Fluorescent probe for detecting cysteine and application thereof
A fluorescent probe, cysteine technology, applied in the field of analytical chemistry, can solve the problems of complex instruments, unsuitable for real-time detection of Cys, etc., and achieve the effect of simple post-processing process and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
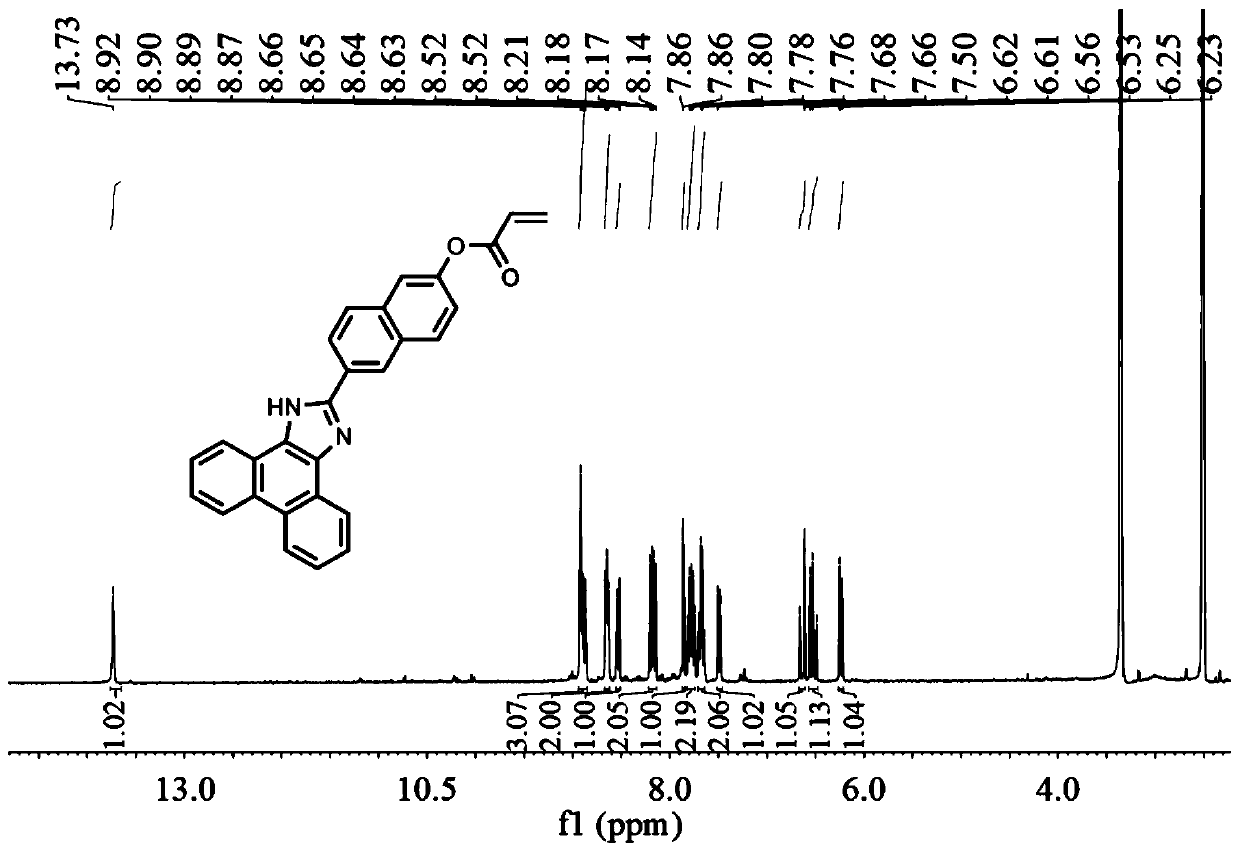
[0025] Example 1 Synthesis of Fluorescent Probes
[0026] Weigh 9,10-phenanthrenequinone (146 mg, 0.7mM, 1eq), 6-hydroxybenzaldehyde (362 mg, 2.1mM, 3 eq), ammonium acetate (185 mg, 1.05 mM, 1.5 eq) in 20 ml of acetic acid, heated to reflux at 110°C for 10 h. Use a TCL plate to detect the reaction. After the reaction is complete, cool to room temperature and pour into water for suction filtration. The obtained crude product is separated with a silica gel column. The silica gel particle size is 200-300 mesh, and the eluent is ethyl acetate / petroleum ether=1: 2. The eluent was spin-dried under reduced pressure to obtain compound 1: , the yield is 67%;
[0027] Dissolve compound 1 (169 mg, 0.47 mM, 1 eq) in 5 ml of dichloromethane, add 2 drops of triethylamine, add acryloyl chloride (72 mg, 0.7 mM, 1.5 eq) dropwise at 0°C, and react at room temperature for 5 h , use a TCL plate to detect the reaction. After the reaction is complete, the solvent is spin-dried under reduced pre...
