Preparation method of aryl-substituted pyrimidinamine acylated derivative
A technology for pyrimidine amide and derivatives, which is applied in the field of preparation of aryl-substituted pyrimidine amide acylation derivatives, can solve problems such as unreported preparation methods, and achieve the effects of easy availability of raw materials, huge economic value and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
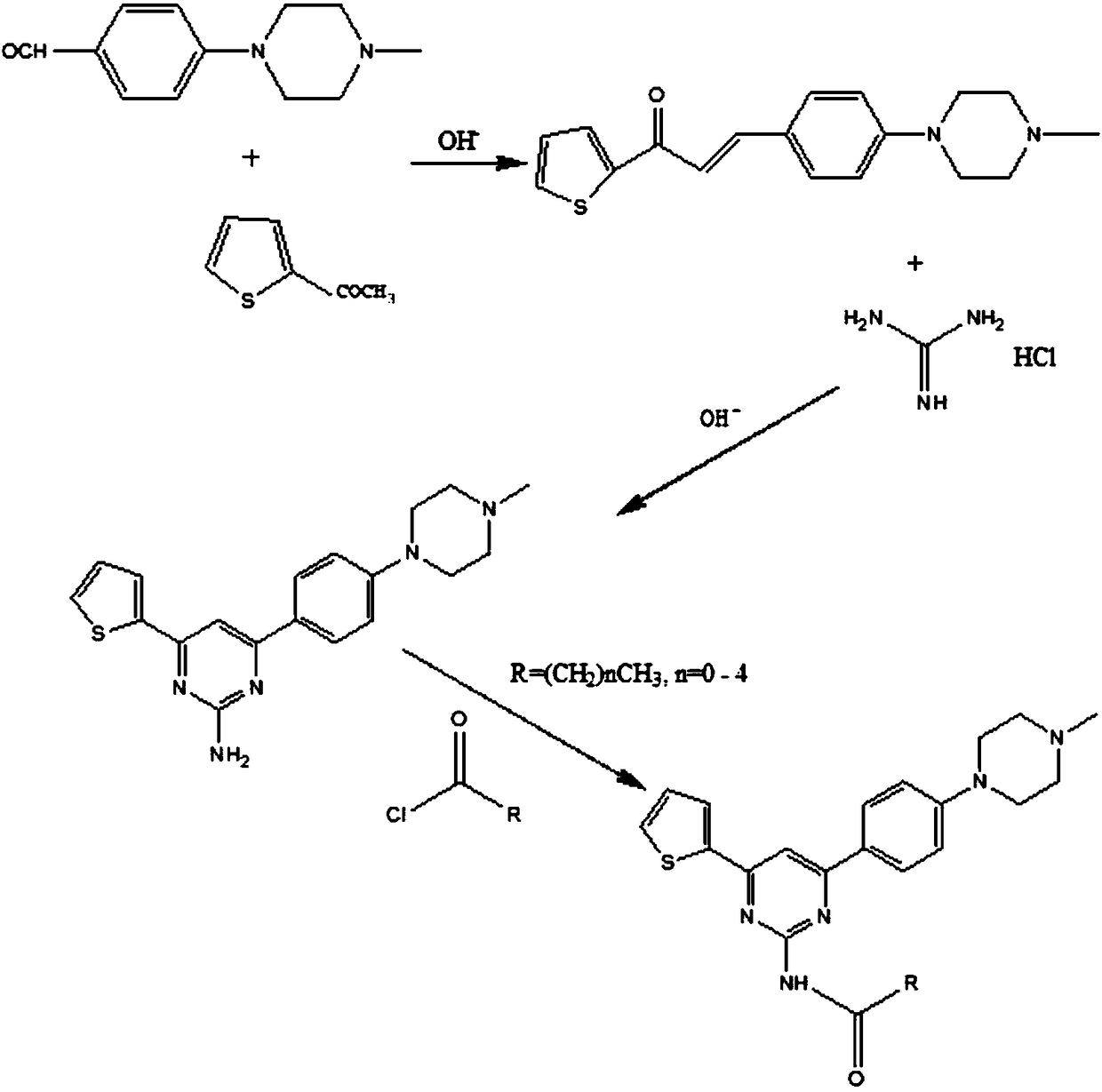
[0024] The preparation method of aryl-substituted pyrimidine amine acylated derivatives of the present invention comprises the preparation of 1-(2-thienyl)-3-(4-(4-methylpiperazinyl)phenyl)-propenone, 4- (2-thienyl)-6-(4-(4-methylpiperazinyl) phenyl)-pyrimidin-2-amine preparation and 4-(2-thienyl)-6-(4-(4- The preparation of methylpiperazinyl) phenyl)-pyrimidine-2-acylated derivative amine, the specific preparation steps are as follows:
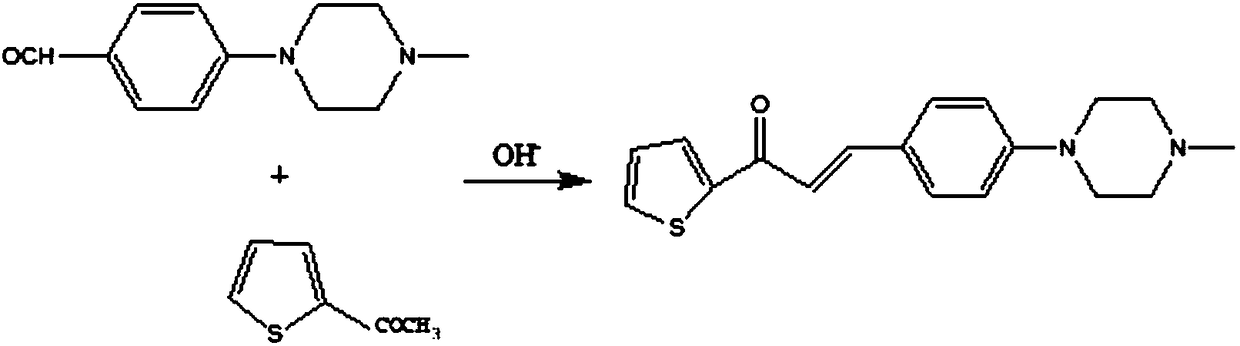
[0025] 1) the preparation of 1-(2-thienyl)-3-(4-(4-methylpiperazinyl) phenyl)-propenone, its synthetic route is:
[0026]
[0027] Specifically: add alkali and organic solvent into the reaction flask, stir evenly at room temperature, then add 4-(4-methylpiperazinyl)benzaldehyde, slowly add 2-acetylthiophene, at a temperature of 30-60°C Stir the reaction, monitor the end point of the reaction by thin-layer chromatography, and filter with suction to obtain 1-(2-thienyl)-3-(4-(4-methylpiperazinyl)phenyl)-propenone as a light yellow solid pow...
