Method of selective cyclization of myrac aldehyde catalyzed by supported tungsten carbide for preparation of cyclic myrac aldehydes
A tungsten carbide catalyzed and supported technology is applied in the preparation of carbon-based compounds, chemical instruments and methods, catalysts for physical/chemical processes, etc. Mild conditions, low cost, high activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] W 2 C / AC(W 2 C is tungsten carbide, and AC is activated carbon) The specific preparation method is as follows:
[0018] Dissolve ammonium metatungstate in water so that the mass concentration of ammonium metatungstate is 0.4g / ml. Then, the solution was impregnated with activated carbon support (AC) by the method of equal volume impregnation. After oven drying at 120 °C for 12 h, the catalyst precursor was placed in H 2 A temperature-programmed carbothermal reaction was carried out in the atmosphere. The specific reaction process was as follows: 1.0 g of precursor was heated from room temperature to 400 °C in a quartz reaction tube for 1 h, then raised to 700 °C at 1 °C / min and kept for 1 h for carbonization. The hydrogen flow rate was 60ml / min. A tungsten carbide loading of 30wt% W 2 C / AC catalyst.
[0019] Other conditions remain the same, only change the concentration of ammonium metatungstate in the impregnation solution to obtain catalysts with different loadi...
Embodiment 2
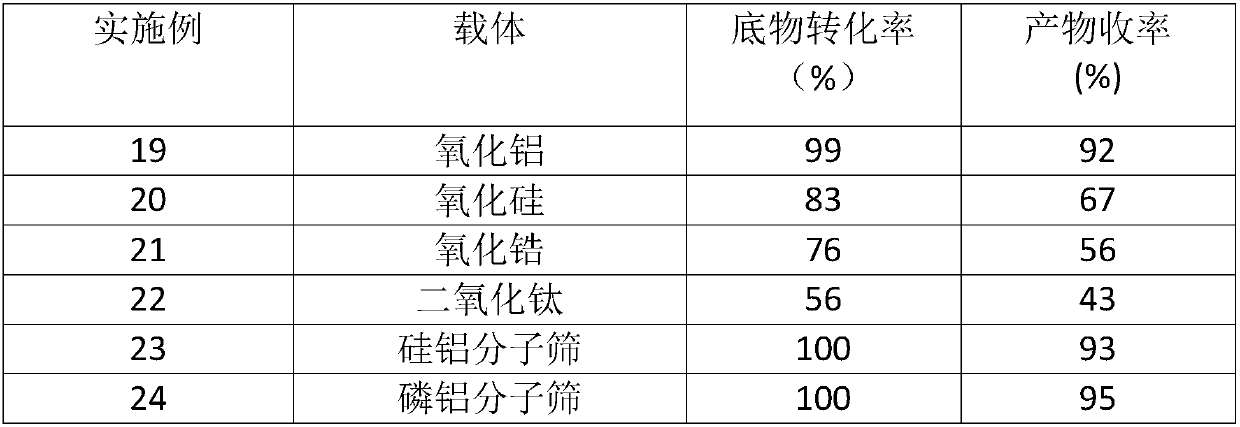
[0021] Tungsten carbide is supported on alumina, silica, titania, zirconia, titania, silica-alumina molecular sieve, and phosphorus-aluminum molecular sieve to prepare supported tungsten carbide catalyst WxC / B (WxC is tungsten carbide, 1≤x≤2; B It is a porous carrier, which is activated carbon, alumina, silica, zirconia, titania, silica-alumina molecular sieve, phosphorus-aluminum molecular sieve). : The preparation process is similar to Example 1, except that the carrier uses alumina, silica, zirconia, titania, silicon-aluminum molecular sieve, phosphorus-aluminum molecular sieve instead of activated carbon, and at the same time, the carbonization gas is changed from hydrogen to CH 4 / H 2 (Volume ratio 1:4), the loading amount of tungsten carbide in the catalyst is 30wt%, thus obtained six kinds of catalysts with tungsten carbide loaded on alumina, silica, zirconia, titania, silica-alumina molecular sieve, and phosphorus-aluminum molecular sieve .
