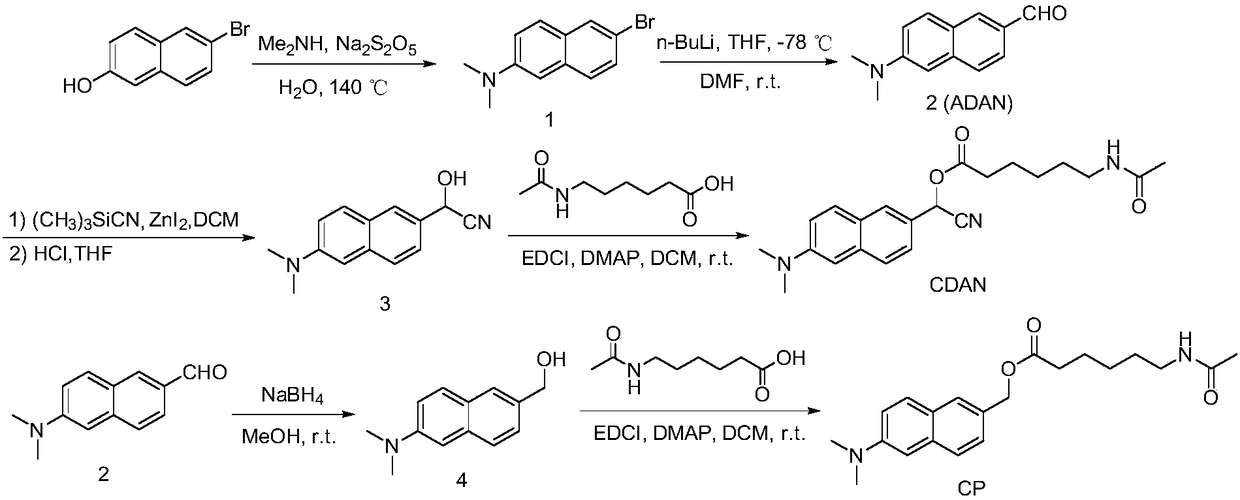
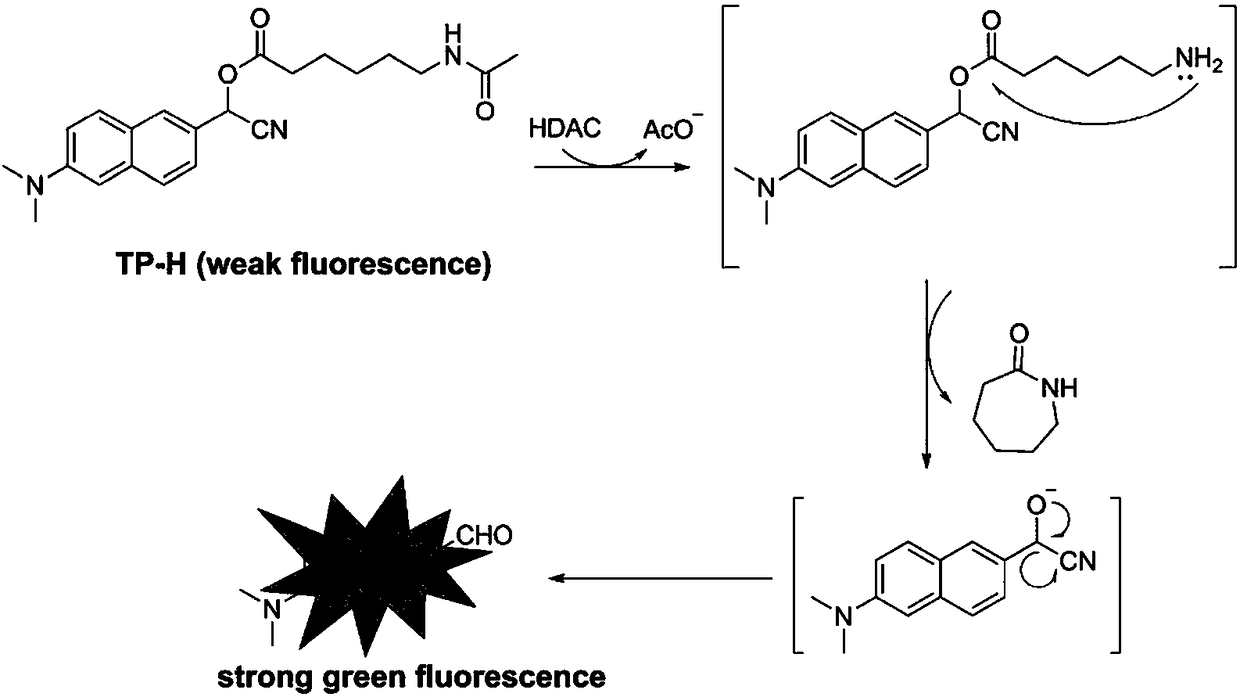
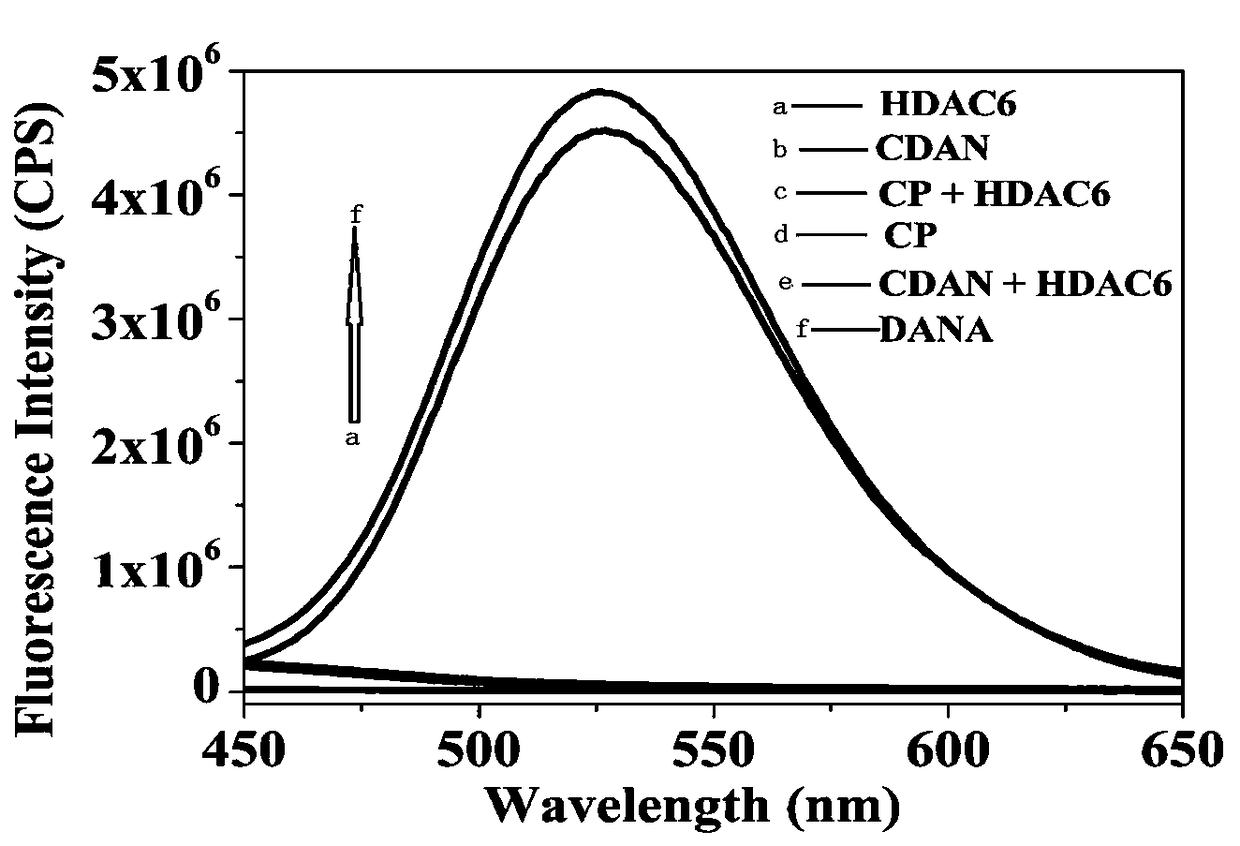
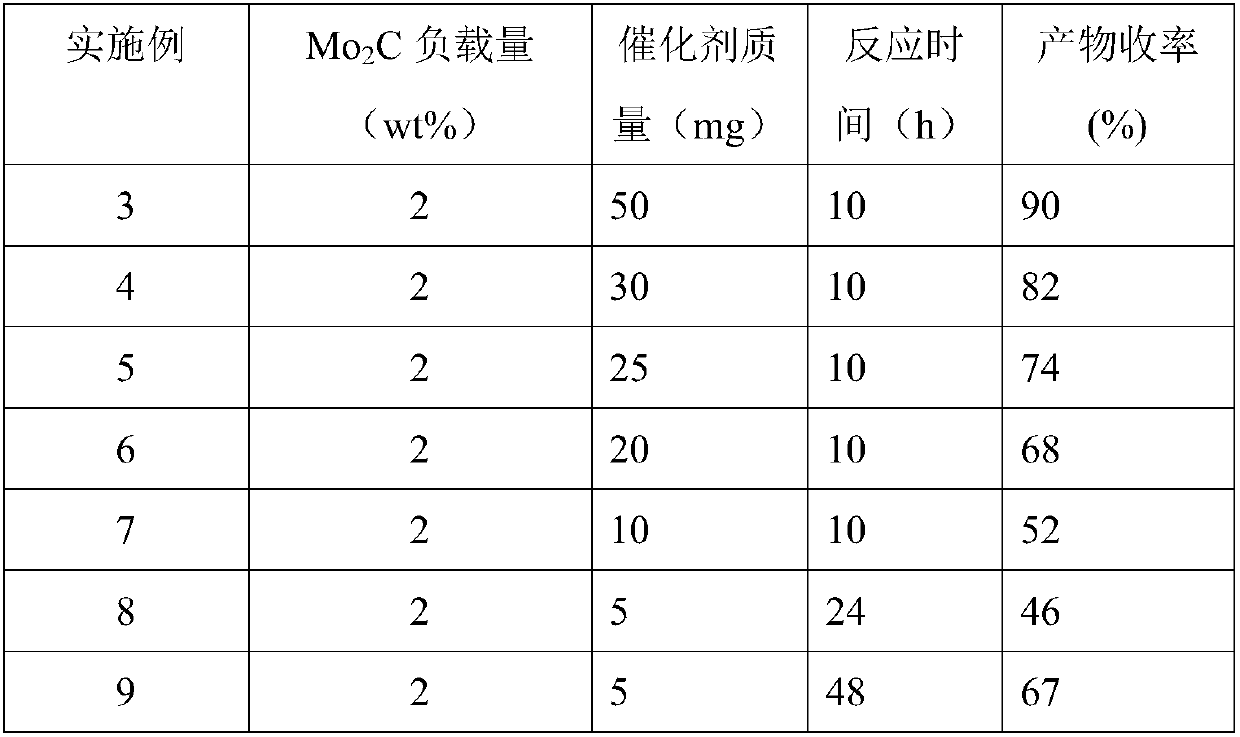
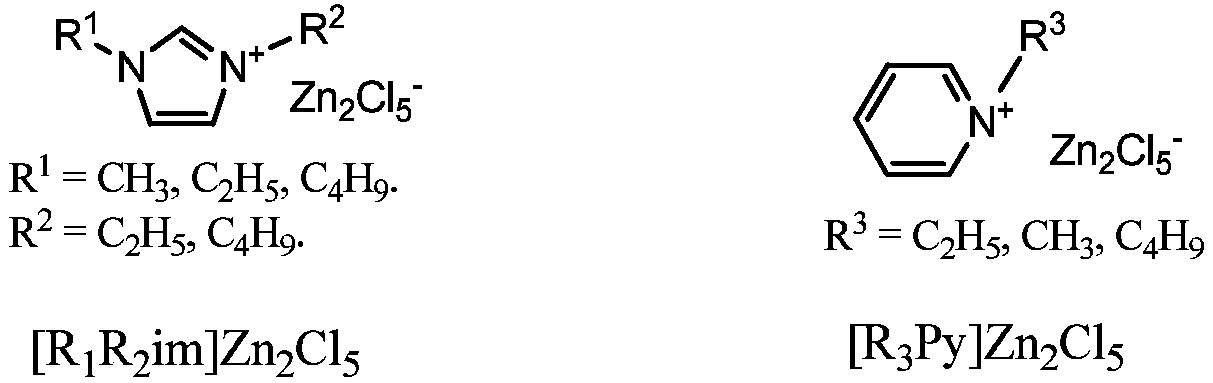Patents
Literature
32 results about "2-naphthaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
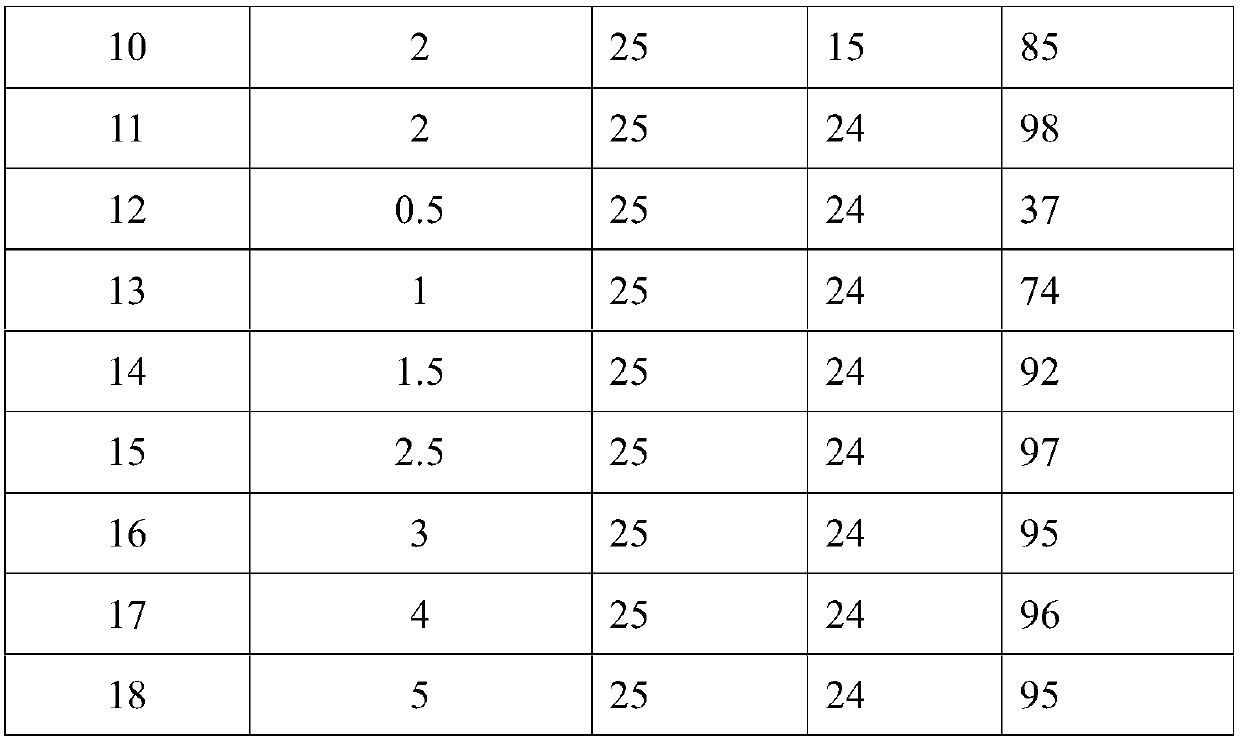
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-naphthaldehyde is a naphthaldehyde that is naphthalene substituted by a formyl group at position 2. It has a role as a mouse metabolite. It has a role as a mouse metabolite. Ontology Summary from ChEBI
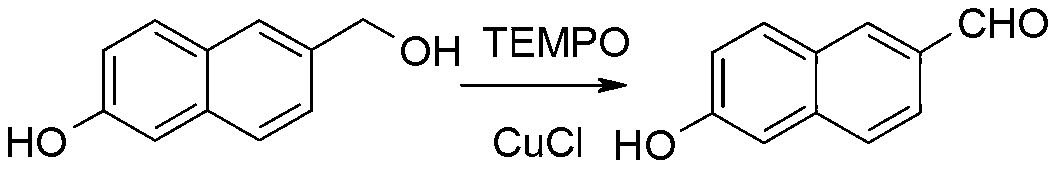
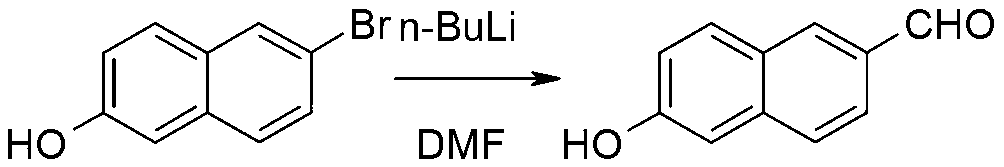
Synthetic method of 6-hydroxy-2-naphthaldehyde
InactiveCN103214358AIndustrial amplification is matureSimple reaction conditionsOrganic compound preparationCarbonyl compound preparationLithium chlorideGrignard reagent
The invention discloses a synthetic method of 6-hydroxy-2-naphthaldehyde, which comprises the following steps: A. beta-naphthol and bromine are reacted for generating 1, 6-dibromo-2-naphthol; then 1, 6-dibromo-2-naphthol and a reducing agent are reacted for obtaining 6-bromine-2-naphthol; B. 6-bromine-2-naphthol and a methyl esterification reagent are reacted for obtaining 6-bromine-2-naphthyl methyl ether; C. 6-bromine-2-naphthyl methyl ether and magnesium are reacted in a Grignard reaction solvent for generating a Grignard reagent; and then the Grignard reagent and N,N-dimethyl formamide are reacted for obtaining 6-methoxy-2-naphthaldehyde; D. 6-methoxy-2-naphthaldehyde and a demethylating reagent lithium chloride are dissolved in a high boiling point aprotic polar solvent for reaction, thereby obtaining a 6-hydroxy-2-naphthaldehyde. Cheap and easily obtained beta-naphthol is used as raw material according to the invention, and after bromination, reduction, methylation, Grignard, demethylating and other reactions, 6-hydroxy-2-naphthaldehyde is obtained; the invention has good advantages of industrial amplification maturation, simple reaction condition and low cost, and the demethylating yield reaches up to 91% and the overall yield reaches up to 62.8%.
