Isolongifolenone oxime ether derivative, and preparation method and application thereof
A kind of technology of leaf ketene oxime ether and isolongifole ketone, which is applied in the field of isolong leaf ketene oxime ether derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
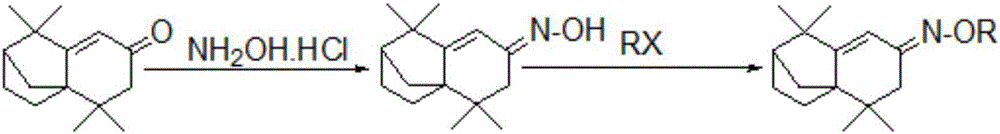
[0027] The present invention uses isolongifolenone as a raw material, synthesizes isolongifolenone oxime through addition elimination reaction, and then performs an alkylation reaction on isolongifolenone oxime to prepare the isolongifolenone oxime ether derivative things.
[0028] The specific overall reaction equation is as follows:
[0029]
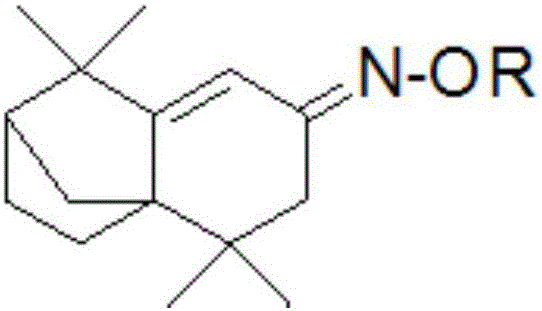
[0030] R is an alkane group or an arene group.
Embodiment 2
[0032] Compound 1: O-(1,2-Epoxy Propyl)1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H- 2,4a-Methanonaphthalen-7-one oxime-ether): preparation.
[0033] Addition Elimination Reaction: Using isolongifolenone as raw material, isolongifolenone oxime was synthesized by addition elimination reaction. Further specifically: using isolongifolenone and hydroxylamine hydrochloride as reactants, organic alcohol (methanol) and deionized water as solvents, and inorganic base (sodium carbonate) to remove the acid generated in the reaction, wherein isolongifolenone The molar ratio of hydroxylamine hydrochloride and inorganic base is: 1:0.1:0.1, and the isolongifolenone oxime can be obtained by carrying out reflux reaction. Further, extraction with ethyl acetate, washing and drying with saturated brine and deionized water in sequence, and then concentration and drying can obtain isolongifolenone oxime crystals.
[0034] Alkylation reaction: using isolongifolenone oxime and epichlorohydrin as r...
Embodiment 3
[0040] Compound 2: Ethyl isolongifolene oxime ether (O-Ethyl 1,2,3,4,5,6-hexahydro-1,1,5,5-tetramethyl-7H-2,4a-MethanonaPht-halen- 7-one oxime-ether) preparation.
[0041]Addition Elimination Reaction: Using isolongifolenone as raw material, isolongifolenone oxime was synthesized by addition elimination reaction. Further specifically: using isolongifolene and hydroxylamine hydrochloride as reactants, organic alcohol (ethanol) and deionized water as solvent, and inorganic base (sodium hydroxide) to remove the acid produced in the reaction, wherein isolongifolene The molar ratio of ketone, hydroxylamine hydrochloride and inorganic base is: 1:0.3:0.2, and the isolongifolenone oxime can be obtained by carrying out the reflux reaction. Further, extraction with ethyl acetate, washing and drying with saturated brine and deionized water in sequence, and then concentration and drying can obtain isolongifolenone oxime crystals.
[0042] Alkylation reaction: using isolongifolene oxime ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com