Applications of phenyl naphthalene type lignin in preparation of anti-rheumatoid arthritis drugs
A technology for rheumatoid and arthritis, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of compound VNL
[0023] (1) Preparation of extracting liquid: After pulverizing the medicinal materials of the fruit of Vitex pratense, put it into the extraction tank, heat extract with 40-90% ethanol for 2-3 times, and the amount of each solvent is about 8-10 times of the crude drug amount, and each extraction The time is 1 to 2 hours, and the extracts are combined;
[0024] (2) Refining, concentrating and drying: the above-mentioned extract is concentrated under reduced pressure, the solvent is recovered, the concentrated solution is degreased with petroleum ether, extracted with ethyl acetate, and concentrated to obtain the ethyl acetate part;
[0025] (3) Separation and purification: dissolve the above-mentioned ethyl acetate part in water and load the macroporous adsorption resin AB-8. After the sample is adsorbed, wash with 5 times the volume of the column bed to remove impurities, and then use the volume fraction of 20 , 40, 60, 8...
Embodiment 2
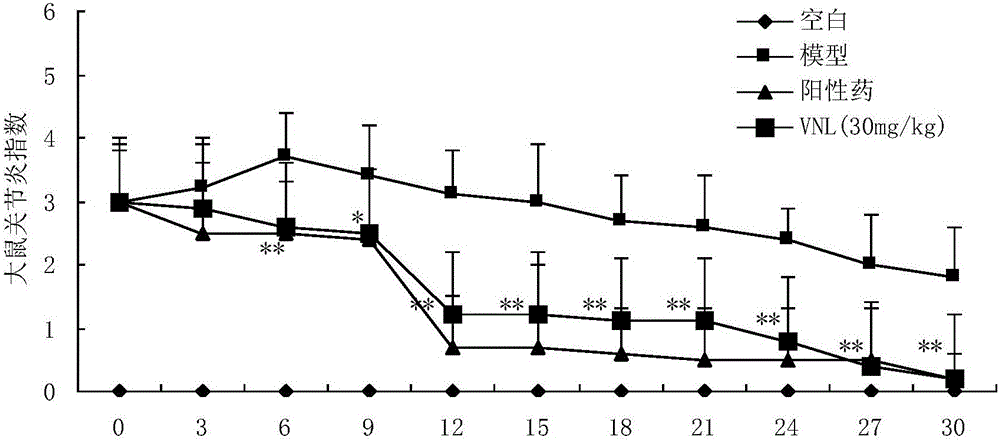
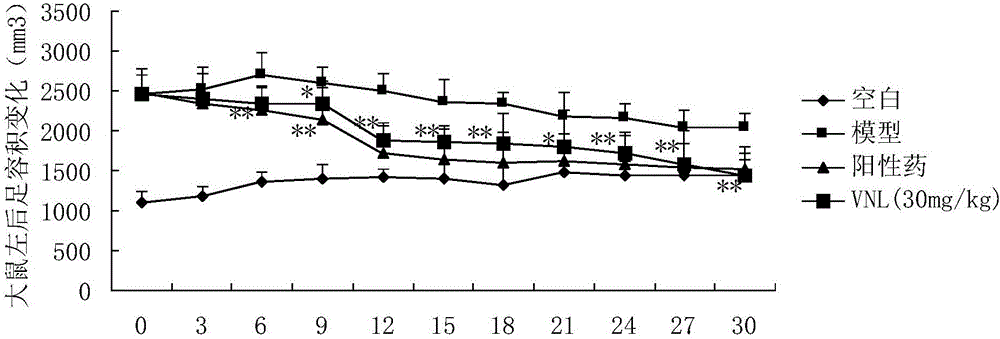
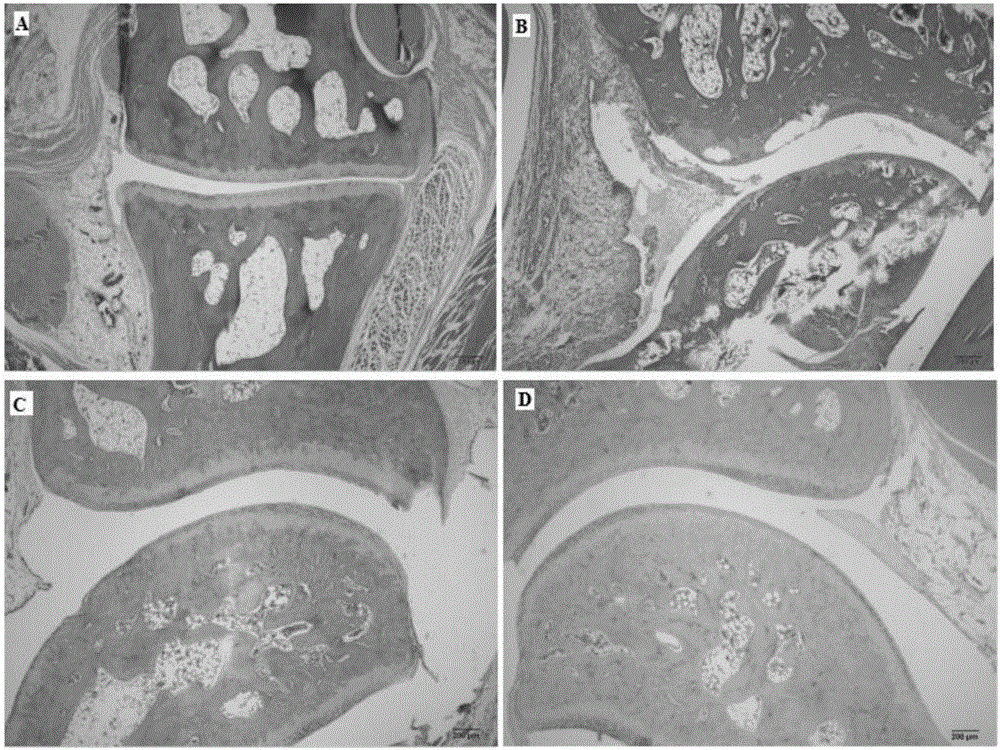
[0028] Example 2: Anti-rheumatoid arthritis pharmacodynamics experiment of compound VNL
[0029] 2.1 Experimental materials
[0030] 40 adult male SD rats of 180-220 g (provided by the Experimental Animal Center of the Second Military Medical University) and ELISA kits for rat serum TNF-α and IL-1 (Sigma, USA). Main instruments: toe volume measuring instrument (YLS-7A, equipment station of Shandong Academy of Medical Sciences), microplate reader (BIO-RAD550), centrifuge (Labofuge400R, Heraeus company), electronic balance (type FA110A, Shanghai Precision Scientific Instrument Co., Ltd. company).
[0031] 2.2 Experimental modeling and administration
[0032] Before the experiment, 40 rats were weighed and randomly divided into 5 groups according to body weight, with 10 rats in each group, free to eat and drink, and to drink water. Divided into blank group, model group, positive drug group and compound VNL group, VitedoinA group.
[0033] Blank group: no treatment;
[0034] ...
Embodiment 3
[0055] Embodiment 3: the acute toxicity test of compound VNL
[0056] 3.1 Experimental animals
[0057] 18-22g ICR mice (provided by the Experimental Animal Center of Second Military Medical University) were 50, half male and half male. 3.2 Drug treatment
[0058] Single intragastric administration of VNL0.125, 0.25, 0.50, 1.00 and 2.00g / kg (maximum administration capacity, maximum drug dissolution concentration) to mice, observe the toxicity and death of animals within 14 days after administration, and determine the LD50 Or the maximum tolerated dose, the dead animals were autopsied within 24 hours, and macroscopic and microscopic examinations were carried out. The surviving animals were weighed on the 15th day and sacrificed, dissected, and macroscopic and microscopic examinations were performed to see if there was any lesion in each organ.
[0059] 3.3 Observation indicators
[0060] (1) Weigh the body weight of the animals at the end of the experiment.
[0061] (2) Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com