Owner:SHANGHAI RECORD PHARM CO LTD
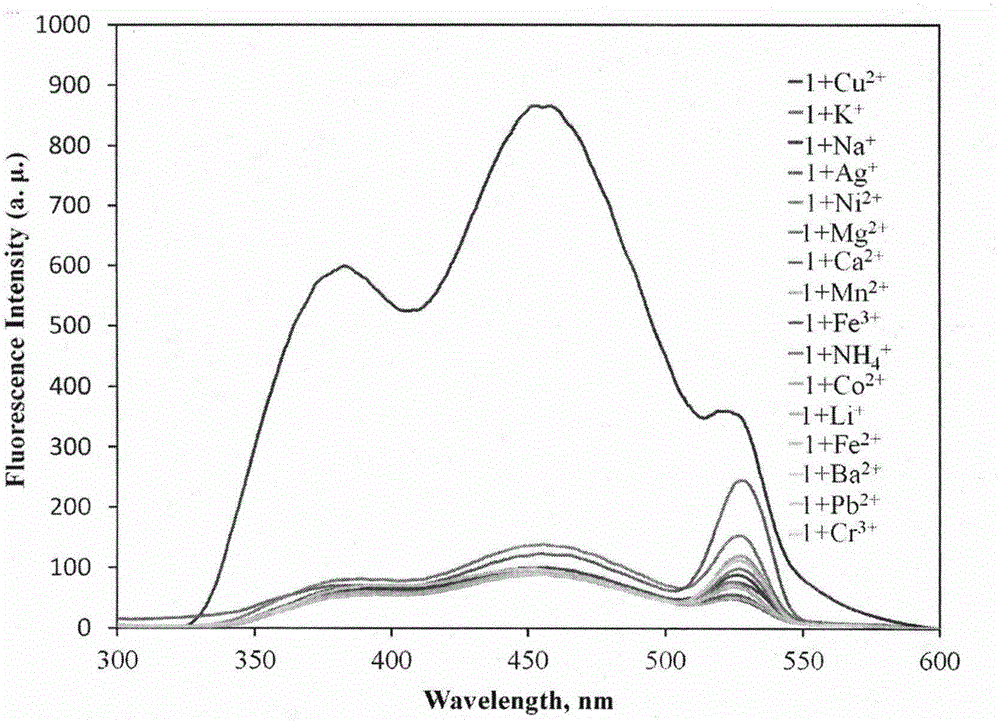
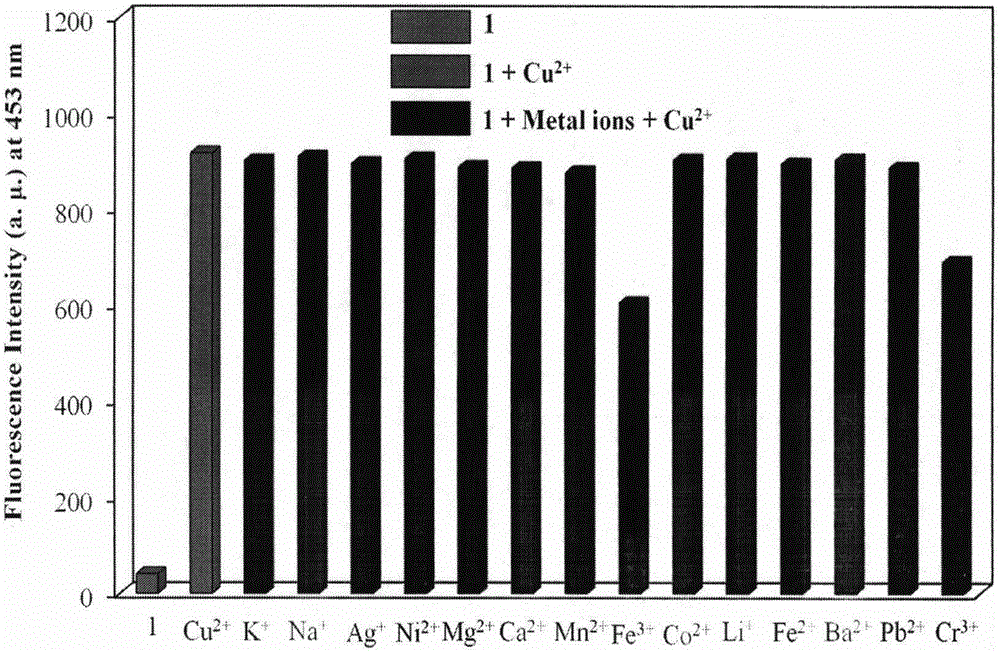
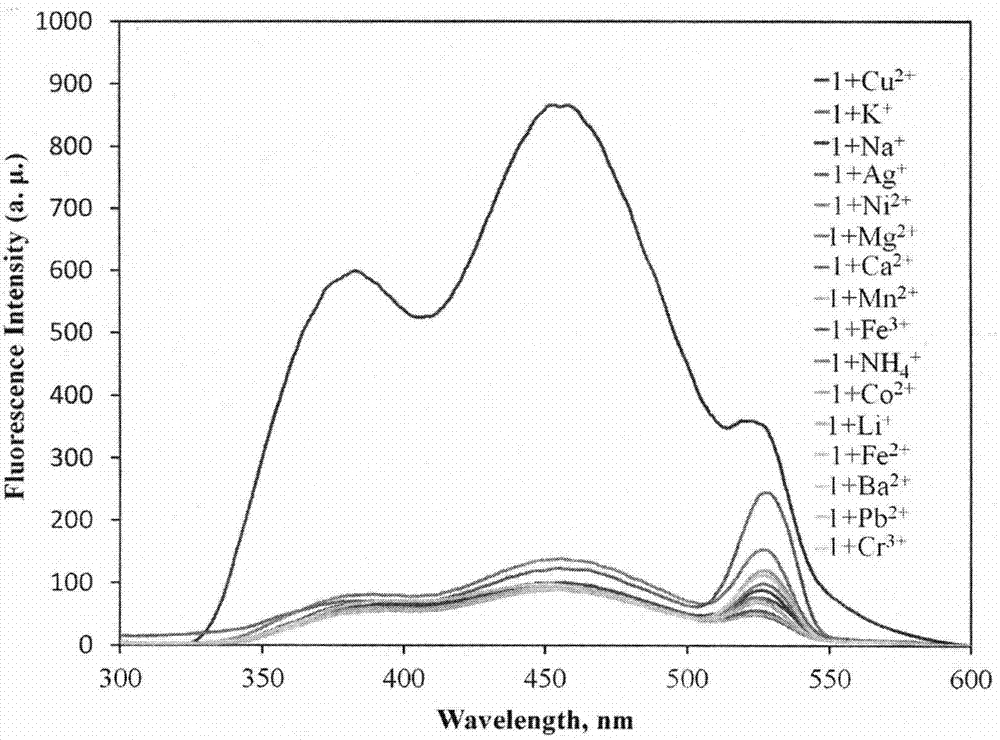
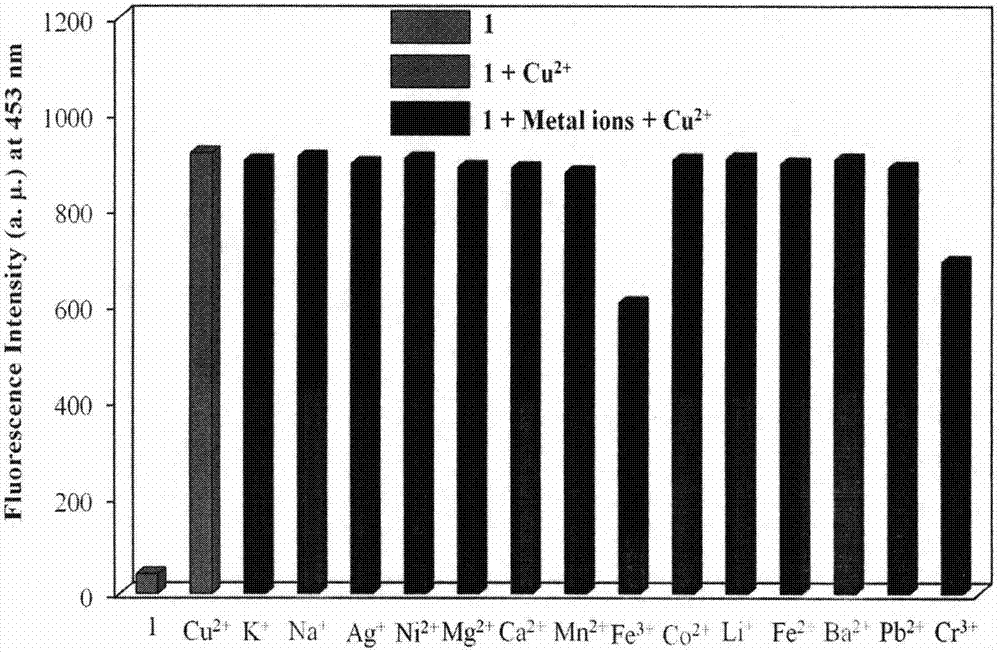
Novel fluorescent molecular probe for copper ions and application of novel fluorescent molecular probe
The invention provides a fluorescent probe for bivalent copper ions and a preparation method of the fluorescent probe. The method comprises the steps of dissolving 6-methoxyl-2-naphthaldehyde in a reaction flask by taking methanol as a solvent, and heating up to 40-50 DEG C; dissolving carbohydrazide by taking water as a solvent, then dropwise adding the dissolved carbohydrazide into the reaction flask, and carrying out reflux reaction for 3h; after the reaction, filtering and drying a mixed solution for removing the solvents to obtain the Schiff base fluorescent probe for the copper ions. The method is simple in operation, convenient and fast, low in cost of a needed intermediate and easy in control of reaction process; the product is easy to separate, high in yield and high in purity. The fluorescent probe has special recognition performance for the copper ions, thus having wide application prospect in the aspects of sensor technologies, photochemistry, electroluminescent devices, and the like.
Owner:WENZHOU MEDICAL UNIV
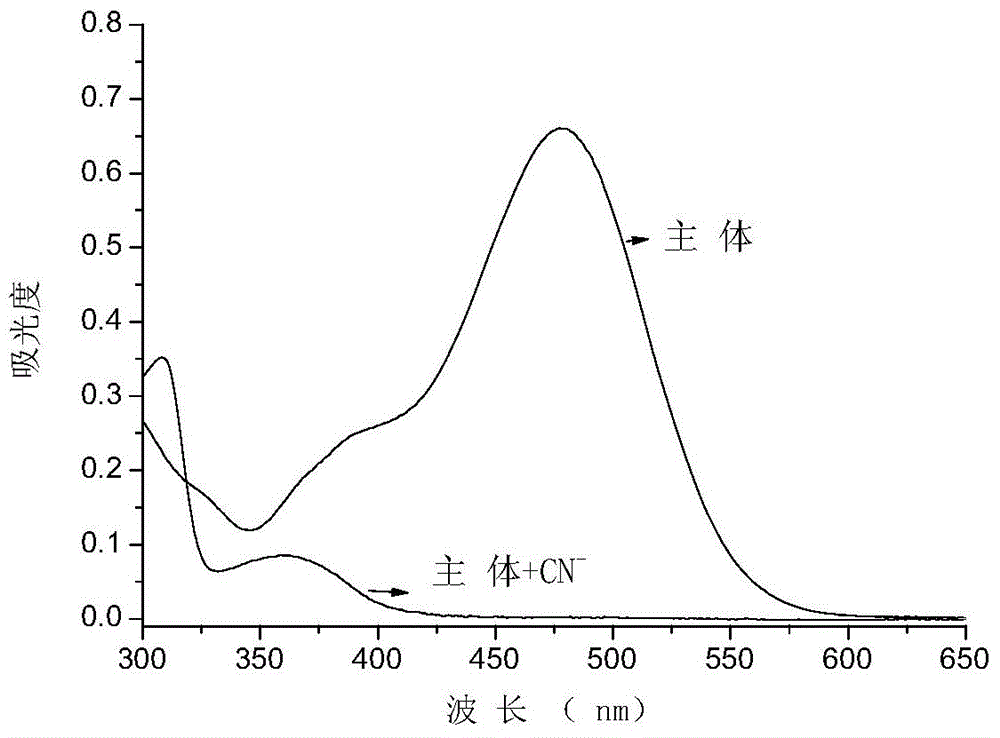
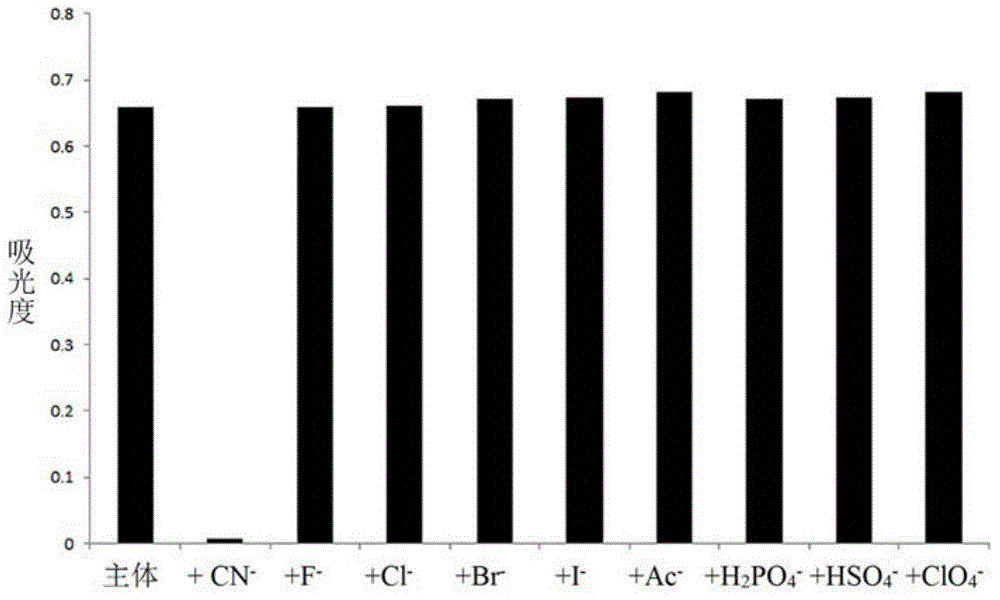
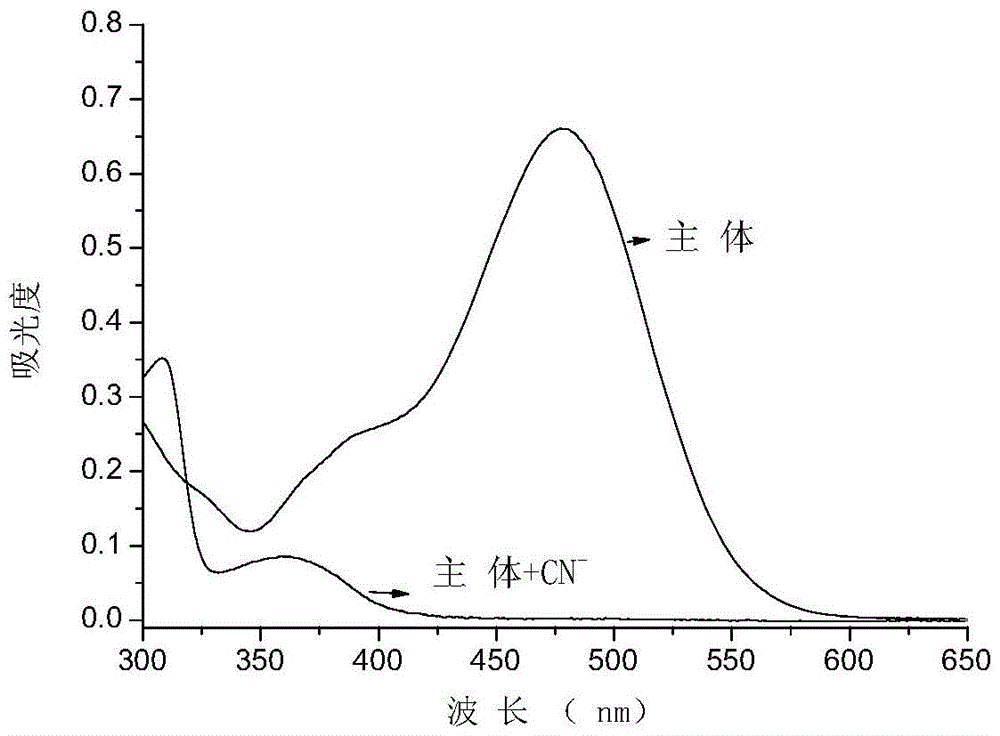
Cyanide receptor compound based on 2-cyano-3-(6-N, N-dimethylamino-2-naphthyl) acrylonitrile, preparation method and application
ActiveCN105037202AEasy to synthesizeRaw materials are easy to getCarboxylic acid nitrile preparationOrganic compound preparationBinding siteAcrylonitrile
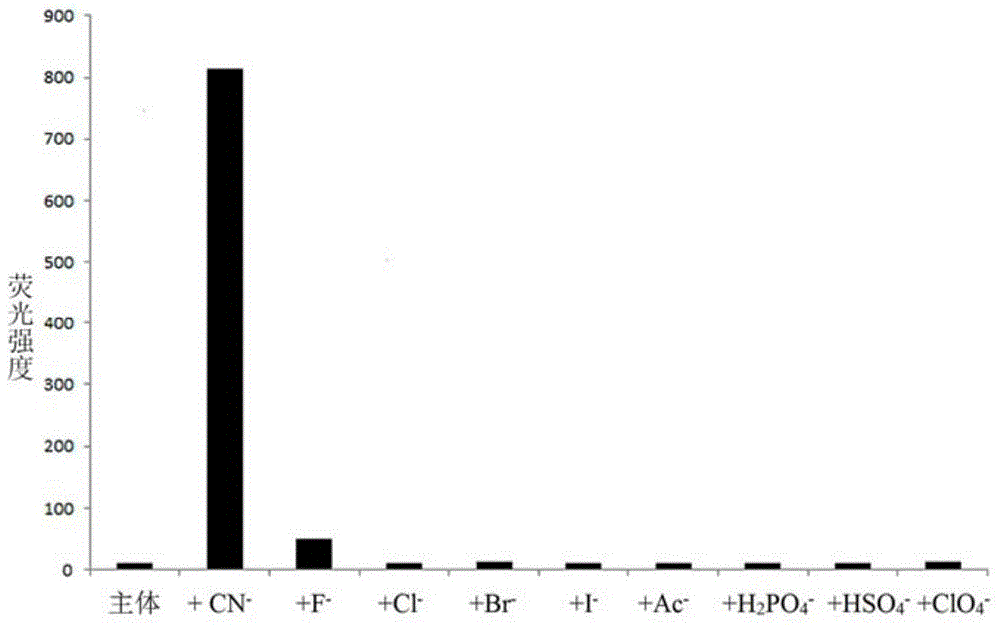
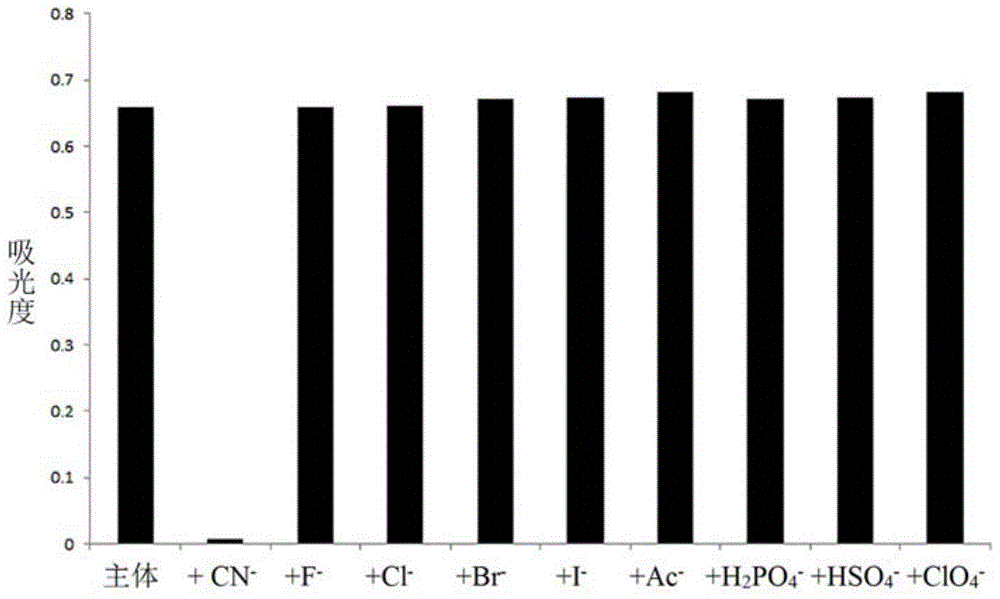
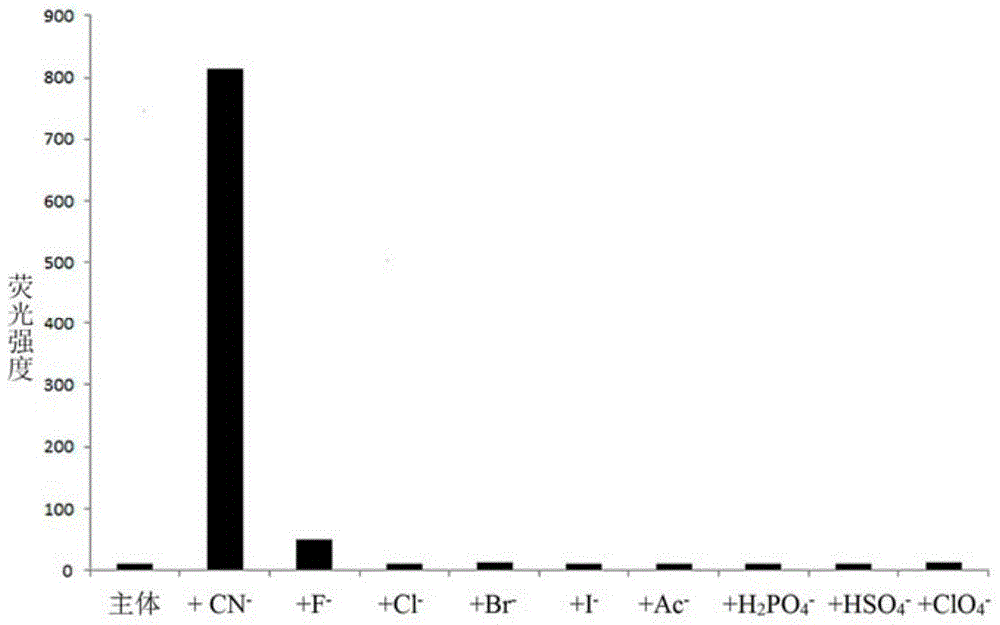
The invention discloses a cyanide receptor compound based on 2-cyano-3-(6-N, N-dimethylamino-2-naphthyl) acrylonitrile, a preparation method and application thereof. The compound takes a C=C double bond as a reactive binding site and takes 6-N, N-dimethylamino-2-naphthaldehyde as a fluorescence signal reporter group. When a receptor encounters cyanide ions, the cyanide ions and the C=C double bond of the receptor can carry out addition reaction so as to cause proton transfer and charge transfer in receptor molecules, so that the receptor molecules have color and fluorescence changes. The invention respectively