Embodiment 2
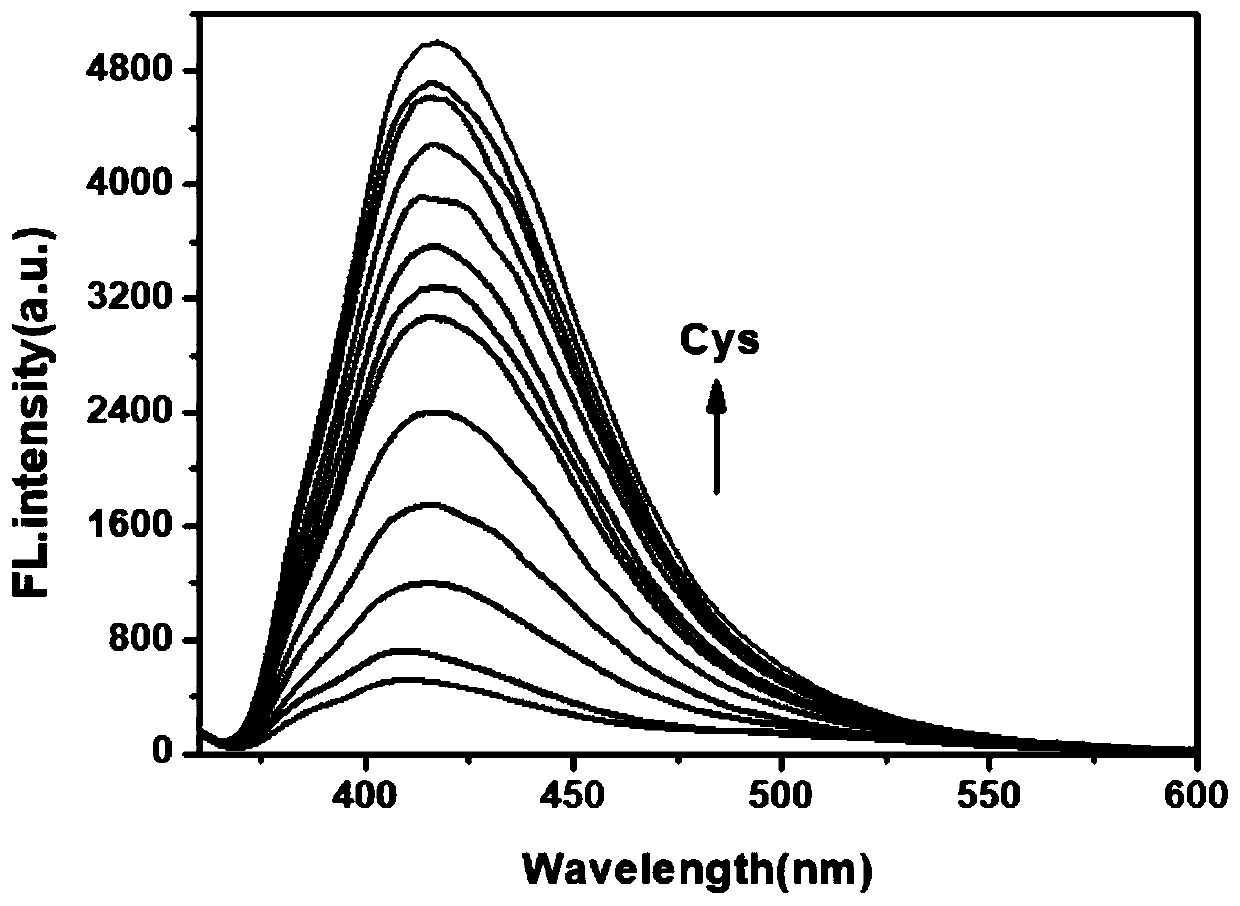
[0028] Example 2 Responses of fluorescent probes to different concentrations of cysteine
[0029] The cysteine fluorescent probe prepared in Example 1 was dissolved in dimethyl sulfoxide (DMSO) to prepare a 1 mmol / L stock solution. Take 20 μL from the stock solution and add it to a 4 mL centrifuge tube, add different equivalents (0-20 eq) of Cys standard solution, use PBS buffer solution (0.1 mol / L, pH=7.5) and DMF volume ratio of 9 :1 solution was diluted to 2 mL, and its fluorescence properties were measured. Fluorescence spectra such as figure 2 shown. Depend on figure 2 It can be seen that with the addition of Cys concentration, the fluorescence intensity gradually increases at 414 nm.
