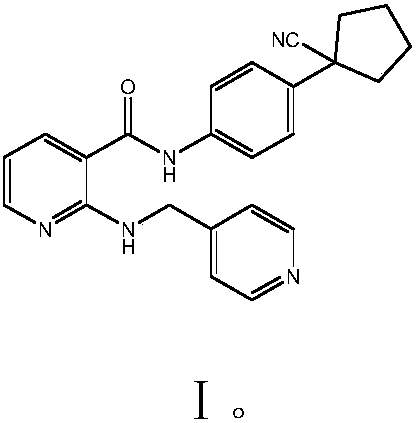
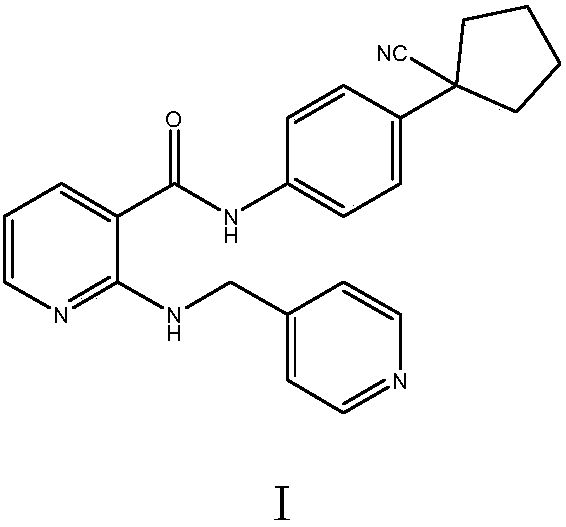
High-selectivity simple preparation method of apatinib
A technology of apatinib and the first amide, applied in the field of medicinal chemistry, can solve the problems of high price of 2-chloronicotinyl chloride, complicated steps, large amount of nitrification reaction wastewater, etc., and achieves high addition reaction activity and technological process. Simple, highly active methylene effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
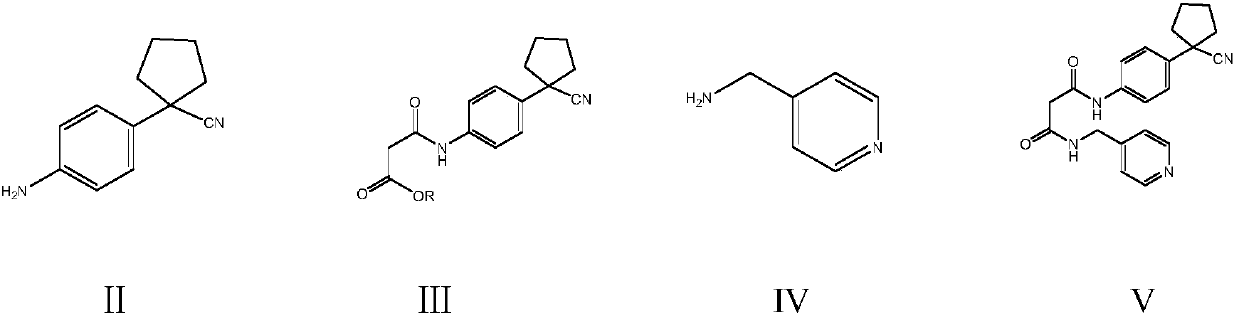
[0046] Example 1: Preparation of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentyl)phenylmalonamide (V)
[0047] Into a 250 ml four-necked flask connected with a stirrer, a thermometer and a reflux condenser, add 120 g of dimethyl malonate and 18.6 g (0.1 mol) of 1-(4-aminophenyl)cyclopentylcarbonitrile II , Stir the dealcoholization reaction at 100~105℃ for 4 hours (first amidation reaction), recover the excess dimethyl malonate by distillation under reduced pressure, cool to 60-70℃, add 13.0g (0.12mol) 4-ammonia The methyl pyridine IV was stirred and dealcoholized at 110-115°C for 4 hours (the second amidation reaction). Cool to 30-40°C, add 80 g of isopropanol to the residue, recrystallize, filter, and dry to obtain 33.5 g of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentan Yl)phenylmalonamide, the yield is 92.6%, and the liquid phase purity is 99.8%.
Embodiment 2
[0048] Example 2: Preparation of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentyl)phenylmalonamide (V)
[0049] Into a 250 ml four-necked flask connected with a stirrer, a thermometer and a reflux condenser, add 150 g of diethyl malonate and 18.6 g (0.1 mol) of 1-(4-aminophenyl)cyclopentylcarbonitrile II , Stirring dealcoholization reaction at 105~110℃ for 4 hours (first amidation reaction), recovering excess diethyl malonate by distillation under reduced pressure, cooling to 60-70℃, adding 13.0g (0.12mol) of 4-ammonia The methyl pyridine IV was stirred and dealcoholized at 110-115°C for 4 hours (the second amidation reaction). Cool to 30-40°C, add 80 g of isopropanol to the residue, recrystallize, filter, and dry to obtain 33.1 g of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentan Base) phenylmalonamide, the yield is 91.3%, and the liquid phase purity is 99.9%.
Embodiment 3
[0050] Example 3: Preparation of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentyl)phenylmalonamide (V)
[0051] Into a 250 ml four-necked flask connected with a stirrer, a thermometer and a reflux condenser, 150 g of di-tert-butyl malonate and 18.6 g (0.1 mol) of 1-(4-aminophenyl)cyclopentylcarbonitrile were added Ⅱ. Stir the dealcoholization reaction at 110~115℃ for 4 hours (the first amidation reaction), recover the excess di-tert-butyl malonate by distillation under reduced pressure, cool to 60-70℃, add 13.0 g (0.12 mole) 4 -Aminomethylpyridine IV, at 120-125°C for 4 hours with stirring and dealcoholization (second amidation reaction). Cool to 30-40°C, add 80 g of isopropanol to the residue, recrystallize, filter, and dry to obtain 32.6 g of N-(4-pyridine)methyl-N'-4-(1-cyanocyclopentan Yl)phenylmalonamide, the yield is 90.1%, and the liquid phase purity is 99.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com