researches the identification effects of the compound to F<->, Cl<->, Br<->, I<->, Ac<->, H2PO4<->, HSO4<->, ClO4<-> and CN<-> through colorimetry, ultraviolet-visible absorption spectroscopy and fluorescent spectrometry. The results show that the receptor compound can singly and selectively identify cyanide ions in an HEPES-DMSO-H2O system and has very high sensitivity to detection of CN<->.
Owner:NANJING UNIV OF SCI & TECH
Dead/living cell distinguishing fluorescence probe and synthesis method and application thereof
InactiveCN108373447AThe synthesis method is simpleHigh yieldOrganic chemistryFluorescence/phosphorescenceFluorescenceSynthesis methods
The invention provides a target and fluorescence color variable fluorescence probe and application thereof in distinguishing of imaging dead and living cells. A chemical name of the fluorescence probeis 2-(6-methoxy-6-naphthylvinyl)-N-methyl-quinoline iodate. The fluorescence probe is synthesized by steps: using 2-methylquinoline and iodomethane to synthesize 1,2-dimethylquinoline iodate; subjecting the 1,2-dimethylquinoline iodate and 6-methoxy-2-naphthaldehyde to condensation reaction at the room temperature to generate a product by taking pyrrolidine as a catalyst. The synthesis method ofthe probe is simple, dead and living cells can be distinguished and marked by two fluorescence colors and two dyeing positions, and a promising application prospect is achieved.
Owner:UNIV OF JINAN
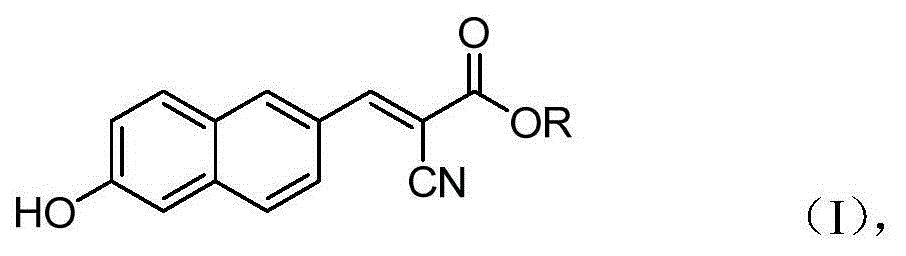
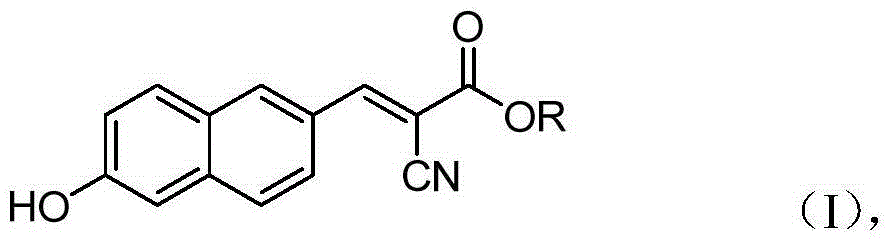
6'-hydroxyl naphthyl-2-cyanoacrylate and application thereof
InactiveCN103709069ACarboxylic acid nitrile preparationOrganic compound preparationChemical structureFluorescence
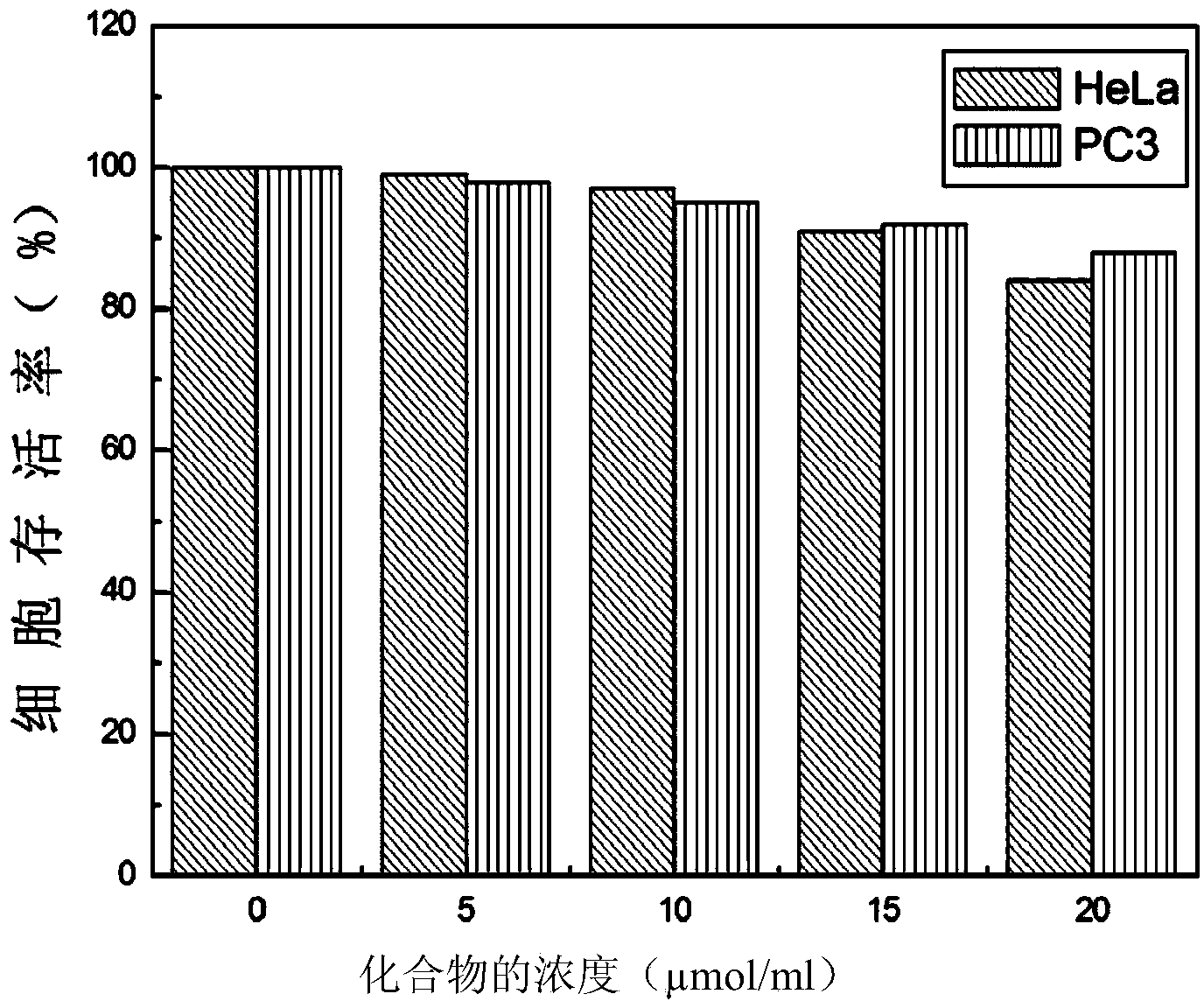
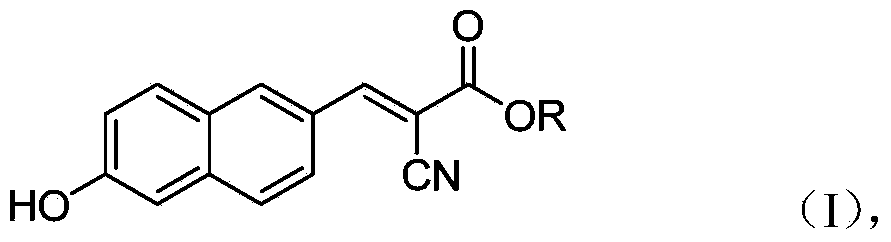
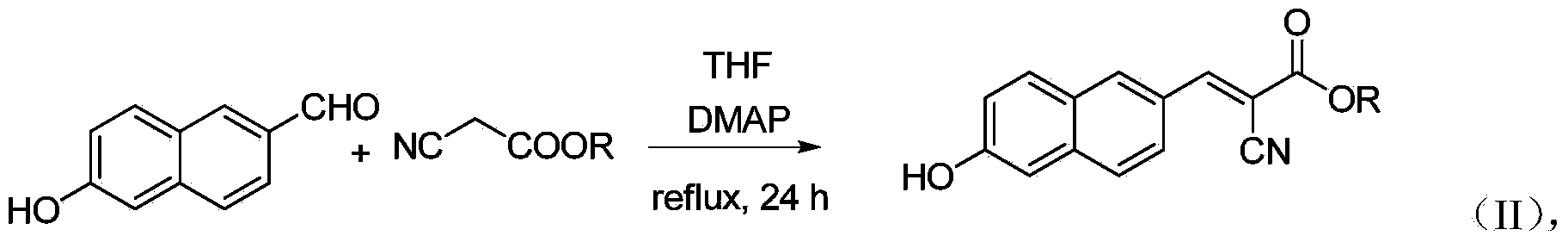
The invention belongs to the field of organic chemistry, and in particular relates to 6'-hydroxyl naphthyl-2-cyanoacrylate. The chemical structure of the 6'-hydroxyl naphthyl-2-cyanoacrylate is shown in formula (I) which is as shown in the description, wherein R is methyl, ethyl, propyl and butyl, 2-(2-methoxy ethyoxyl) ethyoxyl or 2-(N,N-dimethyl) aminoethyl. The 6'-hydroxyl naphthyl-2-cyanoacrylate is obtained by performing reaction on 6'-hydroxyl-2-naphthaldehyde and 2-cyan-acetic ester. The 6'-hydroxyl naphthyl-2-cyanoacrylate has fluorine ion fluorescence recognition performance and can be used for detecting fluorine ions in cells.
