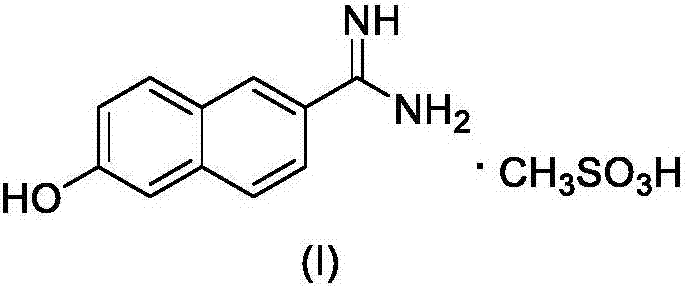
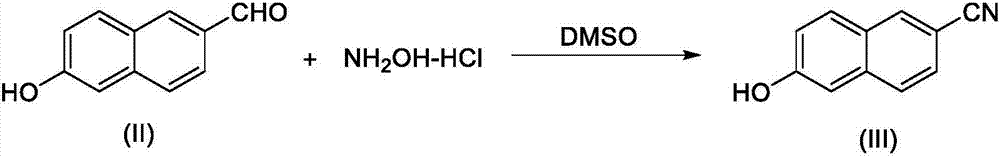
The synthetic method of nafamostat mesylate intermediate-6-amidino-2-naphthol mesylate
A naphthol, amidine-based technology, applied in the field of organic chemistry drug synthesis, can solve problems such as affecting product quality and yield, HCl gas waste, air pollution, etc., and achieve consistent product quality, low environmental pollution, and low equipment requirements. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Add 350 grams of 6-hydroxyl-2-naphthaldehyde (2.0mol), 278 grams of hydroxylamine hydrochloride (4.0mol) and 3500 milliliters of dimethyl sulfoxide in a 5000 milliliter three-necked flask, stir and heat to 100° C., heat preservation reaction for 1 hour, drop After reaching room temperature, the reaction liquid was mixed with a large amount of water, and a solid was precipitated under stirring. After filtering, the filter cake was washed with water to obtain a wet crude product, which was directly recrystallized with ethanol / water solution to obtain 237 g of off-white refined product with a yield of 70%.
Embodiment 2
[0011] Measure 740 ml of anhydrous methanol into a three-necked flask, cool to 0-5° C. under stirring, and add 430 ml of acetyl chloride (5.0 mol) dropwise. After dropping, react for a period of time at 0-5°C, add 85 grams (0.5mol) of 6-cyano-2-naphthol, react at 10°C for 10 hours, add isopropyl ether and stir for 1 hour to filter, and filter cake with isopropyl ether. Wash with propyl ether, dry and mix with methanol directly, heat to 50°C, pass through dry ammonia gas for 3 hours, and evaporate to dryness under reduced pressure to obtain a yellow solid. It was mixed with saturated sodium bicarbonate solution and stirred for a period of time, filtered and washed with water until neutral. Suspend the wet product in methanol, add a certain amount of methanesulfonic acid dropwise to dissolve, add isopropyl ether to precipitate a solid, filter, wash with isopropyl ether, and recrystallize with ethanol to obtain 70 g of a yellow solid with a melting point of 227-229°C and a yield ...
Embodiment 3
[0013] Measure 800 ml of absolute ethanol in a three-necked flask, cool to 0-5°C while stirring, and add 430 ml of acetyl chloride dropwise. After dropping, react at 0-5°C for a period of time, add 85 grams of 6-cyano-2-naphthol, react at 10°C for 10 hours, add methyl tert-butyl ether and stir for 1 hour to filter, filter cake with methyl Wash with tert-butyl ether, dry it, mix it with ethanol directly, heat to 50°C, pass through dry ammonia gas for 3 hours, and evaporate to dryness under reduced pressure to obtain a yellow solid. It was mixed with saturated sodium bicarbonate solution and stirred for a period of time, filtered and washed with water until neutral. Suspend the wet product in methanol, add a certain amount of methanesulfonic acid solution dropwise, add methyl tert-butyl ether to precipitate a solid, filter, wash with methyl tert-butyl ether, and recrystallize with ethanol to obtain 75 grams of a yellow solid with a melting point of 227 ° C ~ 230°C, yield 53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com