Embodiment 1
[0043] (1), the preparation of 1-(2-thienyl)-3-(4-(4-methylpiperazinyl) phenyl)-propenone:
[0044] Add 1.0 mol of sodium hydroxide and 400 ml of anhydrous methanol into the reaction flask, stir at room temperature, then add 0.1 mol of 4-(4-methylpiperazinyl)benzaldehyde, and slowly add 0.1 mol of 2-acetylthiophene dropwise. , Stir the reaction at 40°C, monitor the end point of the reaction by thin-layer chromatography, cool to room temperature, and filter with suction to obtain a light yellow solid powder with a yield of 80-85%.
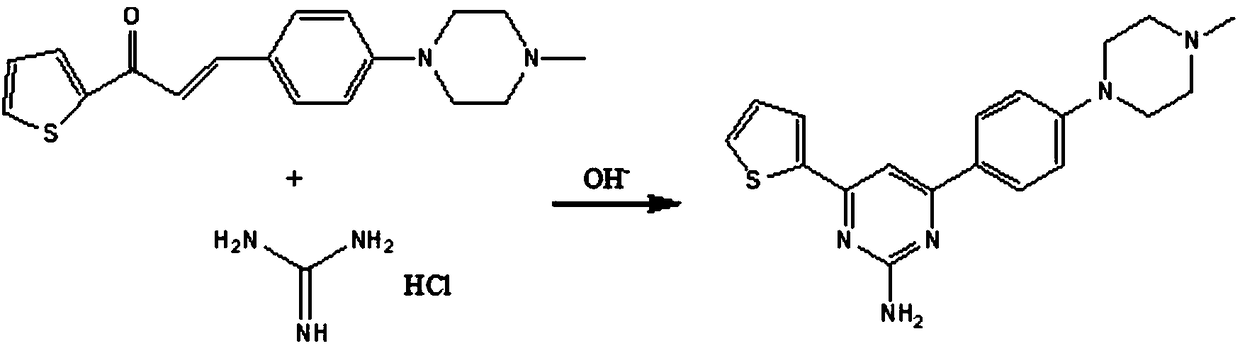
[0045] (2), the preparation of 4-(2-thienyl)-6-(4-(4-methylpiperazinyl)phenyl)-pyrimidine-2-amine:
[0046] Add 0.8mol of sodium hydroxide and 500ml of anhydrous methanol into the reaction flask, stir at room temperature, and then add 1-(2-thienyl)-3-(4-(4-methylpiperazinyl)phenyl)-propene 0.1 mol of ketone and 0.12 mol of guanidine hydrochloride were heated to 65°C and stirred for reaction. The end point of the reaction was monitored by thin-layer...
Embodiment 2
[0052] (1), the preparation of 1-(2-thienyl)-3-(4-(4-methylpiperazinyl) phenyl)-propenone:
[0053] Add 1.25 mol of sodium carbonate and 500 ml of absolute ethanol into the reaction flask, stir at room temperature, then add 0.1 mol of 4-(4-methylpiperazinyl)benzaldehyde, slowly add 0.2 mol of 2-acetylthiophene dropwise, Stir the reaction at 56°C, monitor the end point of the reaction by thin-layer chromatography, cool to room temperature, and filter with suction to obtain a light yellow solid powder with a yield of 80-84%.
[0054] (2), the preparation of 4-(2-thienyl)-6-(4-(4-methylpiperazinyl)phenyl)-pyrimidine-2-amine:
[0055] Add 1.125mol of sodium hydroxide, 300ml of anhydrous methanol and 200ml of anhydrous ethanol into the reaction flask, stir at room temperature, and then add 1-(2-thienyl)-3-(4-(4-methylpiperazinyl) 0.1mol of phenyl)-propenone and 0.12mol of guanidine hydrochloride, heated to 80°C and stirred for reaction, monitored the end point of the reaction by t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com