Owner:SOUTHERN MEDICAL UNIVERSITY
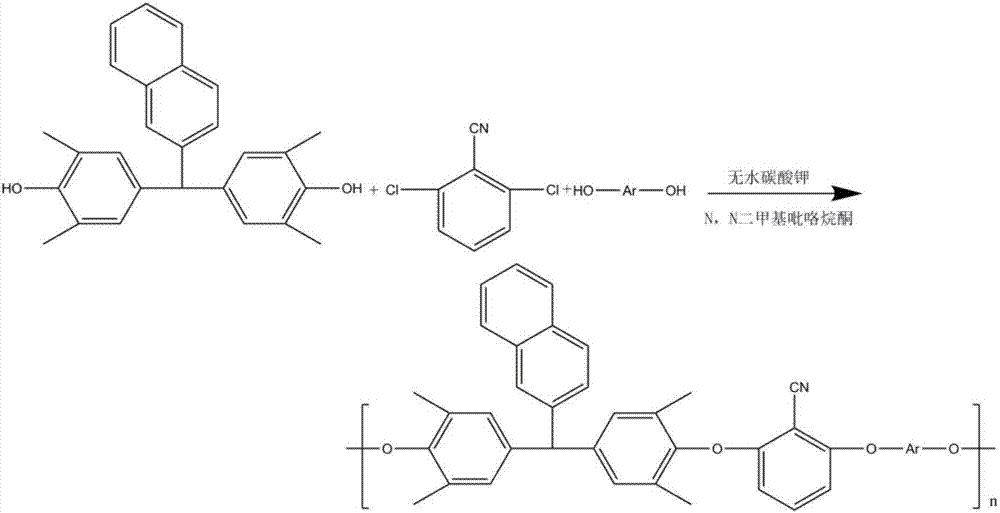
Synthesis method of high-temperature-resistant polyarylene ether nitrile resin
ActiveCN105754089AImprove heat resistanceDoes not affect processing performancePolymer scienceFunctional monomer
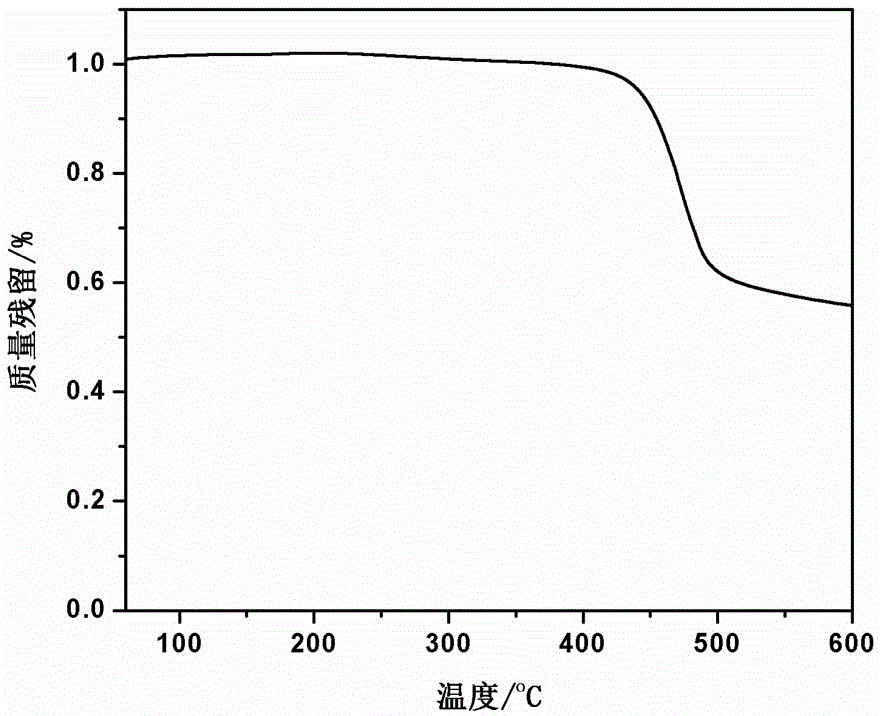
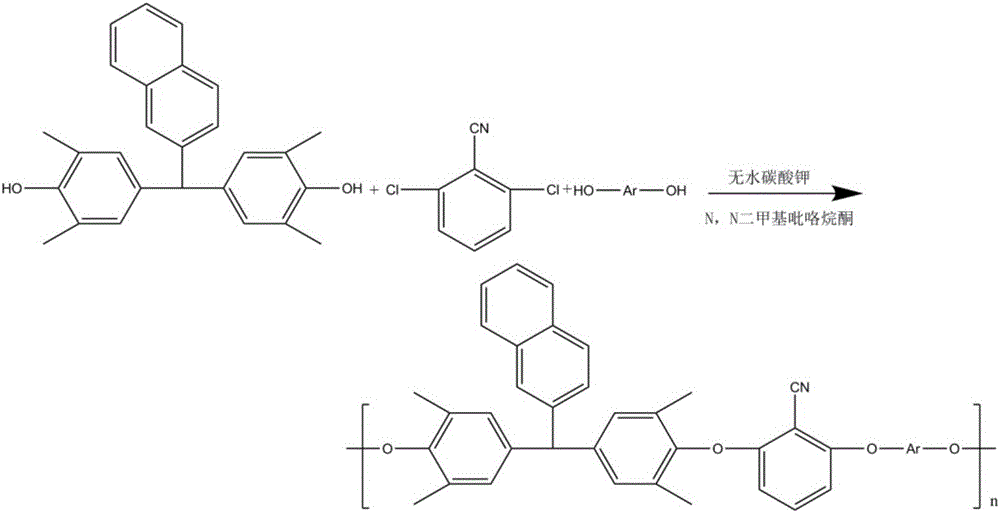
The invention discloses a synthesis method of high-temperature-resistant polyarylene ether nitrile resin. The synthesis method is characterized by comprising the following steps: placing 2-naphthaldehyde, 2,6-xylenol and toluene in a reaction kettle, dropwise adding a sulfuric acid aqueous solution and 3-thiohydracrylic acid under nitrogen atmosphere, heating and concentrating after washing by deionized water and toluene, separating out a crystal and drying to obtain a bisphenol functional monomer; adding 2,6-dichlorobenzonitrile, a diphenol monomer, the bisphenol functional monomer, anhydrous potassium carbonate, N, N-dimethylpyrrolidine and toluene to the reaction kettle, carrying out heat preservation to obtain a polycondensation product; adding the polycondensation product to deionized water or the sulfuric acid aqueous solution to be boiled, performing suction filtration, washing a solid by deionized water and then drying the solid to prepare the high temperature resistant polyarylene ether nitrile resin. According to the invention, the bisphenol monomer of which the side group contains a naphthalene ring structure is introduced into molecules of the traditional polyarylene ether nitrile, so that the prepared polyarylene ether nitrile resin has good heat resistance and processing properties.
Owner:MIANYANG HONGQI NEW MATERIAL SCI & TECH
Fluorescent probe for mitochondrial membrane potential as well as synthesis method and application thereof
InactiveCN109574922ALow toxicityLarge fluorescence wavelength shiftOrganic chemistryFluorescence/phosphorescenceSynthesis methodsFluorescence
The invention provides a fluorescent probe for mitochondrial membrane potential as well as a synthesis method and application thereof. The structural formula of the probe is as shown in the followingdescriptions. The probe can be synthesized with a reaction product 6-hexyloxyl-2-naphthaldehyde of 6-hydroxyl-2-naphthaldehyde and hexane iodide and a reaction product 1,2-dimethyl-quinoline iodate of2-methylquinoline and methyl iodide. The probe provided by the invention can be used for distinguishing different membrane potential. The fluorescent probe provided by the invention is large in fluorescent wavelength displacement when responding to the mitochondrial membrane potential and low in toxicity to a cell; and the fluorescent probe is simple in synthesis method and simple and convenientin purification step. The probe is successfully applied to cell imaging, and can be used for distinguishing the change of the mitochondrial membrane potential.
Owner:UNIV OF JINAN
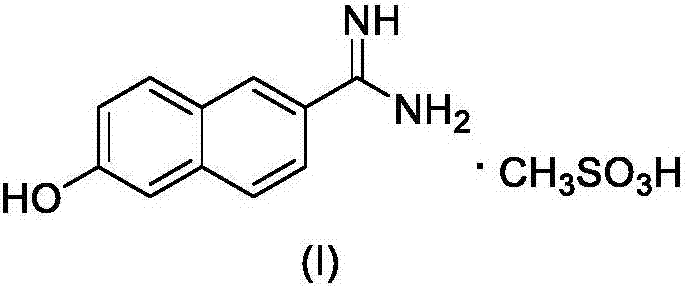
New 6-amidino-2-naphthol methanesulfonate synthesis method
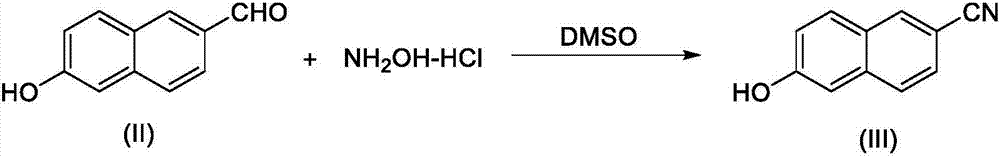
ActiveCN103896809AReduce pollutionMild reaction conditionsSulfonic acids salts preparationSodium bicarbonateHydroxylamine Hydrochloride
The present invention relates to a new synthesis method for a nafamostat mesylate intermediate 6-amidino-2-naphthol methanesulfonate. According to the new method, 6-hydroxy-2-naphthaldehyde is adopted as a raw material, dimethyl sulfoxide is adopted as a reaction solvent, the dimethyl sulfoxide and hydroxylamine hydrochloride are subjected to an addition elimination reaction to obtain 6-cyano-2-naphthol, the 6-cyano-2-naphthol is subjected to a pinner reaction in a HCl / methanol solution to obtain 6-hydroxy-2-naphthalene imino methyl ester hydrochloride, ammonia gas is introduced to carry out an aminolysis reaction to obtain 6-amidino-2-naphthol, and the 6-amidino-2-naphthol sequentially reacts with sodium bicarbonate and methanesulfonic acid to convert into the 6-amidino-2-naphthol methanesulfonate. According to the present invention, in the new synthesis method, the 6-cyano-2-naphthol preparation in the first step adopts the completely-new method, the use of the highly toxic copper cyanide in the traditional method is avoided, the operations are simple, and the conditions are mild; and the second step adopts the improved pinner method, wherein the reaction of acetyl chloride and methanol is adopted to produce HCl to replace direct introduction of HCl gas into the reaction system, such that the improved method has strong operability and industrialization is easily achieved.
Owner:BEIJING LABWORLD BIO MEDICINE TECH
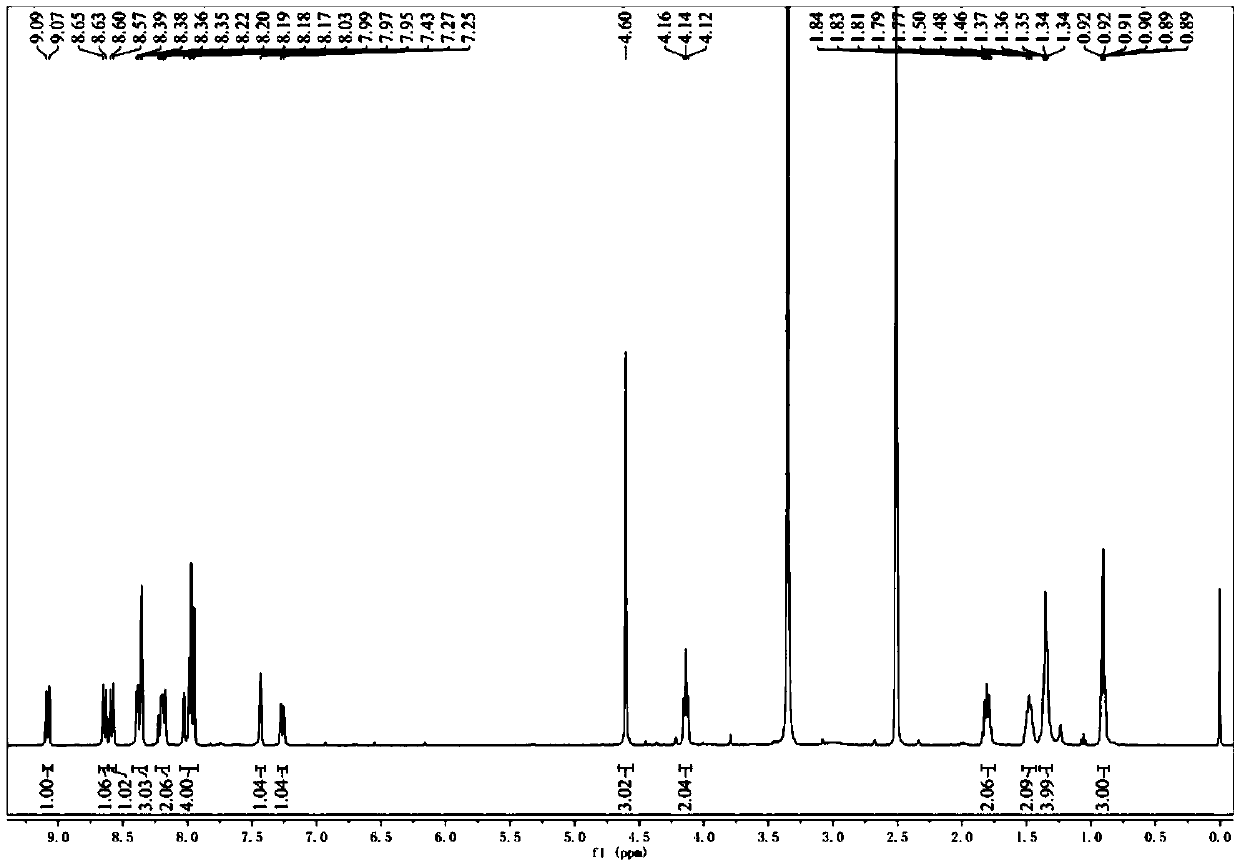
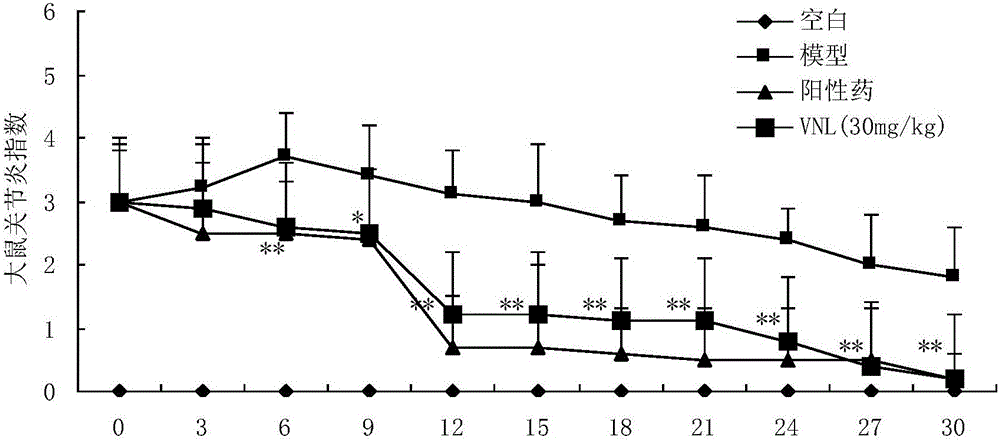
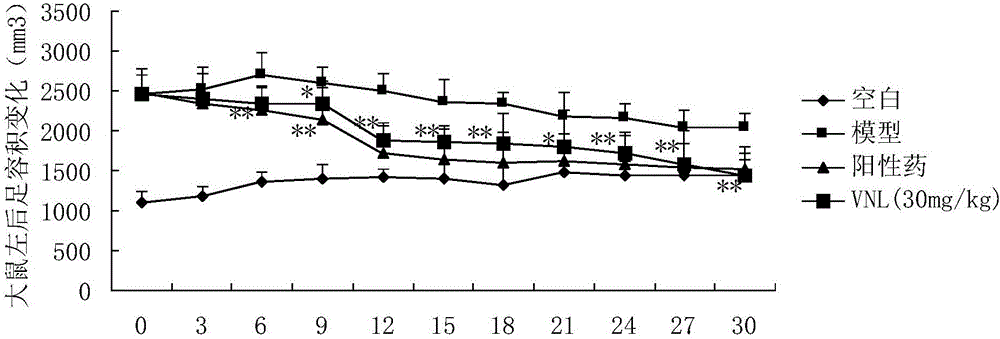
