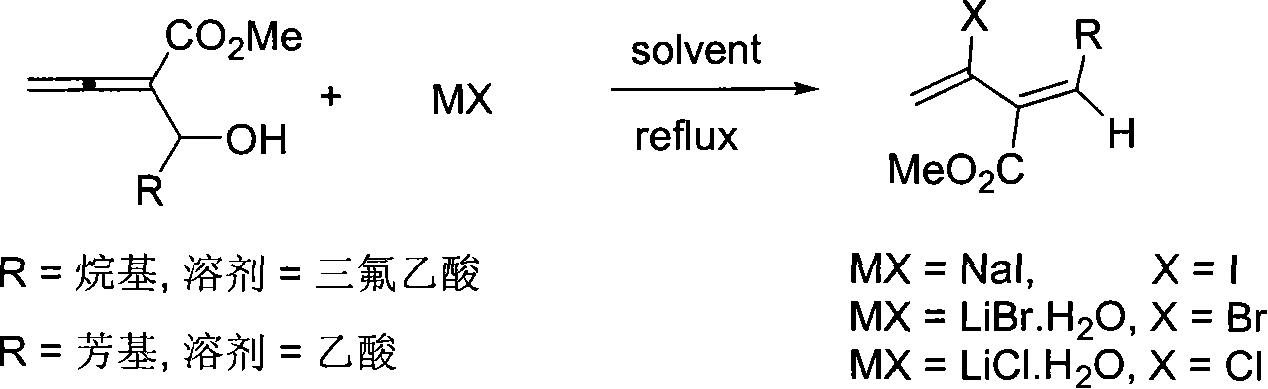
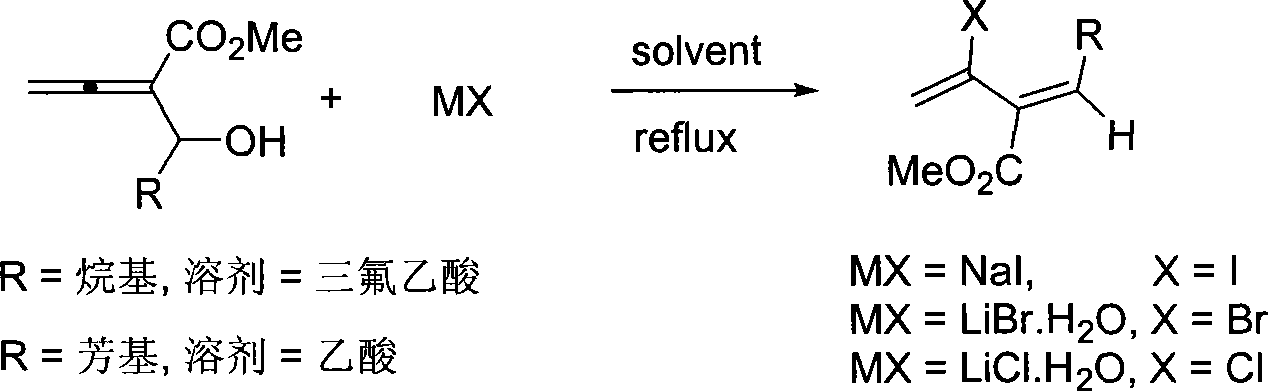
Method of synthesizing 3-carbomethoxy-2-halogen-1,3(Z)-conjugated diolefin
A technology of conjugated dienes and methyl esters, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve the problems of requiring noble metals and low stereoselectivity of reactions, and achieve high stereoselectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add NaI (151.1mg, 1.0mmol), 3-methylester-1,2-hexadien-4-ol (77.0mg, 0.49mmol) and trifluoroacetic acid (1mL) at room temperature, and then reflux at eighty degrees The reaction was complete in 1 hour, cooled to room temperature, quenched with 10 mL of water, neutralized with sodium bicarbonate until almost no bubbles, extracted with ether (3×25 mL), saturated with Na 2 S 2 o 3 The aqueous solution and saturated brine were washed once respectively, dried over anhydrous sodium sulfate, filtered, concentrated, and subjected to flash column chromatography to obtain 65.4 mg of the product with a yield of 50%. The product is a colorless liquid.
[0021] 1 H NMR (300MHz, CDCl 3 )δ6.75(t, J=7.8Hz, 1H), 6.08(d, J=1.1Hz, 1H), 6.03(d, J=1.1Hz, 1H), 3.77(s, 3H), 2.29-2.18( m, 2H), 1.06(t, J=7.7Hz, 3H);
[0022] 13 C NMR (75MHz, CDCl 3 ): δ165.3, 146.6, 135.7, 131.2, 96.3, 52.1, 22.8, 12.3;
[0023] MS (m / z): 267 (M + +1, 100);
[0024] IR (neat, cm -1 ): 2962, 1722, 16...
Embodiment 2
[0027] According to the method described in Example 1, the difference is that the substrate and reagent used are: LiBr H 2 O (105.0mg, 1.0mmol), 3-methylester-1,2-hexadien-4-ol (77.1mg, 0.49mmol) and trifluoroacetic acid (1mL), the product was 60.2mg, the yield was 56% . The product is a colorless liquid.
[0028] 1 H NMR (400MHz, CDCl 3 )δ6.88(t, J=7.8Hz, 1H), 5.84(d, J=2.0Hz, 1H), 5.66(d, J=2.0Hz, 1H), 3.79(s, 3H), 2.32-2.26( m, 2H), 1.07(t, J=7.6Hz, 3H); 13 C NMR (100-MHz, CDCl 3 ): δ165.5, 148.5, 132.9, 122.6, 122.3, 52.2, 22.9, 12.8; MS (m / z): 220 (M + (81Br), 8.22), 218 (M + ( 79 Br), 8.32), 139 (100); IR (neat, cm -1 ): 2971, 2952, 1724, 1645, 1615, 1267, 1245. HRMS calcd for C 8 h 11 BrNaO 2 (M + +Na): 242.9822 ( 81 Br), 240.9835 (79Br), Found: 242.9811 ( 81 Br), 240.9833 ( 79 Br).
Embodiment 3
[0030] According to the method described in Example 1, the difference is that the substrate and reagent used are: LiCl H 2 O (122.1mg, 2.02mmol), 3-methylester-1,2-hexadien-4-ol (78.8mg, 0.51mmol) and trifluoroacetic acid (1mL) yielded 35.5mg of the product, yield 40% . The product is a colorless liquid.
[0031] 1 H NMR (400MHz, CDCl 3 )δ6.94(t, J=7.8Hz, 1H), 5.62(d, J=1.2Hz, 1H), 5.26(d, J=1.2Hz, 1H), 3.79(s, 3H), 2.36-2.27( m, 2H), 1.07(t, J=7.6Hz, 3H); 13 C NMR (100MHz, CDCl 3 ): δ165.6, 149.1, 133.0, 131.4, 118.3, 52.2, 23.0, 13.0; MS (m / z): 176 (M + ( 37 Cl), 3.72), 174 (M + (35Cl), 11.67), 139(100); IR (neat, cm-1): 2971, 2955, 1725, 1644, 1619, 1246. HRMS calcd for C 8 h 11 ClO 2 (M + ): 174.0448 ( 35 Cl), Found: 174.0448 ( 35 Cl).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com