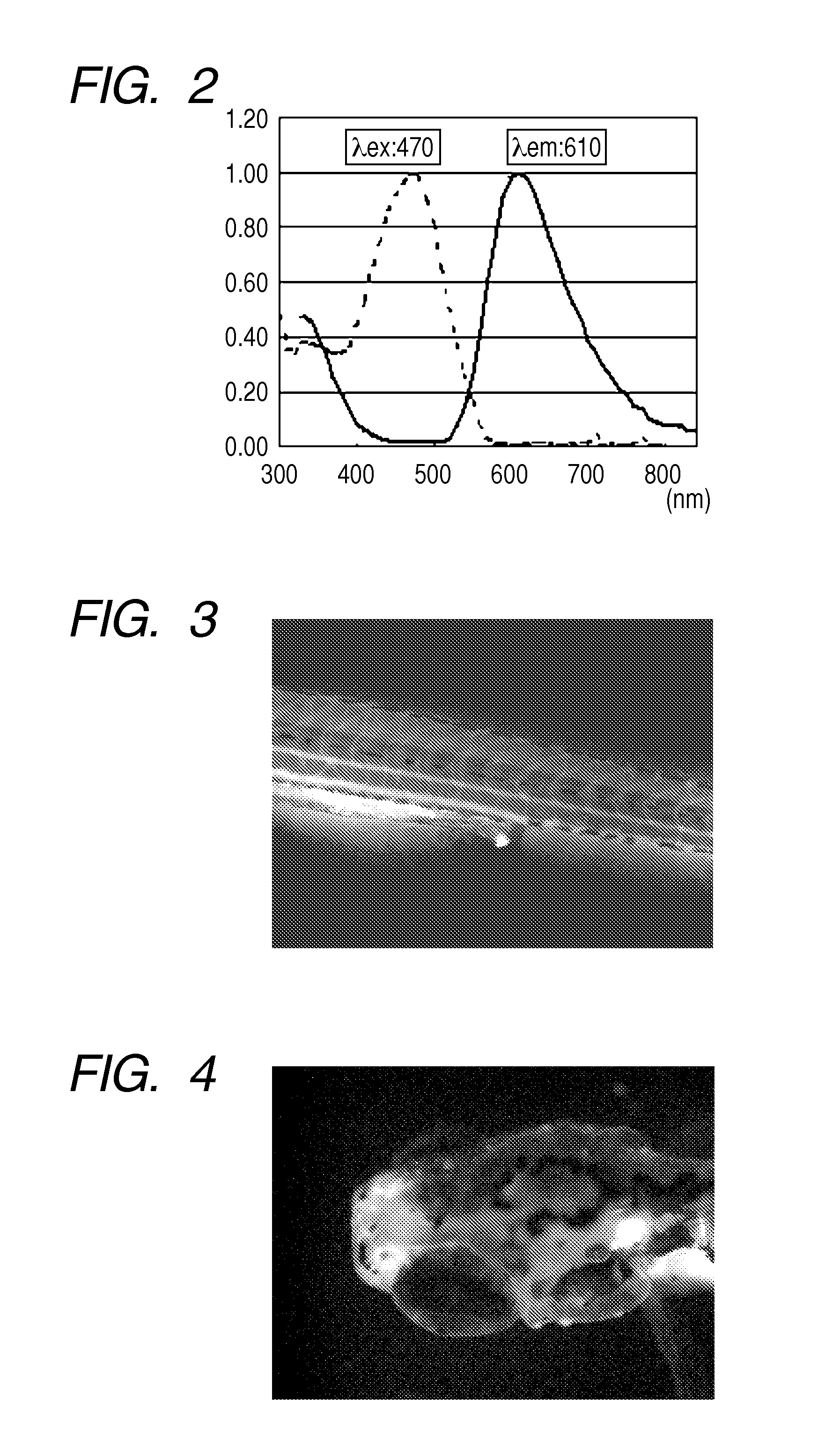
[0158]Five juveniles of Zebrafish were put into one well of a well plate together with rearing water. After rearing water had been discharged, 1 mL of distilled water was added to the well, and a solution of a staining compound (1) in DMSO was further added thereto so as to achieve a concentration of 10 ng / mL, followed by gentle stirring (pipetting). Further, Egg Water was prepared by dissolving artificial seawater SEALIFE (manufactured by Marinetech Co., Ltd.) in distilled water at a concentration of 60 mg / L. After the whole had been left to stand for 1 hour, distilled water in the well was discharged and replaced by 1 mL of fresh Egg Water. In addition, such an operation that Egg Water was discharged and replaced by 1 mL of fresh Egg Water was repeated twice. One of the juveniles was taken out from the well onto a dish, supplemented with 100 μL of a 3% methylcellulose aqueous solution to fix the movement of the juvenile, and phot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Kinematic viscosity | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com