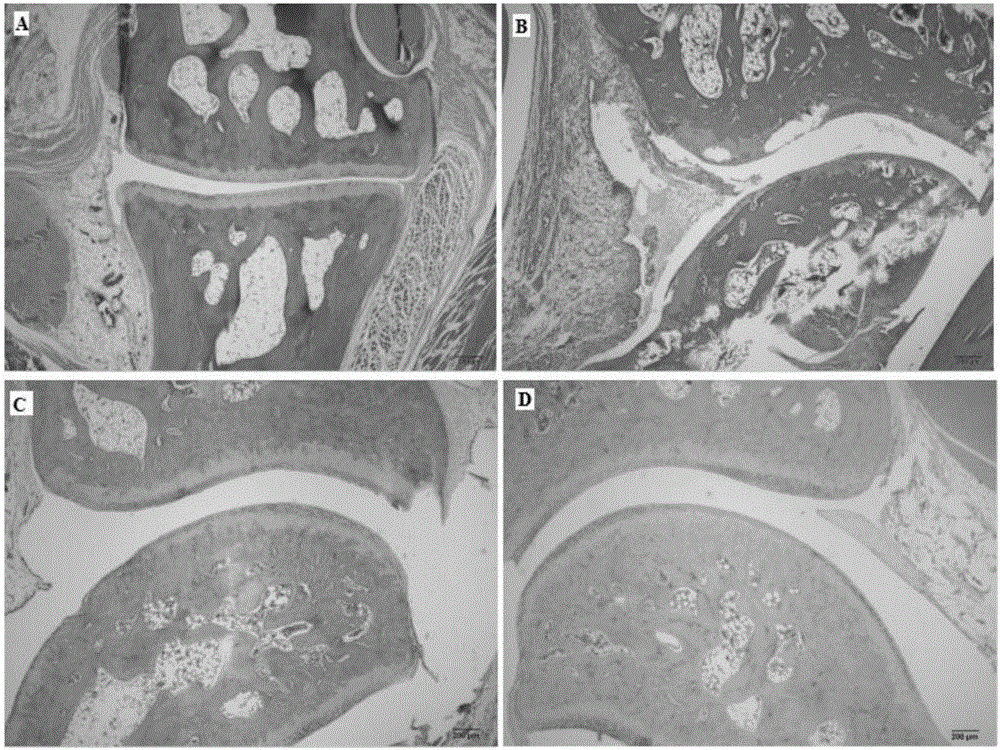
Applications of phenyl naphthalene type lignin in preparation of anti-rheumatoid arthritis drugs
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Mitochondrial pH fluorescent probe based on benzothiazole and preparation method thereof
ActiveCN109293698AExcellent targeting abilityRealize real-time monitoringGroup 5/15 element organic compoundsFluorescence/phosphorescenceRefluxFluorescence
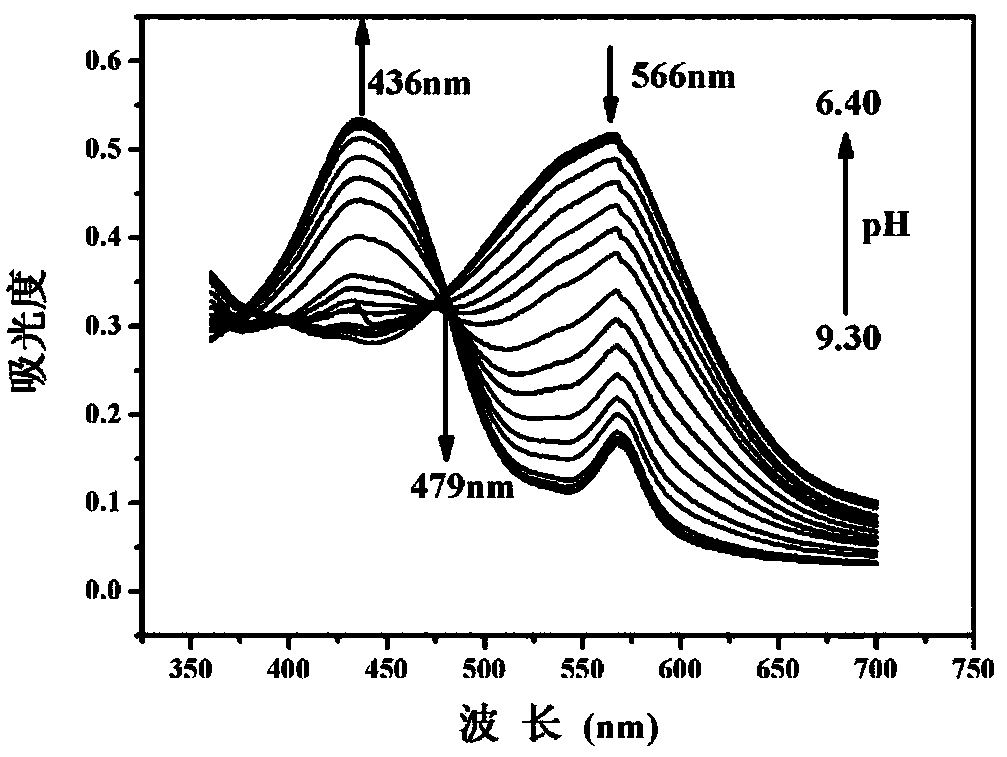
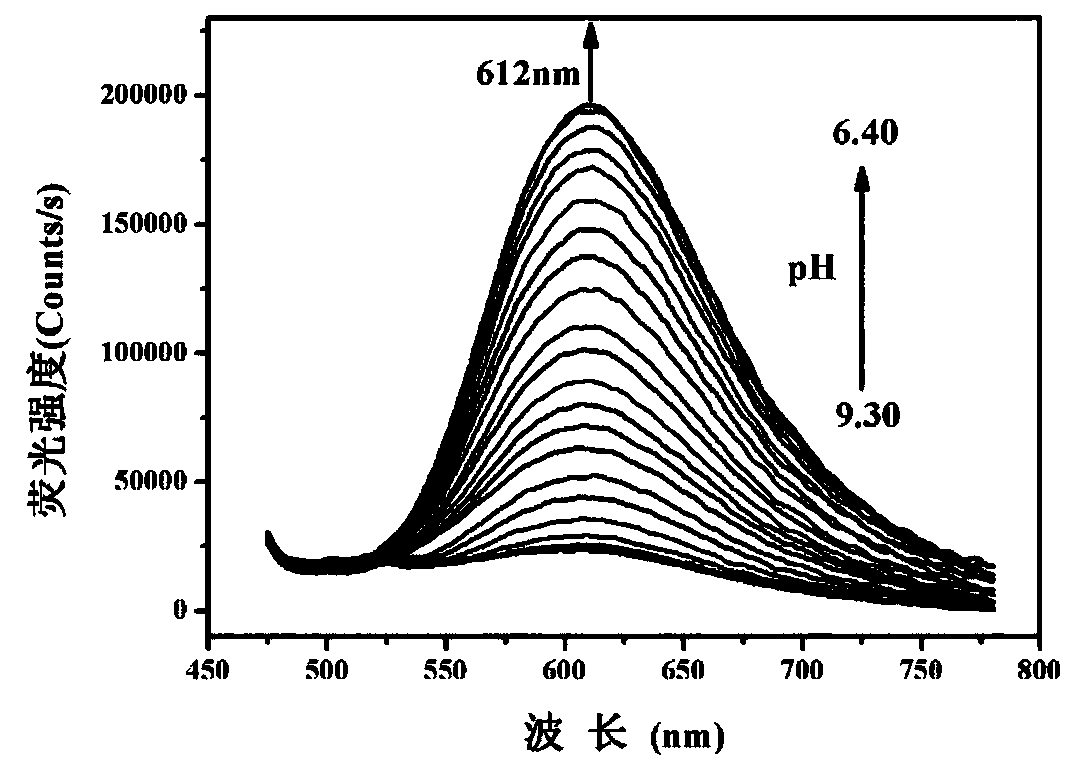
The invention discloses a mitochondrial pH fluorescent probe based on benzothiazole, and a preparation method and an application thereof. The probe is specifically named as 2-(2-(6-hydroxynaphthalene-2-yl)vinyl)-3-(6-(triphenylphosphonyl)hexyl)benzothiazole-3-bromide (HTBT2). The preparation method comprises the steps: firstly, carrying out a reaction of 2-methylbenzothiazole and 1,6-dibromohexaneunder heating conditions to prepare 3-(6-bromohexyl)-2-methylbenzothiazole-3-bromide (BMBI), and then carrying out mixed reflux of the prepared BMBI with triphenylphosphine and acetonitrile to prepare 2-methyl-3-(6-(triphenylphosphorus)hexyl)benzothiazole-3-bromide (MTBI); and finally, dissolving MTBI and 6-hydroxy-2-naphthaldehyde in ethanol, and adding a small amount of piperidine, refluxing, and then separating and purifying to obtain HTBT2. The probe has the pKa value of 8.04+ / -0.02 and is quite close to that of a mitochondrial matrix (with the pH of 8.0). At the same time, the probe hasthe advantages of high sensitivity to the changes of the pH, good selectivity and large Stokes displacement, and can be used for monitoring the changes of the pH in cell mitochondria.
Owner:SHANXI UNIV
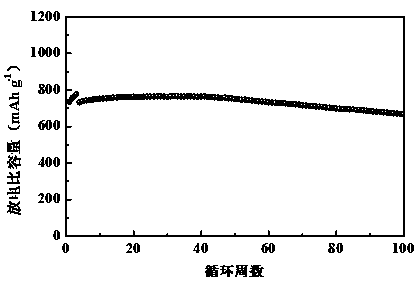
Polyimine conductive binder for silicon-based negative electrode of lithium ion battery
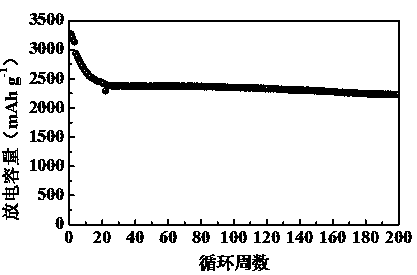
ActiveCN110277559ALess inactive componentsEasy to operateFinal product manufactureCell electrodesConductive polymerLithium-ion battery
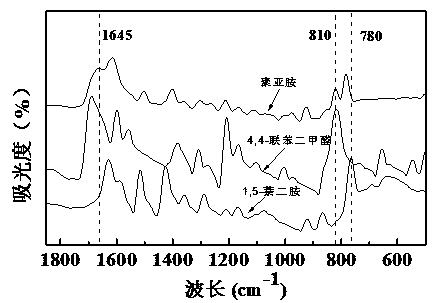
The invention relates to a polyimine conductive binder for a silicon-based negative electrode of a lithium ion secondary battery, which is characterized in that a conductive polymer is copolymerized by using a monomer A and a monomer B as raw materials, wherein the monomer A is one or a mixture of 1,5-naphthylene diamine, 4,4'-diaminodiphenyl or 3,3'-dihydroxybenzidine; the monomer B is one or a mixture of naphthalene-2,3 dialdehyde, 4, 4-biphenyldicarboxaldehyde or 6-hydroxy-2-naphthaldehyde; and the infrared spectrum test shows that there is a characteristic absorption peak of the imino at 1645(+ / -5)cm<-1>. The invention can completely abandon the use of the traditional conductive agent and greatly improve the load capacity of active substance in the electrode; through abundant hydrogen bonds and physical crosslinking in a molecular chain, an abundant crosslinking network is formed, the volume expansion of a silicon-based material is inhibited, and the electrode structure is stabilized. The polyimine conductive binder is especially suitable for the silicon-based negative electrode of the lithium ion secondary battery. The electrode has the characteristics of good rate performance, high cycling stability, long cycling life, simple preparation process, ability of being suitable for industrial production and the like.
Owner:NANKAI UNIV
Benzothiazole derivative and preparation method and application thereof
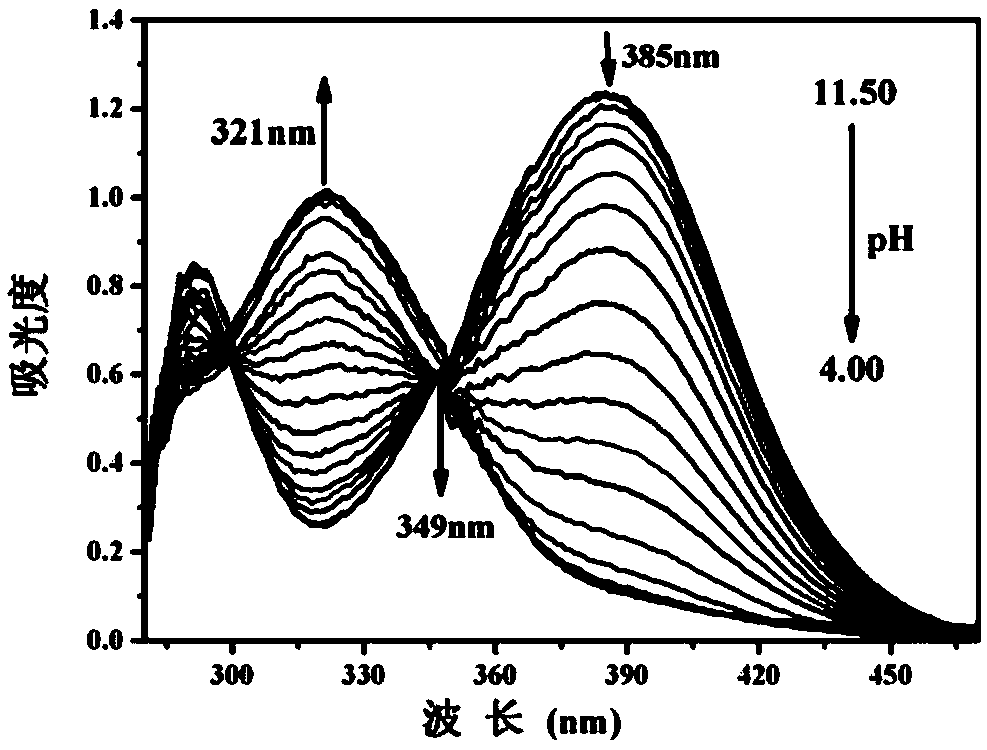
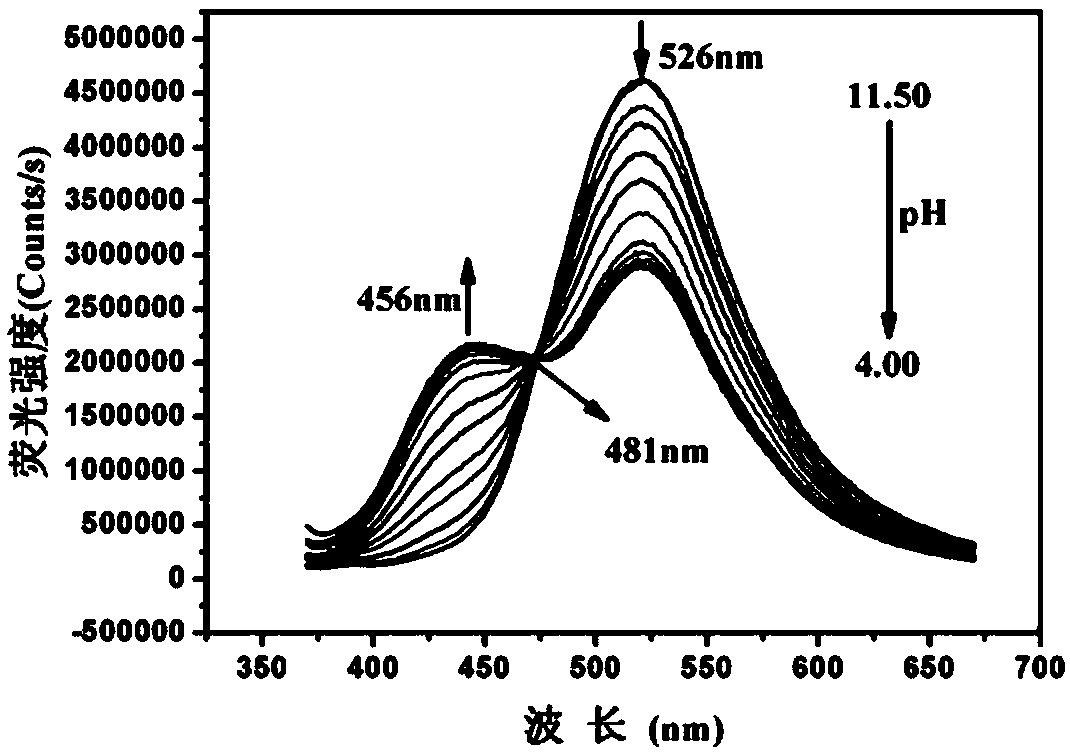
ActiveCN109369565AImprove permeabilityCell membrane permeability is goodOrganic chemistryFluorescence/phosphorescenceQuantum yieldCell membrane
The invention discloses a benzothiazole derivative and a preparation method and application thereof. The derivative is specifically named as 6-(2-(benzothiazolyl-2-yl)vinyl)naphthalene-2-phenol (BTNO). The preparation method comprises the following steps: dissolving 6-hydroxy-2-naphthaldehyde and 2-methylbenzothiazole in a very small amount of dichloromethane, adding trimethylchlorosilane and heating to obtain a crude product; removing a solvent from the crude product and conducting silica gel column separation to obtain a pure product. With decrease of the pH of BNTO from 11.50 to 4.00, fluorescence spectrums have blue shift and exhibit ratio fluorescence emission (F456nm / F526nm) characteristics. At the same time, the advantages of high sensitivity to pH response, good selectivity, high quantum yield and large Stokes displacement and the like can be achieved. In addition, the BTNO acts as a probe to mark cells by permeating cell membranes and can be used for monitoring pH fluctuationswithin the scope of cytoplasm.