Embodiment 3-18
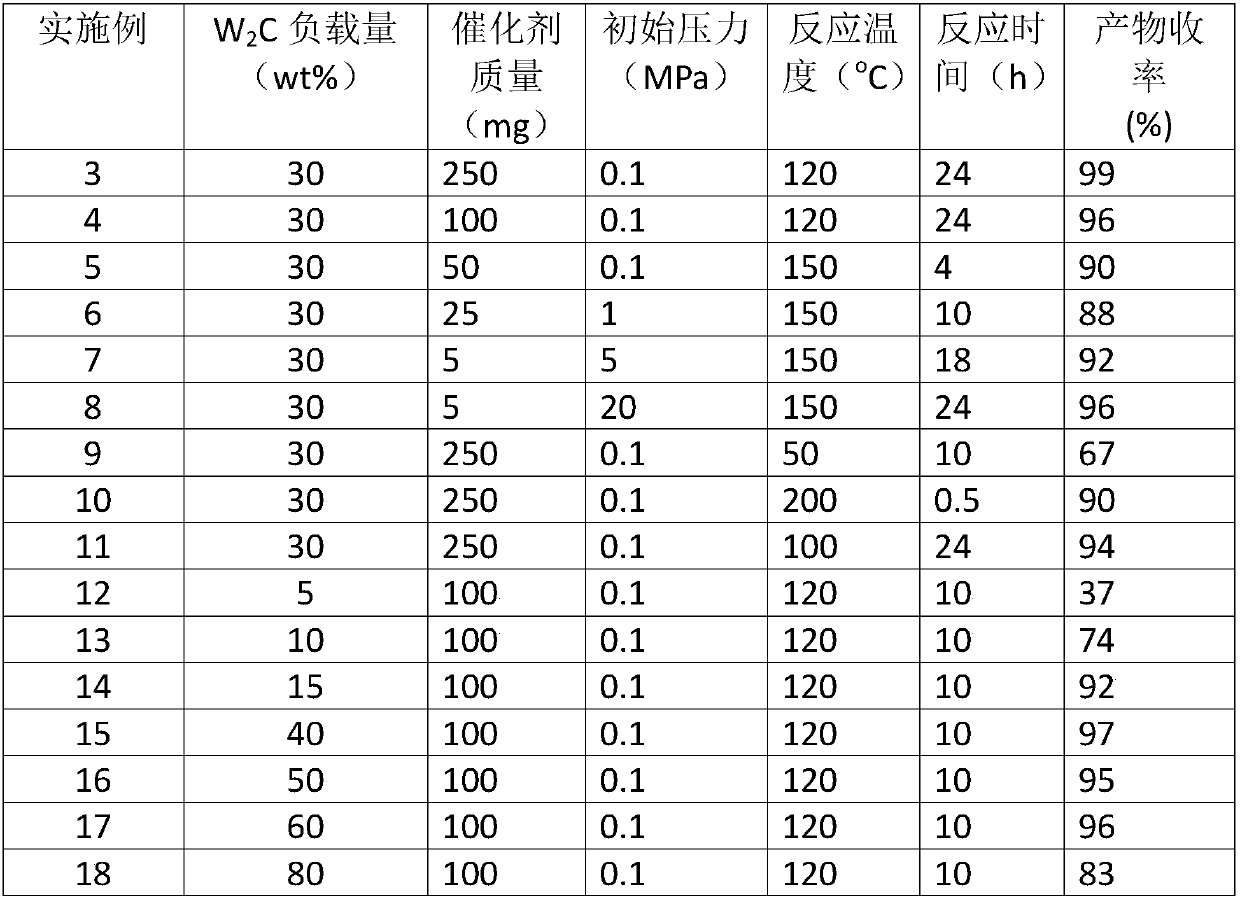
[0023] W 2 C / AC catalyzed the selective cyclization of p-citrus aldehyde to prepare citrus aldehyde: 0.5 g of citrus aldehyde was mixed with a certain mass of W in the reaction kettle 2 The C / AC catalysts were dissolved in 50ml of toluene, and replaced with nitrogen five times to make the initial pressure of nitrogen 0.1MPa-20MPa, the temperature was raised to 50-200°C, and the stirring reaction was carried out at a speed of 1000 rpm for 0.5-24h. After the reaction was completed, the temperature was lowered to room temperature, and the supernatant was filtered and then sampled for analysis. Qualitative analysis of the product was carried out by GC-MS technology and standard sample control, and quantitative analysis was realized by gas chromatography internal standard method. The reaction results are shown in Table 1.
[0024] Table 1 W under different conditions 2 C / AC catalyzed the cyclization reaction of 4-(4-methyl-3-pentenyl)-3-cyclohexenal (p-citral) to generate cycloc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com