Simple preparation method of palbociclib
A compound and solvent technology, which is applied in the field of simple preparation of Palbociclib, can solve the problems of difficult industrialized operation, increase synthesis cost and the like, and achieve the effects of low cost, less waste water and waste liquid, and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
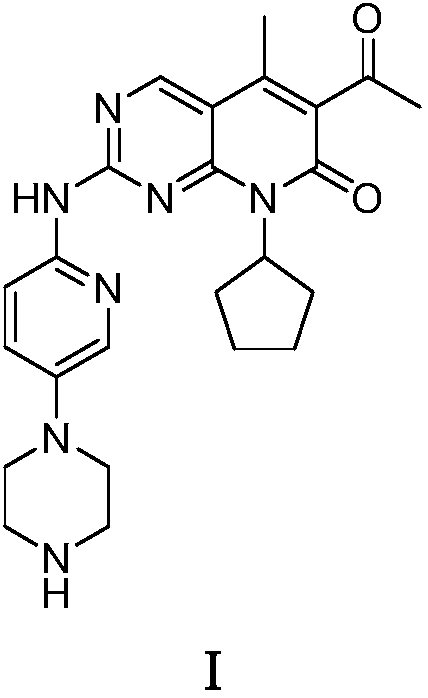
[0052] Embodiment 1: the preparation of palbociclib (I)
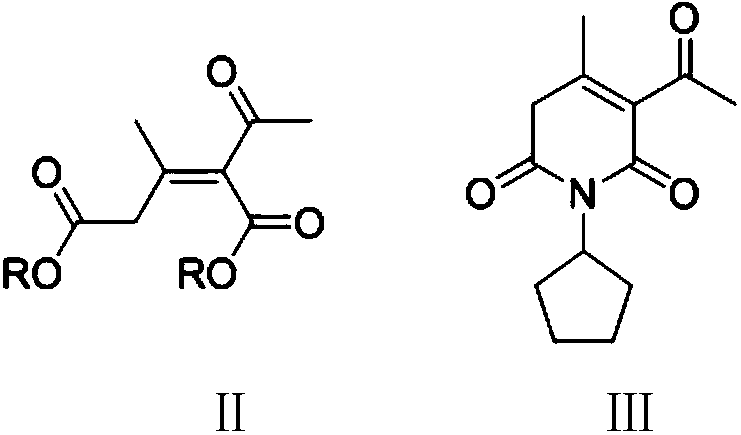
[0053] Step (1): Preparation of 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H)-dione (Ⅲ)
[0054] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, water separator, reflux condenser and dropping funnel, add 220 gram toluene, 0.2 gram methylbenzenesulfonic acid, 26.0 gram (0.2 moles) ethyl acetoacetate, 110 to 115 ° C stirring and reflux dehydration reaction for 5 hours. Cool to 50°C, add 10.0 g (0.12 moles) of cyclopentylamine, stir and react at 90 to 95°C for 4 hours, while distilling off the ethanol generated. The solvent was recovered by distillation under reduced pressure, and the residue was recrystallized with 50 g of methyl tert-butyl ether to obtain 21.7 g of white solid 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H) - diketone, yield 92.5%, liquid phase purity 99.8%.