Owner:SHANXI UNIV
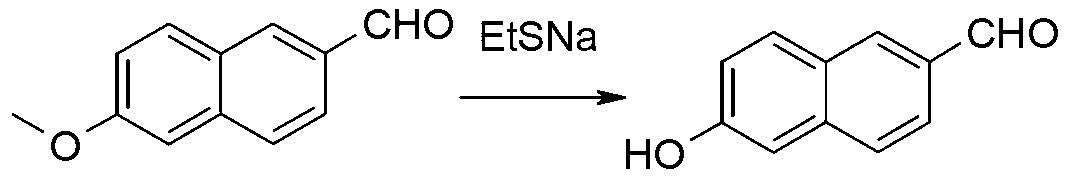
Method for synthesizing 6-methoxy-2-naphthaldehyde
The present invention discloses a method for synthesizing 6-methoxy-2-naphthaldehyde. The method comprises allowing bromization and reduction reaction of 2-methoxynaphthalene to produce 6-bromo-2-methoxynaphthalene, and allowing reaction of 6-bromo-2-methoxynaphthalene and N,N-dimethylformamide to obtain the final product. The method has no need for column chromatography to obtain high-purity 6-methoxy-2-naphthaldehyde, and improves yield.
Owner:SUZHOU HEALTH COLLEGE
Histone deacetylase fluorescence probe, and preparation method and applications thereof
ActiveCN108218743AHigh sensitivityFast responseCarboxylic acid nitrile preparationOrganic compound preparationAlcoholFluorescence
The invention provides a histone deacetylase fluorescence probe, and a preparation method and applications thereof. The structure formula of the histone deacetylase fluorescence probe is disclosed inthe invention. In synthesis, 6-(dimethylamino)-2-naphthaldehyde with two-photon effect is selected as a dye, so that further nucleophilic reaction is realized after deacetylation reaction, and fluorescence signals are generated; aldehyde group carbon is modified into cyanogroup alcohol structures, hydroxyl is connected with HDACs responsive groups, and the histone deacetylase fluorescence probe isused for detecting and imaging of the activity of histone deacetylase in tissue. According to the histone deacetylase fluorescence probe, cyanogroup with excellent electron-withdrawing capability isintroduced, so that after response of HDACs with the histone deacetylase fluorescence probe, intramolecular cyclization reaction is carried out rapidly, activation of fluorescence signals is realized,detecting and imaging on histone deacetylase in living cells and tissue are realized, and excellent clinical application value is achieved.
Owner:湖南融健生物科技有限公司
Method for preparing 6-methoxyl-2-naphthaldehyde
InactiveCN104447252AEmission reductionReduce manufacturing costOrganic compound preparationCarboxylic acid esters preparationAlcoholEsterification reaction
The invention discloses a method for preparing 6-methoxyl-2-naphthaldehyde. The method comprises the following steps: (1) contacting 6-methoxyl-2-naphthoic acid with alcohol to obtain 6-methoxyl-2-naphthoate through esterification reaction; (2) contacting the 6-methoxyl-2-naphthoate resulting from step (1) with a reducing agent to acquire 6-methoxyl-2-naphthalene methanol through reduction reaction; (3) contacting the 6-methoxyl-2-naphthalene methanol resulting from step (2) with an oxidizing agent to obtain 6-methoxyl-2-naphthaldehyde through oxidation reaction. According to the invention, the method has advantages of less discharge of industrial waste, low production cost and being environment-friendly and safe.
Owner:ZHEJIANG TIANXIN PHARMA
Method used for preparation of cyclized myrac aldehyde through catalytic selective cyclization of myrac aldehyde with supported molybdenum carbide
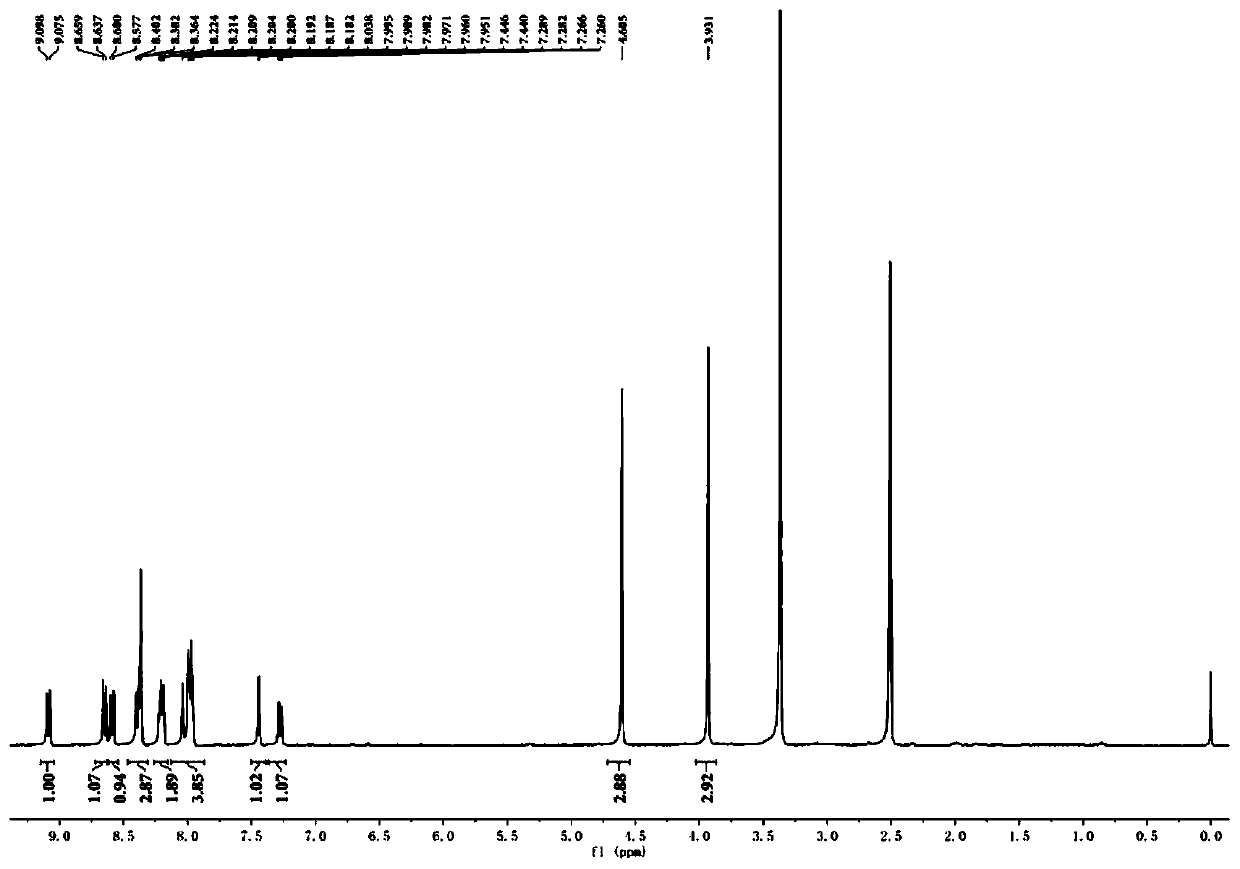
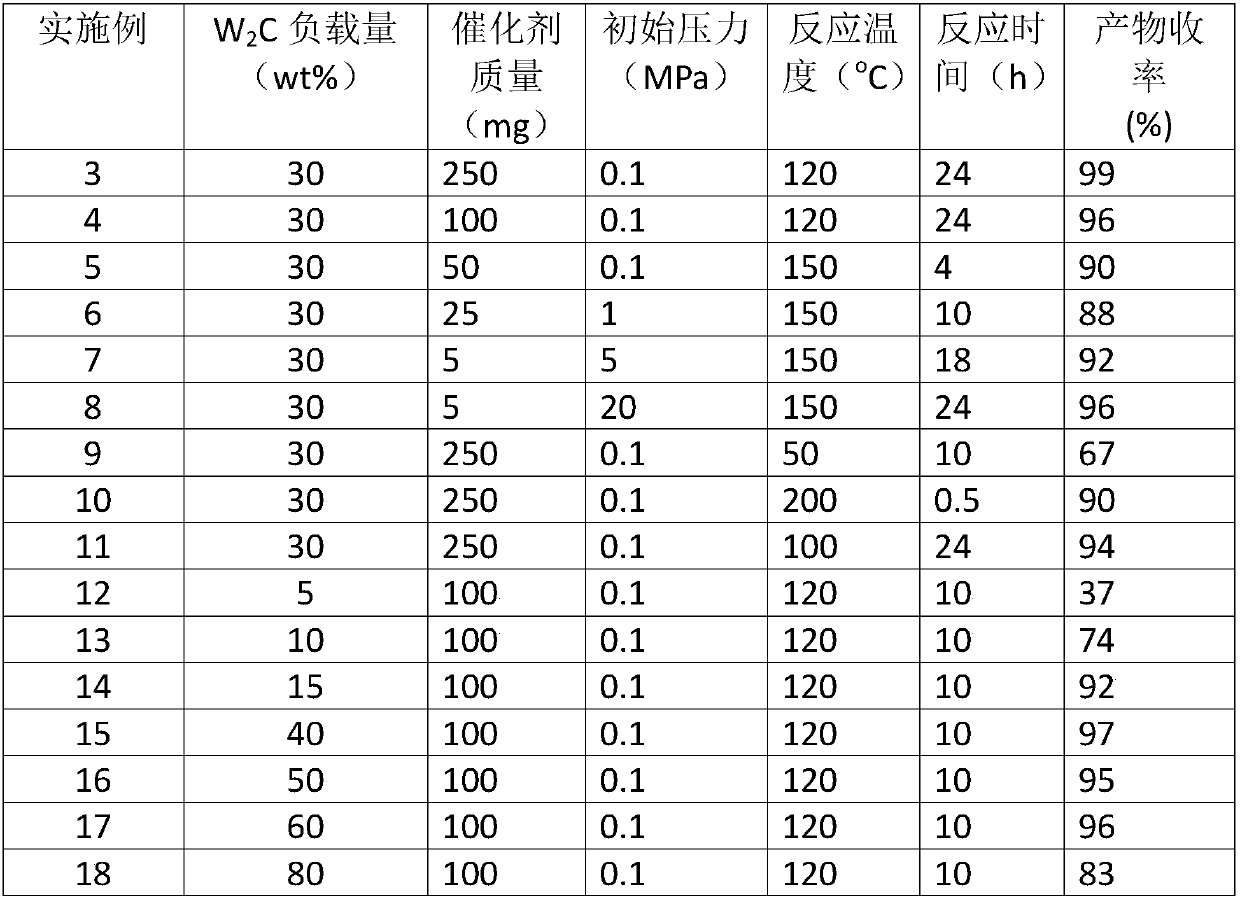
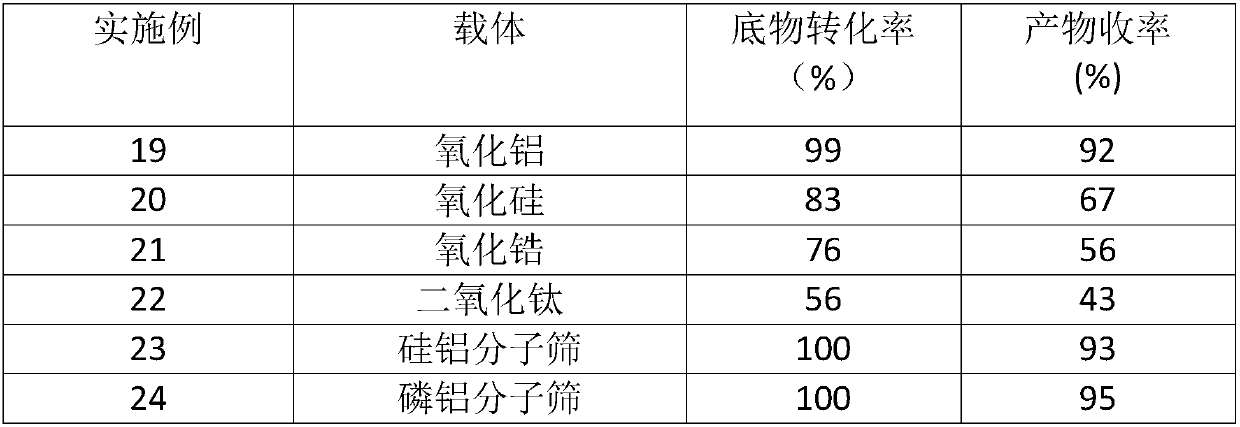
ActiveCN109851487ALow costAvoid problems such as difficulty in recycling and environmental pollutionMolecular sieve catalystsOrganic compound preparationCatalytic methodOrganic solvent
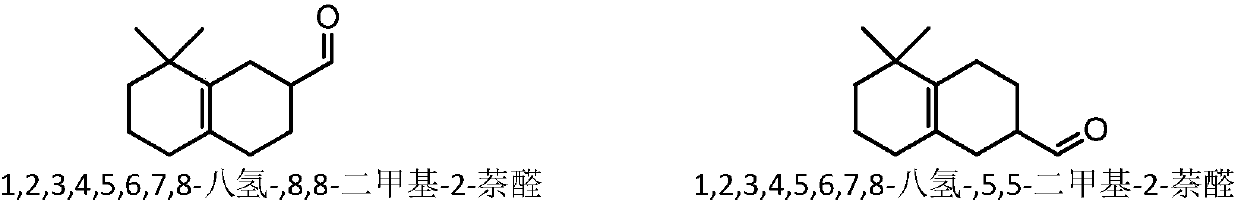
The invention relates to molybdenum carbide catalyzed myrac aldehyde cyclisation reaction, and more specifically relates to a method used for preparation of cyclized myrac aldehydes (1, 2, 3, 4, 5, 6,7, 8-octahydro-, 8, 8-dimethyl-2-naphthaldehyde and 1, 2, 3, 4, 5, 6, 7, 8-octahydro-5, 5-dimethyl-2-naphthaldehyde) through catalytic selective cyclization of myrac aldehydes (4-(4-methyl-3-pentenyl)-3-cyclohexene aldehyde (p-myrac aldehyde) and 3-(4-methyl-3-pentenyl)-3-cyclohexene aldehyde (m-myrac aldehyde)) with a supported molybdenum carbide catalyst under relatively mild conditions. According to the method, p-myrac aldehyde is taken as a raw material, in an organic solvent, at room temperature, production of 1, 2, 3, 4, 5, 6, 7, 8-octahydro-, 8, 8-dimethyl-2-naphthaldehyde through highselective cyclization reaction is realized; the highest substrate conversion rate is 100%, and the highest target product yield is 99%. Compared with conventional catalytic route, supported non-precious metal molybdenum carbide is taken as a catalyst, water is taken as reaction solvent, using of inorganic acid or base is not needed, and generation of a large amount of acid solution in conventional catalytic method is avoided; reaction conditions are mild, the catalyst is cheap, is high in activity and selectivity, and can be recycled, and the reaction process is friendly to the environment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of cable material for rare earth based precision equipment
InactiveCN108559244ARaw materials are easy to getSimple processPlastic/resin/waxes insulatorsMANGANESE ACETATEHexadienoic Acid
The invention discloses a preparation method of a cable material for rare earth based precision equipment. The preparation method comprises the following steps: preparing an activated adhesive from vinyltriethoxysilane, polymethylphenyl siloxane fluid, sodium metabisulfite, cyclosiloxane fatty ester, dimethyl phthalate, manganese acetate, sodium chromate, hexamethylenetetramine and the like as rawmaterials, and then, enabling the activated adhesive and raw materials including rare earth, ethyl methacrylate, polycarbonate, polysulfone, polymethyl methacrylate, polyalkylene glycol, rosin resin,barium ferrite, azobisisobutyronitrile, 6-methoxy-2-naphthaldehyde, hexadienoic acid, diethylene triaminepentaacetic acid, triethyl phosphate and the like to be respectively subjected to high-temperature internal mixing, magnetic stirring, mold casting, static cooling, water-cooled drawing, solid solution and aging and the like, thereby obtaining the cable material for the rare earth based precision equipment. The prepared cable material for the rare earth based precision equipment has good electrochemical properties and material stability and can be suitable for multiple precision equipment.