Embodiment 3
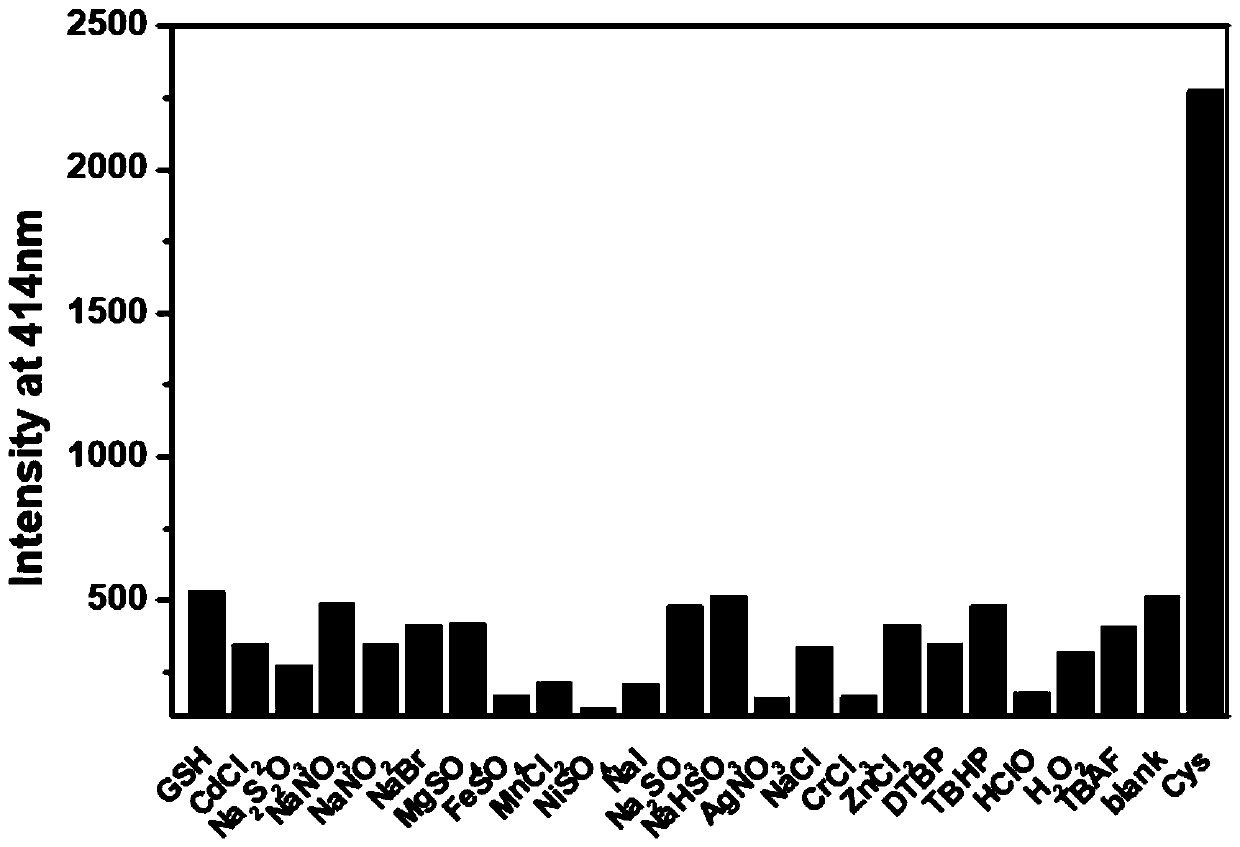
[0030] Example 3 Selectivity of fluorescent probes to different ions
[0031] Take 20 μL from the fluorescent probe stock solution in Example 2 and add it to a 4 mL centrifuge tube, add equimolar amounts of different competitor molecule standard solutions, one of which is added with an equimolar amount of Cys standard solution, and detect after 30 min The fluorescence emission spectrum of the solution changes, image 3 It can be found that other ions have almost no effect on the fluorescence of the compound, but the addition of Cys solution significantly changes the fluorescence of the compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com