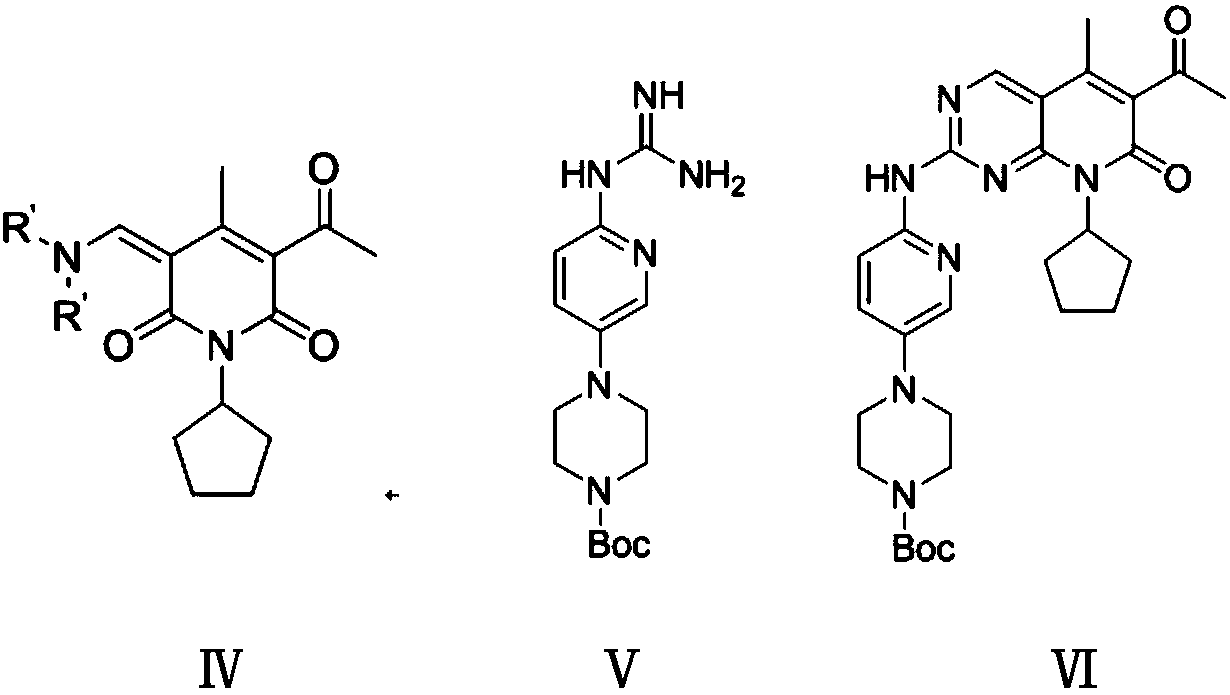
[0055] Step (2): Preparation of Boc-protected palbociclib (Ⅵ)
[0056] In a 500 ml four-necked...
Embodiment 2
[0063] Embodiment 2: the preparation of palbociclib (I)
[0064] Step (1): Preparation of 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H)-dione (Ⅲ)
[0065] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, water separator, reflux condenser and dropping funnel, add 220 gram toluene, 0.15 gram 98wt% sulfuric acid, 23.2 gram (0.2 moles) methyl acetoacetate, 110 to Stir and reflux dehydration reaction at 115°C for 5 hours. Cool to 50° C., add 10.0 g (0.12 moles) of cyclopentylamine, stir and react at 100 to 105° C. for 3 hours, and simultaneously distill off the generated methanol. The solvent was recovered by distillation under reduced pressure, and the residue was recrystallized with 50 g of methyl tert-butyl ether to obtain 21.3 g of white solid 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H) - diketone, yield 90.6%, liquid phase purity 99.3%.
[0066] Step (2): Preparation of Boc-protected palbociclib (Ⅵ)
[0067] In a 500 ml fo...
Embodiment 3
[0070] Embodiment 3: the preparation of palbociclib (I)
[0071] Step (1): Preparation of 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H)-dione (Ⅲ)
[0072] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, water separator, reflux condenser and dropping funnel, add 220 grams of toluene, 0.25 grams of p-toluenesulfonic acid, 23.2 grams (0.2 moles) of methyl acetoacetate , 110 to 115 ° C stirring reflux dehydration reaction for 5 hours. Cool to 50° C., add 10.0 g (0.12 moles) of cyclopentylamine, stir and react at 100 to 105° C. for 3 hours, and simultaneously distill off the generated methanol. The solvent was recovered by distillation under reduced pressure, and the residue was recrystallized with 50 g of methyl tert-butyl ether to obtain 21.6 g of white solid 3-acetyl-1-cyclopentyl-4-methylpyridine-2,6-(1H,5H) - diketone, yield 91.9%, liquid phase purity 99.5%.
[0073] Step (2): Preparation of Boc-protected palbociclib (Ⅵ)
[0074]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com