Owner:苏州耐思特塑胶有限公司
The synthetic method of nafamostat mesylate intermediate-6-amidino-2-naphthol mesylate
ActiveCN103896809BReduce pollutionMild reaction conditionsSulfonic acids salts preparationSodium bicarbonateHydroxylamine Hydrochloride
Owner:BEIJING LABWORLD BIO MEDICINE TECH
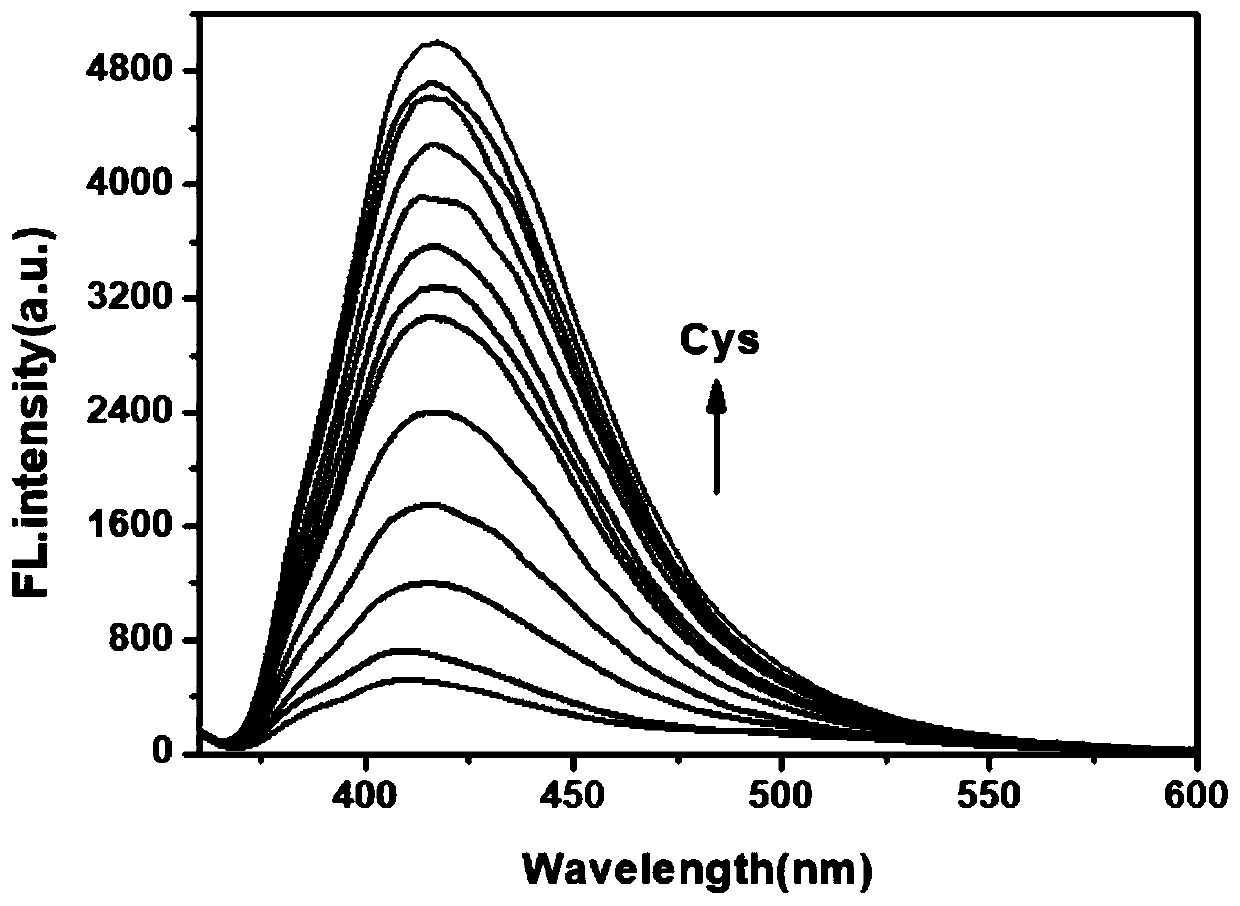
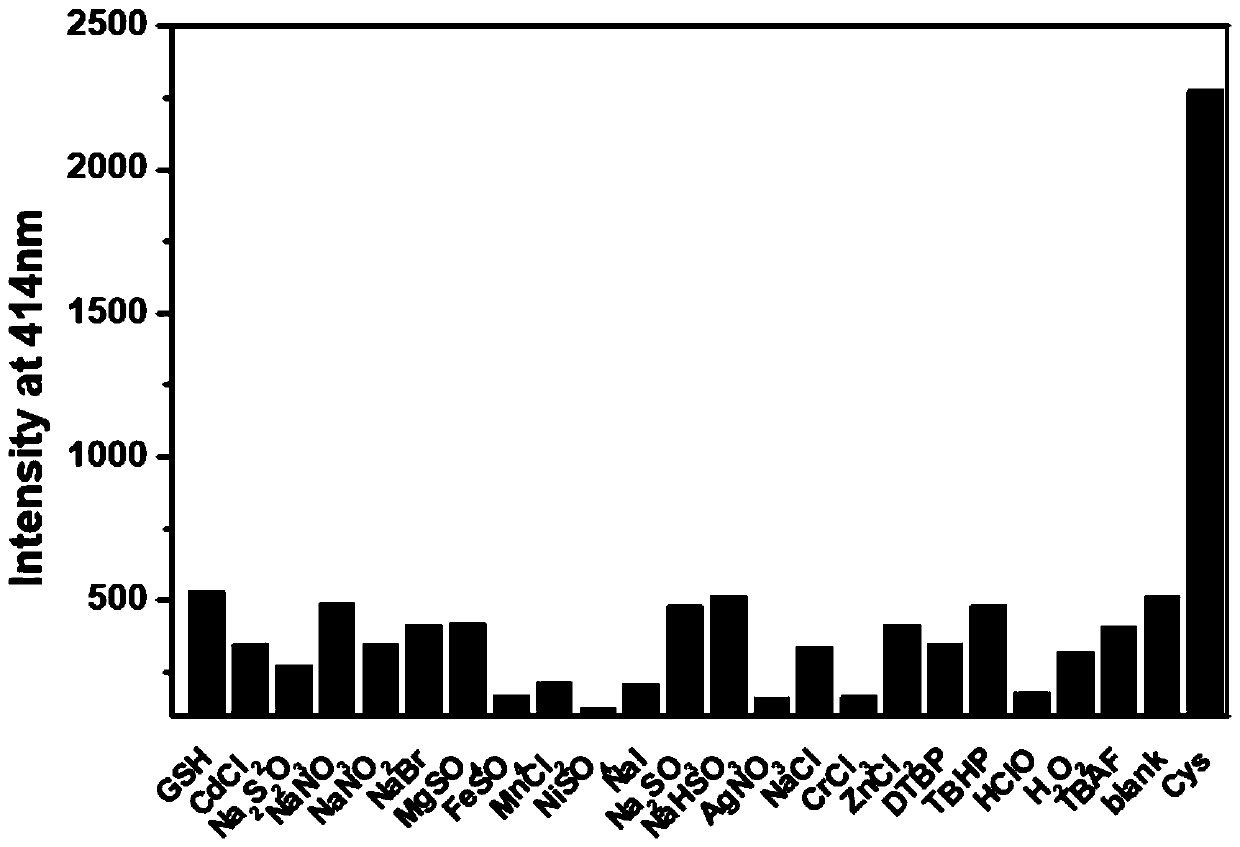
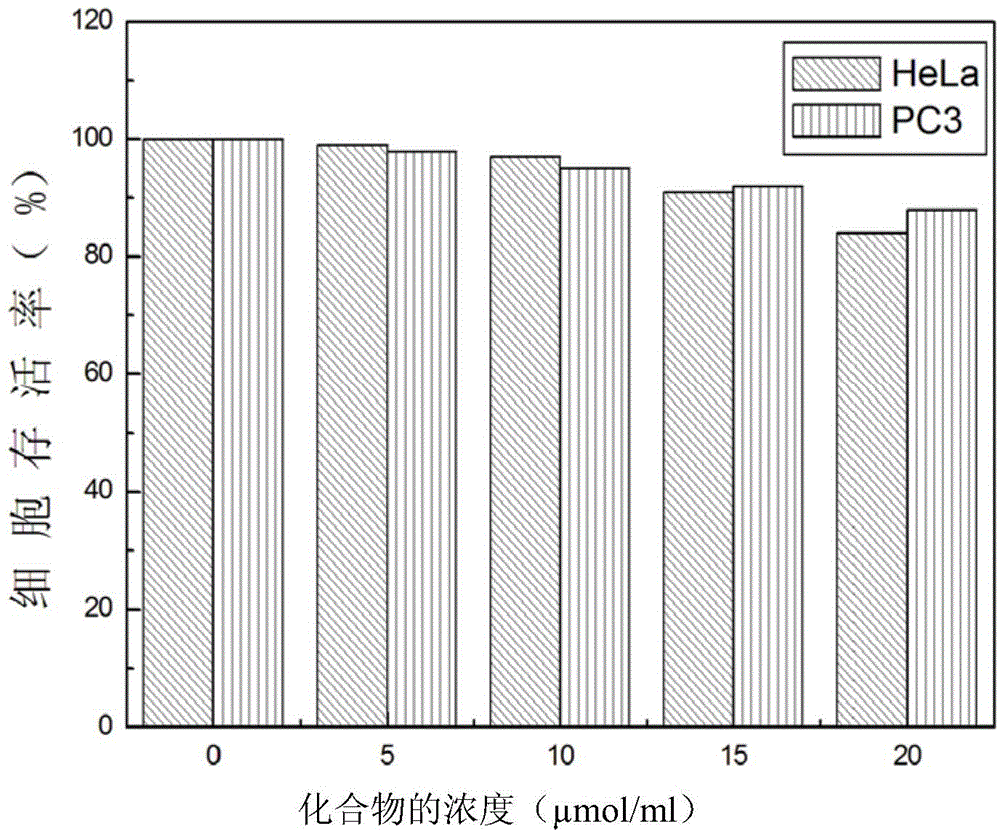
Fluorescent probe for detecting cysteine and application thereof
InactiveCN110156695AIncreased sensitivityHigh selectivityOrganic chemistryFluorescence/phosphorescenceFluorescenceStructural formula
The invention provides a fluorescent probe for detecting cysteine. The probe has the chemical structural formula shown in the specification. The probe can be obtained by carrying out a reaction of thereaction product of 9,10-phenanthrenequinone, 6-hydroxy-2-naphthaldehyde and ammonium acetate with acryloyl chloride. The probe can realize the high sensitivity and high selectivity of a cysteine probe, and can be used as an indicator for indicating the change of cysteine in an aqueous solution and inside biological cells, and can perform the real-time qualitative and quantitative visual colorimetry detection. The probe is a simple, rapid and sensitive cysteine specific detection reagent, and has broad application prospects in the biomolecule detection field.
Owner:UNIV OF JINAN
6'-hydroxyl naphthyl-2-cyanoacrylate and application thereof
InactiveCN103709069BCarboxylic acid nitrile preparationOrganic compound preparationChemical structureFluorescence
The invention belongs to the field of organic chemistry, and in particular relates to 6'-hydroxyl naphthyl-2-cyanoacrylate. The chemical structure of the 6'-hydroxyl naphthyl-2-cyanoacrylate is shown in formula (I) which is as shown in the description, wherein R is methyl, ethyl, propyl and butyl, 2-(2-methoxy ethyoxyl) ethyoxyl or 2-(N,N-dimethyl) aminoethyl. The 6'-hydroxyl naphthyl-2-cyanoacrylate is obtained by performing reaction on 6'-hydroxyl-2-naphthaldehyde and 2-cyan-acetic ester. The 6'-hydroxyl naphthyl-2-cyanoacrylate has fluorine ion fluorescence recognition performance and can be used for detecting fluorine ions in cells.
Owner:SOUTHERN MEDICAL UNIVERSITY
A Novel Copper Ion Fluorescent Molecular Probe and Its Application
The invention provides a fluorescent probe for bivalent copper ions and a preparation method of the fluorescent probe. The method comprises the steps of dissolving 6-methoxyl-2-naphthaldehyde in a reaction flask by taking methanol as a solvent, and heating up to 40-50 DEG C; dissolving carbohydrazide by taking water as a solvent, then dropwise adding the dissolved carbohydrazide into the reaction flask, and carrying out reflux reaction for 3h; after the reaction, filtering and drying a mixed solution for removing the solvents to obtain the Schiff base fluorescent probe for the copper ions. The method is simple in operation, convenient and fast, low in cost of a needed intermediate and easy in control of reaction process; the product is easy to separate, high in yield and high in purity. The fluorescent probe has special recognition performance for the copper ions, thus having wide application prospect in the aspects of sensor technologies, photochemistry, electroluminescent devices, and the like.
