Steroidal c- 17 benzoazoles
A composition and compound technology, applied in the direction of steroids, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
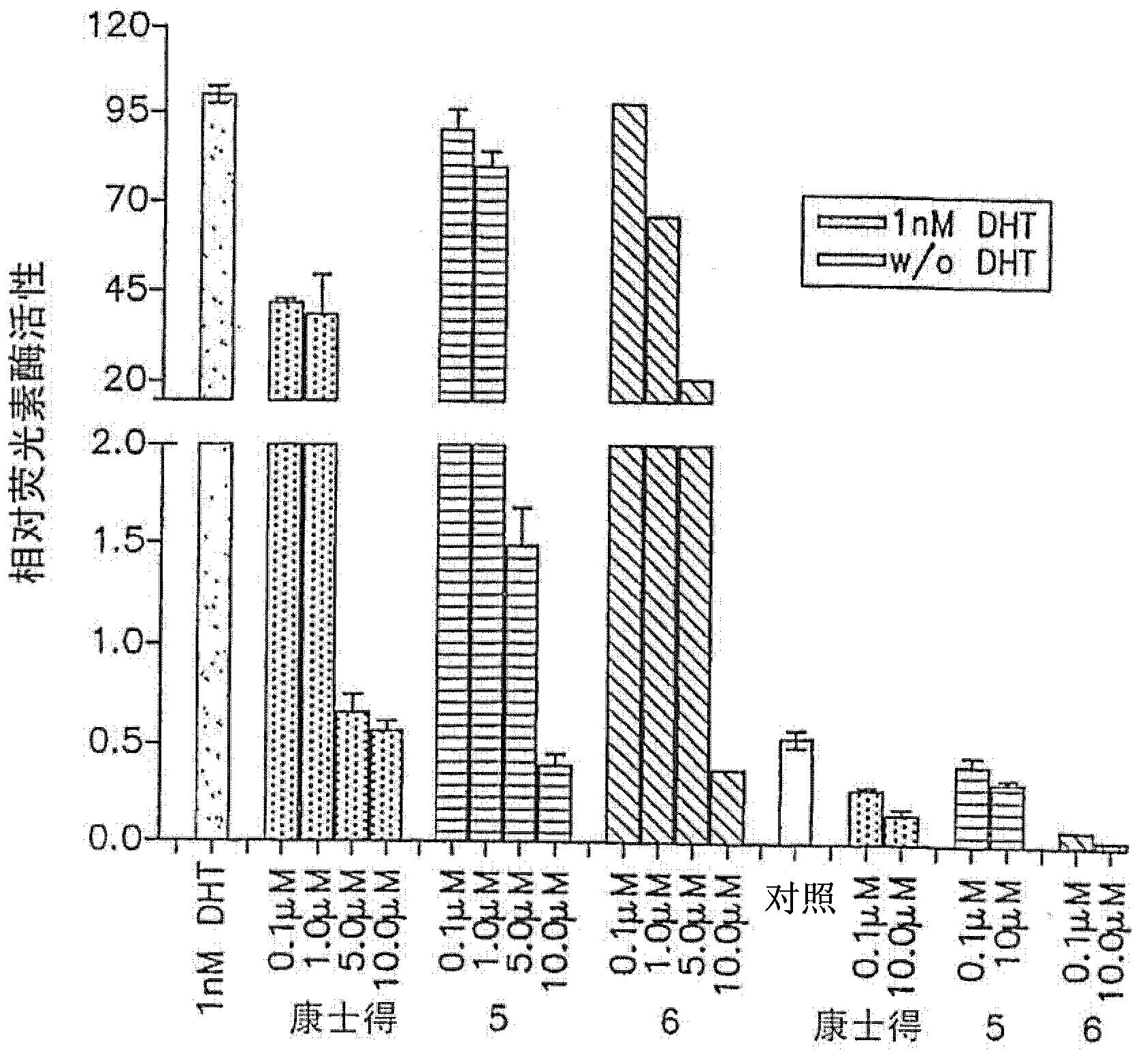
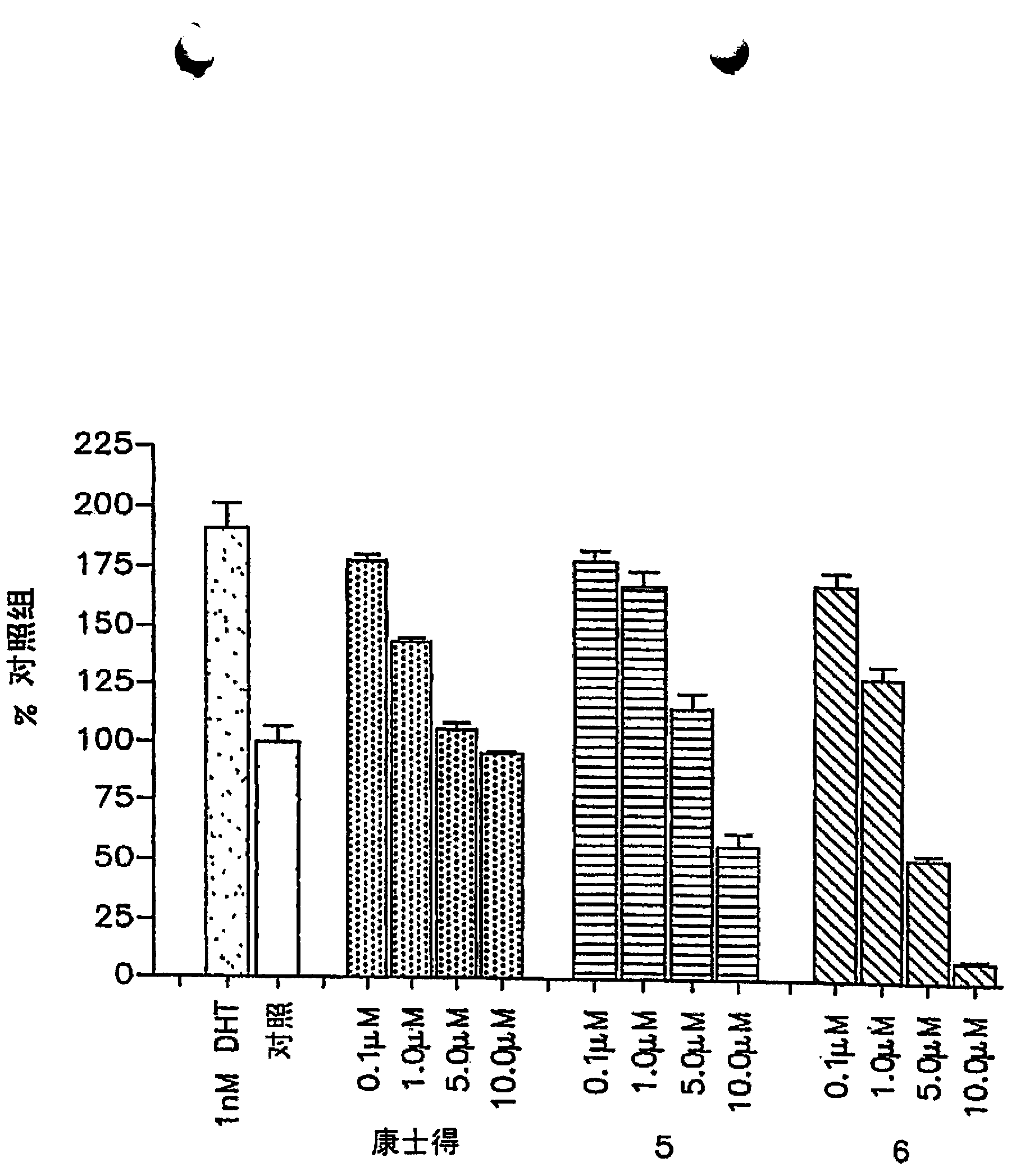
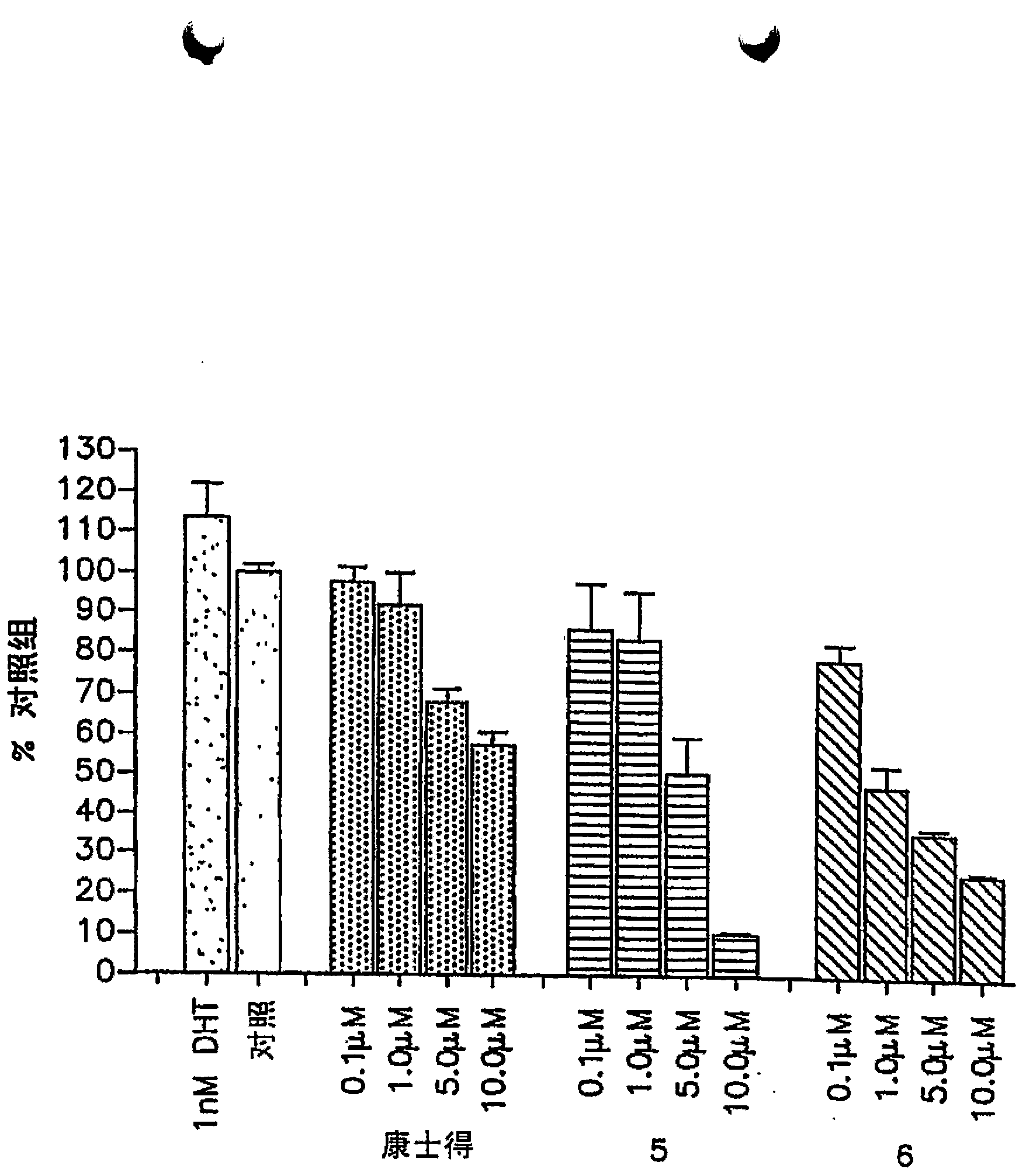
[0073] biological research
[0074] CYP17 inhibition studies: The CYP17 inhibition coordination assay was done following our previously published procedure, where intact cytochrome P450cl7-expressing E. coli was used as the enzyme source (Grigoryev et al., "Cytochrome P450cl7-expressing Escherichia coli as a first-step screening system for 17[alpha]-hydroxylase-C17,20-lyase inhibitors", Anal.Biochem.;1999,267,319-330; and "Effects of new 17α-hydroxylase / C17,20-lyase inhibitors on LNCaP prostate cancer cell growth in vitro and in vivo", Br.J.Cancer, 1999, 81, 622-630. The IC of the compound 50 Values were determined from dose-response curves and are listed in Table 1. Ketoconazole, IC of Abiraterone 50 values (one CYP17 inhibitor in clinical trials (O'Donnell above), Chart 1) and 3β-hydroxy-17-(lH-imidazol-l-yl)androst-5,16-diene (VN / 85-1, compound 16, Diagram 1, is considered to be the most potent CYP17 inhibitor (Njar et al, mentioned above, Current Pharm.Design, 1999...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com