Owner:WENZHOU MEDICAL UNIV
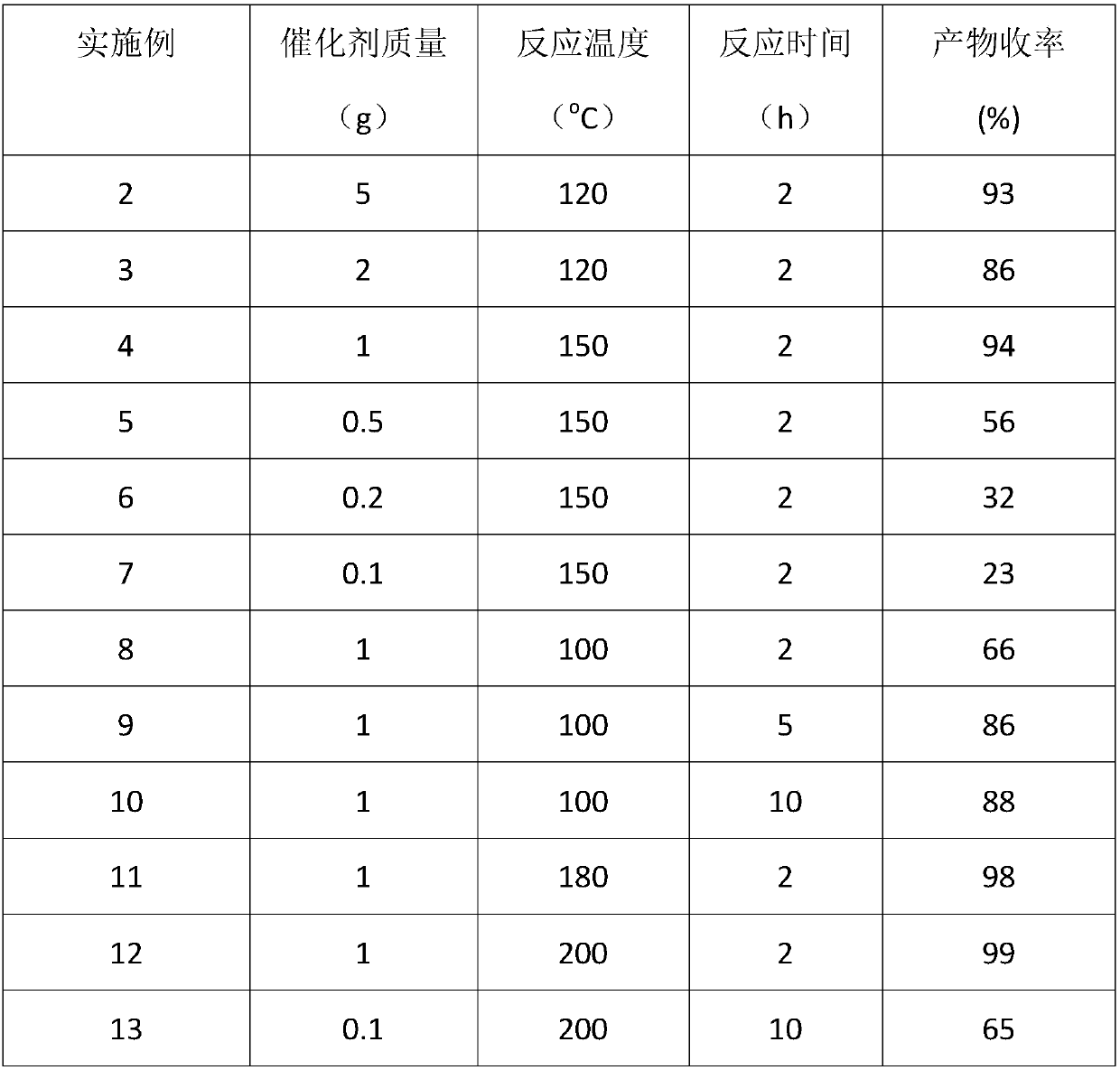
Method for preparation of cyclic myrac aldehydes from myrac aldehyde catalyzed by zinc-containing ionic liquid
ActiveCN109651122AAvoid it happening againHigh selectivityOrganic compound preparationCarbonyl compound preparationOrganic solventZinc
The invention provides a novel method for catalyzing cyclization reaction of myrac aldehyde for conversion to cyclic myrac aldehydes by using a Lewis acidic ionic liquid as a catalyst. According to the method, by using a zinc-containing ionic liquid as a catalyst and under the mild condition, 4-(4-methyl-3-pentenyl)-3-cyclohexenal(p-myrac aldehyde) and 3-(4-methyl-3-pentenyl)-3-cyclohexenal (m-myrac aldehyde) undergo selective cyclization to prepare cyclic myrac aldehydes (1,2,3,4,5,6,7,8-octahydro-,8,8-dimethyl-2-naphthaldehyde and 1,2,3,4,5,6,7,8-octahydro-5,5-dimethyl-2-naphthaldehyde). Incomparison with a traditional cyclic myrac aldehydes production method, the invention has the following distinctive characteristics: the use of a volatile organic solvent is avoided in the reaction; the reaction condition is mild; the target product has high selectivity, and the reaction is rapid; after the reaction, the ionic liquid catalyst and the product are automatically layered and are convenient to separate; the ionic liquid catalyst is recyclable; generation of a waste acid solution by traditional catalysis is avoided; and the method is environmentally friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
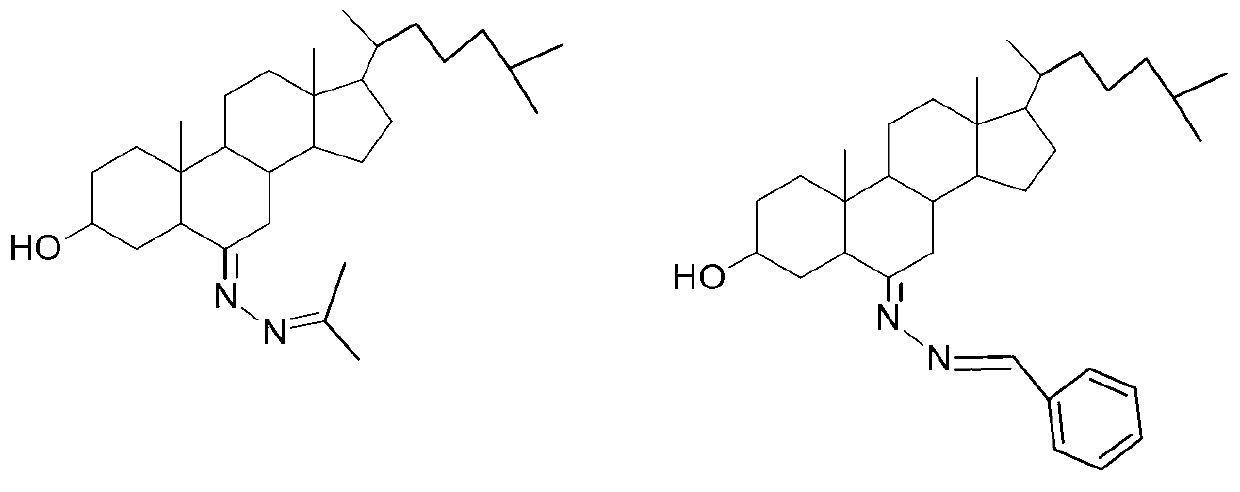
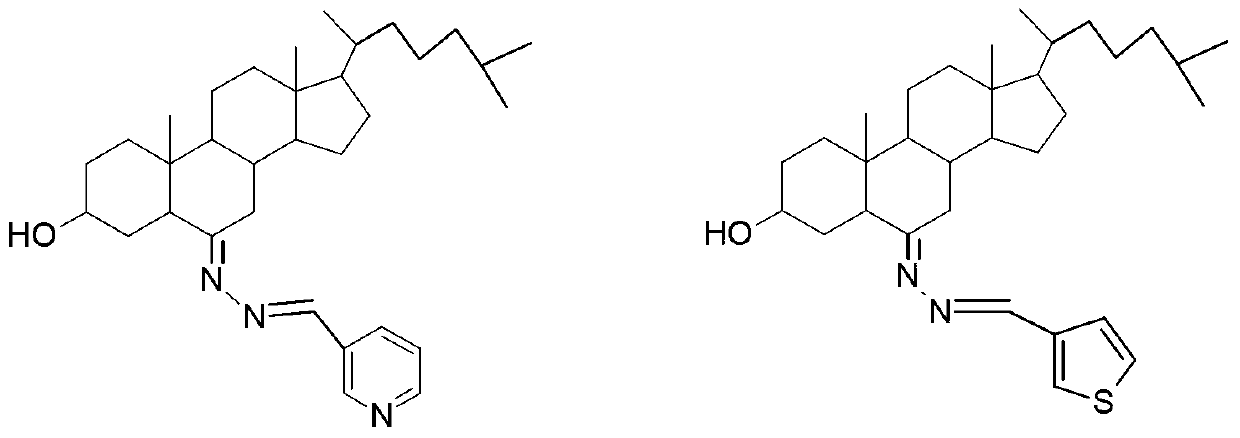
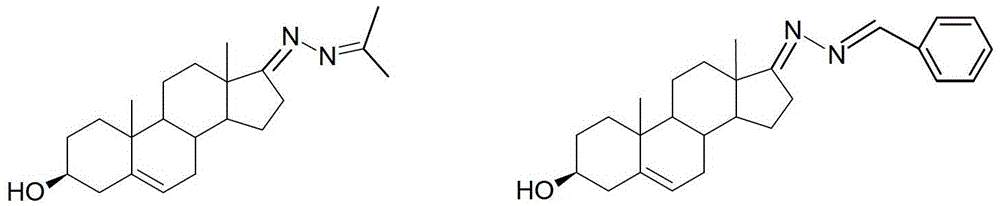
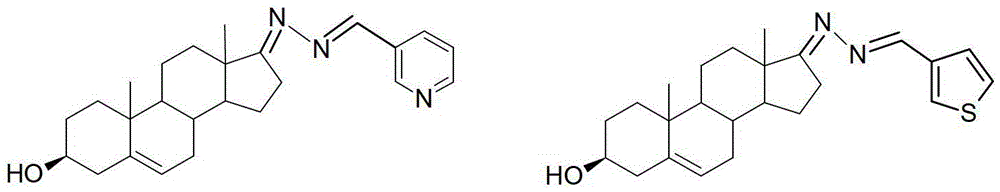
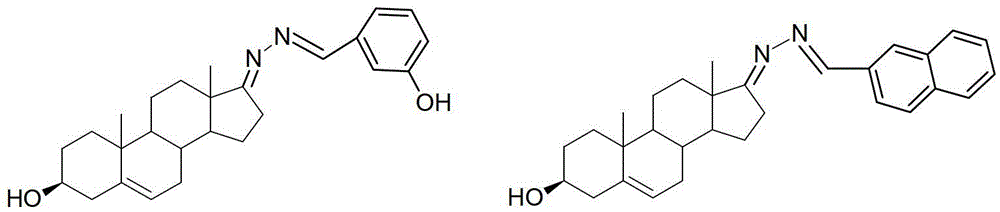
3-hydroxyl cholest-6-keto aromatic aldehyde azine steroidal compound, synthetic method of steroidal compound and application of steroidal compound in preparation of anti-tumour drug
InactiveCN103275161AGood inhibitory effectOrganic active ingredientsSteroidsBenzaldehydeStructural formula
The invention relates to a 3-hydroxyl cholest-6-keto aromatic aldehyde azine steroidal compound with an azine structure. The structural formula of the steroidal compound is described in the specification. 3-hydroxyl cholest-6-keto acetone acetone azine (1), 3-hydroxyl cholest-6-keto benzaldehyde azine (2), 3-hydroxyl cholest-6-keto-3-pyridine methanol azine (3), 3-hydroxyl cholest-6-keto-3-thiophene methanol azine (4), 3-hydroxyl cholest-6-keto-3-hydroxy benzaldehyde azine (5), 3-hydroxyl cholest-6-keto-2-naphthaldehyde azine (6), 3-hydroxyl cholest-6-keto-4-quinolinecarboxaldehyde azine (7), and 3-hydroxyl cholest-6-keto-6-methoxy naphthaldehyde azine (8) are shown in the specification. Experiments prove that the 3-hydroxyl cholest-6-keto aromatic aldehyde azine steroidal compound has an obvious inhibiting effect on multiple tumour cell strains and can be applied to preparation of a drug used for treating cancer.
Owner:GUANGXI TEACHERS EDUCATION UNIV
A fluorescent probe for distinguishing dead/living cells and its synthesis method and application
InactiveCN108373447BThe synthesis method is simpleHigh yieldOrganic chemistryFluorescence/phosphorescenceFluorescenceSynthesis methods
The invention provides a target and fluorescence color variable fluorescence probe and application thereof in distinguishing of imaging dead and living cells. A chemical name of the fluorescence probeis 2-(6-methoxy-6-naphthylvinyl)-N-methyl-quinoline iodate. The fluorescence probe is synthesized by steps: using 2-methylquinoline and iodomethane to synthesize 1,2-dimethylquinoline iodate; subjecting the 1,2-dimethylquinoline iodate and 6-methoxy-2-naphthaldehyde to condensation reaction at the room temperature to generate a product by taking pyrrolidine as a catalyst. The synthesis method ofthe probe is simple, dead and living cells can be distinguished and marked by two fluorescence colors and two dyeing positions, and a promising application prospect is achieved.
Owner:UNIV OF JINAN
Method of selective cyclization of myrac aldehyde catalyzed by supported tungsten carbide for preparation of cyclic myrac aldehydes
ActiveCN109651123ALow costMild reaction conditionsMolecular sieve catalystsOrganic compound preparationOrganic solventNon noble metal
The invention relates to a cyclization reaction of myrac aldehyde catalyzed by tungsten carbide, specifically to a method of selective cyclization of myrac aldehyde catalyzed by supported tungsten carbide for preparation of cyclic myrac aldehydes. According to the method, para- / meta- myrac aldehyde is used as a raw material to undergo a highly selective reaction in an organic solvent at 50-150 DEGC to generate cyclic myrac aldehydes 1,2,3,4,5,6,7,8-octahydro-,8,8-dimethyl-2-naphthaldehyde and 1,2,3,4,5,6,7,8-octahydro-5,5-dimethyl-2-naphthaldehyde. The conversion rate of the substrate reachesup to 100%, and yield of the target product reaches up to 99%. Compared with the conventional catalytic route, the supported non-noble metal tungsten carbide is used as a catalyst without the use ofinorganic acid or alkali in the invention, thus avoiding generation of a large amount of acid liquor by traditional catalysis. The invention has the following characteristics: the reaction condition is mild; the catalyst is cheap and recyclable and has high activity and selectivity; and the reaction process is environmentally-friendly.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Cyanide acceptor compound, preparation method and application based on 2-cyano-3-(6-n,n-dimethylamino-2-naphthyl)acrylonitrile
ActiveCN105037202BEasy to synthesizeRaw materials are easy to getCarboxylic acid nitrile preparationMaterial analysis by observing effect on chemical indicatorBinding siteAcrylonitrile
The invention discloses a cyanide receptor compound based on 2-cyano-3-(6-N, N-dimethylamino-2-naphthyl) acrylonitrile, a preparation method and application thereof. The compound takes a C=C double bond as a reactive binding site and takes 6-N, N-dimethylamino-2-naphthaldehyde as a fluorescence signal reporter group. When a receptor encounters cyanide ions, the cyanide ions and the C=C double bond of the receptor can carry out addition reaction so as to cause proton transfer and charge transfer in receptor molecules, so that the receptor molecules have color and fluorescence changes. The invention respectively researches the identification effects of the compound to F<->, Cl<->, Br<->, I<->, Ac<->, H2PO4<->, HSO4<->, ClO4<-> and CN<-> through colorimetry, ultraviolet-visible absorption spectroscopy and fluorescent spectrometry. The results show that the receptor compound can singly and selectively identify cyanide ions in an HEPES-DMSO-H2O system and has very high sensitivity to detection of CN<->.
Owner:NANJING UNIV OF SCI & TECH
Dehydroepiandrosterone aromatic aldehyde azine steroid compound and its synthesis method and application in the preparation of antitumor drugs
The invention relates to a dehydroepiandrosterone aromatic aldehyde azine steroidal compound with an azine structure. The chemical structural formula is as shown by dehydroepiandrosterone acetone azine (1), dehydroepiandrosterone benzaldehyde azine (2), dehydroepiandrosterone-3-pyridylaldehyde azine (3), dehydroepiandrosterone-3-thiophenecarboxaldehyde azine (4), dehydroepiandrosterone-3-hydroxybenzaldehyde azine (5), dehydroepiandrosterone-2-naphthaldehyde azine (6), dehydroepiandrosterone-4-quinolinecarboxaldehyde azine (7) and dehydroepiandrosterone-6-methoxy-naphthaldehyde azine (8) described in the specification. Experiments prove that the dehydroepiandrosterone aromatic aldehyde azine steroidal compound has an obvious inhibitory effect on various tumor cell strains, and can be applied to preparation of drugs for treating cancers.
Owner:NANJING GREEPHARMA INC LTD
A kind of synthetic method of high temperature resistant polyarylether nitrile resin
ActiveCN105754089BImprove heat resistanceDoes not affect processing performanceFunctional monomerPolymer science
The invention discloses a synthesis method of high-temperature-resistant polyarylene ether nitrile resin. The synthesis method is characterized by comprising the following steps: placing 2-naphthaldehyde, 2,6-xylenol and toluene in a reaction kettle, dropwise adding a sulfuric acid aqueous solution and 3-thiohydracrylic acid under nitrogen atmosphere, heating and concentrating after washing by deionized water and toluene, separating out a crystal and drying to obtain a bisphenol functional monomer; adding 2,6-dichlorobenzonitrile, a diphenol monomer, the bisphenol functional monomer, anhydrous potassium carbonate, N, N-dimethylpyrrolidine and toluene to the reaction kettle, carrying out heat preservation to obtain a polycondensation product; adding the polycondensation product to deionized water or the sulfuric acid aqueous solution to be boiled, performing suction filtration, washing a solid by deionized water and then drying the solid to prepare the high temperature resistant polyarylene ether nitrile resin. According to the invention, the bisphenol monomer of which the side group contains a naphthalene ring structure is introduced into molecules of the traditional polyarylene ether nitrile, so that the prepared polyarylene ether nitrile resin has good heat resistance and processing properties.
Owner:MIANYANG HONGQI NEW MATERIAL SCI & TECH
Preparation process of 3,6-dihydroxy-2-naphthaldehyde
InactiveCN110218149AHigh yieldFew reaction stepsOrganic compound preparationCarbonyl compound preparationBromineOrganic synthesis
The invention belongs to the field of organic synthesis, and particularly relates to a preparation process of 3,6-dihydroxy-2-naphthaldehyde. The process includes a step 1) of subjecting 2,7-dihydroxynaphthalene to bromination with liquid bromine to form a first compound the structure of which is shown as a formula I; a step 2) of subjecting the first compound to a nucleophilic substitution reaction to generate the 3,6-dihydroxy-2-naphthaldehyde. The process solves a problem that preparation processes in the prior art have long synthesis steps and unsatisfactory yields.
Owner:GUANGDONG UNIV OF TECH
High-performance nano conductive adhesive and preparation method thereof
InactiveCN106189965AImprove conductivityImprove stabilityNon-macromolecular adhesive additivesMacromolecular adhesive additivesSodium acetateIndium
The invention discloses a high-performance nano conductive adhesive and a preparation method thereof. The conductive adhesive is prepared from sulfonated phenolic resin, fluoro-siloxane resin, maleic resin, bismuth oxychloride, nanometer silver powder, nanometer ruthenium dioxide, nanometer zinc dioxide, nanometer indium powder, 6-methoxy-2-naphthaldehyde, phosphorus trichloride, 2-cyanophenylboronic acid, cashew nut shell oil, N,N-dioctadecylmethylamine, 3,3'-dichloro-4.4'-diaminodiphenyl-methane, calcium hydroxide, diethylene glycol monobutyl ether, bismuth octoate, naphtha, 801 rubber powder, carnauba wax, allylpolyethyleneglycol, magnesium aluminum silicate, hydrogenated castor oil, sodium acetate and ethyl acetate. According to the high-performance nano conductive adhesive and the preparation method thereof, the volume resistivity of the conductive adhesive is low, the conductivity property is excellent, the stability is good, the adhesive property and heat-conducting property are good, the conductive adhesive can be widely applied to conductive connection of electronic industries such as integrated circuits, LED and PCB, and a very wide application prospect is achieved.
Owner:王泽陆
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com